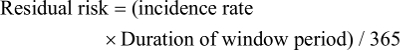Back to Journals » Journal of Blood Medicine » Volume 10
Residual risk of HIV, HCV, and HBV transmission by blood transfusion between 2015 and 2017 at the Regional Blood Transfusion Center of Ouagadougou, Burkina Faso
Authors Yooda AP, Sawadogo S , Soubeiga ST, Obiri-Yeboah D , Nebie K, Ouattara AK , Diarra B, Simpore A , Yonli YD , Sawadogo AG, Drabo BE, Zalla S, Siritié AP , Nana RS , Dahourou H, Simpore J
Received 2 October 2018
Accepted for publication 18 December 2018
Published 1 February 2019 Volume 2019:10 Pages 53—58
DOI https://doi.org/10.2147/JBM.S189079
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin Bluth
Arzouma Paul Yooda,1–3 Salam Sawadogo,3 Serge Théophile Soubeiga,1,2 Dorcas Obiri-Yeboah,4 Koumpingnin Nebie,3 Abdoul Karim Ouattara,1,2 Birama Diarra,1,2 Abibou Simpore,3 Yetema Dieudonné Yonli,3 Abdoul-Guaniyi Sawadogo,3 Bia Emile Drabo,3 Seimbou Zalla,3 Anita Pierrette Siritié,3 Rodrigue Sosthène Nana,3 Honorine Dahourou,3 Jacques Simpore1,2
1Laboratory of Molecular Biology and Genetics (LaBioGene), Training and Research Unit in Life and Earth Sciences, University Ouaga I Professor Joseph Ki-Zerbo, Ouagadougou, Burkina Faso; 2Pietro Annigoni Biomolecular Research Center (CERBA), Ouagadougou, Burkina Faso; 3National Blood Transfusion Center (NBTC), Ouagadougou, Burkina Faso; 4Department of Microbiology and Immunology, School of Medical Sciences, University of Cape Coast, Ghana
Introduction: In sub-Saharan Africa, the high endemicity of blood-borne infections is a serious threat to transfusion safety. In order to improve transfusion safety, Burkina Faso has undertaken in recent years a reorganization of its blood-transfusion system through the creation of a National Blood Transfusion Center, which is the only blood operator in the whole country. This study aimed to estimate the residual risk of transmission of HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV) by blood transfusion at the Regional Blood Transfusion Center (RBTC) of Ouagadougou.
Methods: This was a retrospective study conducted at the RBTC of Ouagadougou between 2015 and 2017. Prevalence of infectious markers was calculated for first-time donors and incidence rates calculated for repeat donors who had made at least two donations of blood over the study period. Residual risks were estimated for the three viruses (HIV, HBV, and HCV) by multiplying the incidence rate per 100,000 person-years by the respective durations of serological windows.
Results: Between 2015 and 2017, of a total of 84,299 blood donors, 68,391 (81.13%) were first-time donors compared to 15,908 (18.87%) repeat donors. The seroprevalence of HBV (8.56%) was twice that of HCV (4.40%) and fourfold that of HIV (1.80%). Incidence rates were 1,215, 2,601, and 1,599 per 100,000 donations for HIV, HCV, and HBV, respectively. In contrast, the estimated residual risk for HCV (1 in 213 donations) was double that of HBV (1 in 408 donations) and four times that of HIV (1 in 1,366).
Conclusion: The residual risk of transmission of these viruses by blood transfusion remains high in repeat donors. An effective donor-retention and education policy could help to reduce this residual risk.
Keywords: infectious diseases, prevalence, incidence, residual transfusion risk
Introduction
Blood transfusion contributes to saving lives every day around the world, but it can also be a source of transmission of infectious agents, including HIV, HBV, and HCV. Although the performance of serological tests has been considerably improved in recent years, there remains a residual risk of transmission of viruses by blood transfusion. This residual risk is essentially related to the serological window, the time between infection and when the serological test can reliably detect that infection.1 These infectious agents are major concerns for transfusion safety, especially in sub-Saharan Africa. Indeed, the highest prevalence of these three viruses is found in sub-Saharan Africa,1,2 where 12.5% of transfused patients are at risk of posttransfusion hepatitis. Moreover, 5%–10% of HIV1 infection in this region is attributable to unsafe blood transfusion.1,3 In Burkina Faso, studies have reported very high seroprevalence of these infections in both the general population and blood donors.4–6
In Burkina Faso, studies5–7 have reported very high seroprevalence of these infections in both the general population and blood donors. In addition, a survey8 conducted in 2005 among blood donors who attended the blood bank of Yalgado Ouédraogo Teaching Hospital concluded that there was insufficient knowledge of blood donors about transfusion-transmitted infections (TTIs). About 14.4% of blood donors were motivated at first donation by the result of HIV testing, and 40.3% did not have adequate knowledge of the concept of the HIV window. Most of thought that serological tests could reliably detect the infection immediately after exposure. Indeed, about 30% said that they would donate blood immediately if they were exposed to a risk of HIV infection. In order to improve transfusion safety, Burkina Faso, as recommended by the WHO,9,10 has undertaken since 2000 the reorganization of its transfusion system through the creation of a NBTC, which is the only blood operator in the country. The NBTC coordinates transfusion activities through four RBTCs, located in Ouagadougou, Bobo Dioulasso, Koudougou, and Fada N’Gourma. Since the operationalization of these RBTCs in 2005, blood policy has been continuously changed with integration of the recommendations of the WHO,9,10 such as recruitment of unpaid voluntary donors from populations at low risk of TTIs, medical predonation selection, retention of blood donors, and the use of fourth-generation serological reagents for screening of TTIs.
In addition, the NBTC is committed to a continuous quality-control approach aimed at improving the quality of blood products and its services. This study aimed to estimate the residual risk of transmission of HIV, HBV, and HCV by blood transfusion at the RBTC of Ouagadougou (RBTC/O) and to propose additional measures to prevent transfusion risks.
Methods
Type and population study
This was a retrospective study conducted at the RBTC/O (the largest transfusion center in Burkina Faso) from January 1, 2015 to December 31, 2017. It involved 84,299 subjects accepted for blood donation after medical predonation selection at fixed sites and mobile collection sites. Medical selection was performed by qualified health workers based on a standardized predonation questionnaire designed to identify situations and behavior at risk for HIV, HBV, and HCV.
Data collection
At the RBTC/O, at each stage of the transfusion chain (collection, biological qualification of donations, blood-component preparation and distribution), information related to blood donors and their donations is recorded and managed by medical software (CTS Inlog server, France). Therefore, donors who have been previously tested positive for HIV, HBV, and HCV are detected and excluded when they return for another donation. For this study, donations and donor information collected included years of collection, sex, age, address of the donor, status of donor (first time or repeated), collection site (fixed or mobile collection), and results (positive or negative) of serological tests for HIV, HBV, HCV, and syphilis. A repeated donor was define as a donor who had donated blood twice during the study period. Otherwise, they were considered a first-time donor.
Serological tests
Screening tests
HIV, HBV, and HCV serological tests were performed according to an algorithm defined by the NBTC. Initially, all donations tested with an Architect 1000 SR (Abbott Laboratories, Abbott Park, IL, USA). Architect HIV Ag/Ab Combo kits (Abbott), Architect HBsAg Qualitative II (Abbott), and Architect Anti-HCV (Abbott) were used to detect anti-HIV1 HIV1 antibodies and antigen P24 of HIV, surface-antigen HBs of HBV, and anti-HCV antibodies, respectively.
Confirmatory strategies
Due to limited resources, positive samples were not confirmed by reference tests, such as Western blot, for HIV or nucleic acid testing. Nevertheless, the algorithm applied by the NBTC defined confirmatory strategies. For HIV, initial reactive samples were retested in duplicate using the same kit. Negative results in duplicate were considered negative. In cases of positive reaction, a parallel algorithm sampling two rapid diagnostic tests (RDTs), a screening test (Determine HIV1/2; Alere, Waltham, MA, USA) and an HIV1 and HIV2 discriminant test (ImmunoComb II HIV1/2; Orgenics, Yavne, Israel) was applied. Positive samples in both RDTs were considered positive. Discordant samples in both RDTs or between RDT and Architect were considered indeterminate.
For HBV and HCV, initial reactive samples were retested using Alere Determine HBsAg and SD Bioline HCV (Standard Diagnostics, Yongin, South Korea), respectively. Positive samples in both tests were considered positive, and discordant samples in both tests were considered indeterminate. Blood units detected positive or indeterminate for one TTI were discarded. Donors with indeterminate results were invited for a control test 3 months later.
Calculation of prevalence, incidence, and residual risk
The annual prevalence rates calculated for first-time donor by dividing the total number of 1-year positive donors for each virus (HIV, HCV, and HBV) by total number of first-time donors in the same year. The incidence rates were calculated for repeat donors who had made at least two donations in the study period as number of seroconverting (negative donation followed by positive) donors divided by the total number of person-years (PY). Number of PY was calculated by summing intervals in days between the first and the last donation for each donor during the study period (2015–2017), divided by 365. The residual risk of transmission of a viral infection related to the window period was estimated from the equation:11
|
Window durations were obtained from literature data:11,12
- 22 days for HIV
- 66 days for HCV
- 56 for HBV
To take into account the transient nature of HBV antigenemia described in the literature,11,13 we multiplied the incidence rate by 0.4.
Statistical analyses
Data were analyzed using Epi Info version 7.0 statistical analysis software.
Ethical considerations
This study received the approval of the Ethics Committee for Health Research of Burkina Faso (deliberation 2015-6-080). Donors gave written informed consent, and the study was conducted according to the Declaration of Helsinki. The anonymity and confidentiality of the serological results of the donors were respected. To do this, tests were performed in the laboratory using donation identification numbers. The results were returned to donors by doctors or health workers authorized for this purpose.
Results
Basic characteristics of population under study
A total of 84,299 blood donors were included in this study. The age of donors ranged from 17 to 65 years, with an average of 27.29±8.81 years, 59,979 (71.15%) were men, and 68 391 were donors for the first time (Table 1). Of repeat donors, 9,970 (62.67%) had made 2 donations, 2,829 (17.78%) 3 donations, 1,191 (7.49%) 4 donations, 635 (3.99%) 5 donations, and 1,283 (8.07%) had donated between 6 and 13 donations.
  | Table 1 Basic characteristics of the population under study |
Seroprevalence of HIV, HCV, and HBV among first-time donors
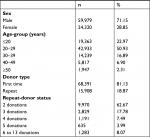
Of 68,391 first-time donors, 1,231 (1.8%) tested positive for HIV, 3,011 (4.4%) for HCV, and 5,854 (8.56%) for HBV (Table 2). HIV–HBV, HBV–HCV, HIV–HCV, HIV–HBV–HCV coinfections comprised 73 (0.11%), 143 (0.21%), 39 (0.06%), and 18 (0.03%) cases, respectively.
  | Table 2 Seroprevalence of HIV, HCV, and HBV in first-time donors at the RBTC/O Abbreviation: RBTC/O, RBTC of Ouagadougou. |
Incidence rate and residual risk of transmission of HIV, HCV, and HBV among repeat donors to the RBTC/O during 2015–2017
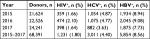
Between 2015 and 2017, of a total of 15,908 repeated donors, incident cases were 240 for HIV, 514 for HCV, and 316 for HBV. Nine cases of coinfection were observed: seven HBV–HCV and two HIV–HBV. Incidence rates were estimated at 1,215 per 100,000 PY for HIV, 2,601 per 100,000 PY for HCV, and 1,599 per 100,000 PY for HBV (Table 3). The residual risk of transmission was estimated at 1 in 1,366 donations for HIV, 1 in 213 donations for HCV, and 1 in 408 donations for HBV.
Discussion
In this study, we determined the residual risk of HIV, HBV, and HCV transmission by blood transfusion using the window-period method. This approach considered only donors who had donated blood at least twice (which represented only 18% of our study population). Therefore, this risk could have been underestimated. In addition, the confirmatory strategy defined by the algorithm for serological testing does not include gold-standard tests, such as Western blot or nucleic acid testing. This limits the reliability of our findings.
HIV seroprevalence (1.80%) in our study was lower than the 9.8%14 in 2002 and 4.6%15 2006 reported among blood donors in Ouagadougou, but it was similar to findings reported in 2009.16 HIV seroprevalence in blood donors reflected the seroprevalence in the general population in Ouagadougou, our study area. Indeed, in this region, HIV seroprevalence is 2% and remains higher compared to the national prevalence (0.8%)17 in 2016.
The seroprevalence of HBV (8.56%) in our study was lower than previously reported: 19.2% in 2002,14 17.3% in 2006,15 and 13.4% in 200916 among blood donors in Ouagadougou. These findings were consistent with the evolution of HBV seroprevalence in the general population in Ouagadougou (14.5%18 in 2014 to 10.4%7 in 2017). In the general population, the prevalence of HBV was 9.1%.19 The trend in the epidemiology of HBV in Burkina Faso could be explained by the impact of awareness campaigns organized in recent years on modes of transmission of this virus and preventive measures, such as vaccination against HBV.
There was no difference in HCV seroprevalence in our study (4.4%) and that reported among blood donors in Ouagadougou in 2002 (5.2%),14 2009 (6.3%),15 and 2011 (4.4%).20 In the general population, the prevalence of HCV is 3.6%.19 The situation described regarding the prevalence of HIV, HBV, and HCV in blood donors would rather indicate a positive impact of measures to control these infections in the general population, not effectiveness of education and medical selection of blood donors. Moreover, a study21 reported that the predonation selection criteria were not effective to exclude all at-risk donors, showing that there was no significant difference between the positivity rate of TTIs among donors accepted for blood donation and those who had been denied.
Over the 2015–2017 period, incidence rates per 100,000 PY were estimated at 1,215 for HIV, 2,601 for HCV, and 1,599 for HBV. In contrast to the seroprevalence where HBV was higher thant HIV and HCV, the incidence rate was the highest for HCV. This observation was also reported in a recent study conducted at the RBTC of Bobo Dioulasso22 (second-largest center in the country). This may be due to the longer HCV window period (66 days) than that for HBV (56 days). Also, the risk in collecting blood from donors in the window is higher for HCV than for HBV. In addition, serological anti-HCV antibody tests without confirmation tests has led to large proportions of false-positive results.20,23 These incidence rates were lower than those obtained in blood donors in 2009 in Burkina Faso,16 (3679, 7,381, and 7,567 per 100,000 PY, respectively, for HIV, HCV, and HBV). In addition, incidence rates of HBV and HCV were comparable to those obtained in blood donors at the RBTC of Bobo Dioulasso.22 Our results show that despite the efforts made in recent years, including the recruitment of unpaid volunteer donors, medical predonation selection, and donor awareness of risk factors for HIV, HBV, and HCV, a high number of new infections were detected among repeated donors in Burkina Faso.
However, these incidence rates in our study were significantly higher than those reported in France24 (1.05 per 100,000 PY for HIV, 0.53 per 100,000 PY for HCV, and 0.72 per 100,000 PY) over the period 2008–2010. They were also higher than those found in the USA25 (2.98 per 100,000 PY for HCV) over the period 2007–2008. Over the 2015–2017 period, the estimated residual risk for HCV (1 in 213 donations) was double that for HBV (1 in 408 donations) and four times that for HIV (1 in 1,366).
The residual risk of HIV was higher than in other countries of sub-Saharan Africa – 4 times higher than in Côte d’Ivoire,26 20 times higher than in Senegal,27 11 times higher than in Gabon28 – indicating that Burkina Faso is one of the African countries with the highest risk of transmission of HIV by blood transfusion. This result is a real concern for transfusion safety for our country, and requires more efforts in donor education and medical selection. Also, it would be necessary to investigate the donor population to identify risk factors for HIV infection that are not taken into account in the current medical selection criteria.
For HCV and HBV, the estimated residual risk rates of 1 in 213 donations for HCV and 1 in 408 donations for HBV were comparable to those reported at the Bobo-Dioulasso RBTC22 (HCV, 1 in 313; HBV 1/302) and in Côte d’Ivoire,26 a neighboring country of Burkina Faso (HCV, 1 in 406; HBV, 1 in 383). This could be explained by the similar seroprevalence in both countries. On the other hand, these rates were higher than those in other countries in sub-Saharan Africa: Senegal27 (HBV, 1 in 976) and Gabon28 (HCV, 1 in 4,808; HBV, 1 in 1,775).
Residual risk rates for HIV, HCV, and HBV in our study were also higher than in France24 (1 in 2,900,000 for HIV, 1 in 7,000,000 for HCV, and 1 in 1,350,000 donations for HBV) over the period 2008–2010 and in the USA25 (1 in 1,000,000 donations for HCV, 1 in 300,000 donations for HBV) over the 2007–2008 period. These large differences reflect the difference in the seroprevalence of these infections between developed and developing countries. Today, with the improvement in donor selection, advances in the biological qualification of donations, and efforts to prevent these infections in the general population, the residual risks of transmission of HIV, HCV, and HBV by blood transfusion are very low in developed countries.24 In contrast, posttransfusion infectious risk remains a major public health concern in sub-Saharan Africa, because of the very high prevalence of these infections and the lack of financial resources for the establishment of adequate infrastructure and logistics, as well as to train staff in blood-transfusion services. Moreover, insufficient donor knowledge about blood donation, blood transfusion, and transfusion-transmissible diseases does not advocate for effective medical predonation selection.8 This demonstrates the need to strengthen the education and information of blood donors and the general population in Burkina Faso.
Conclusion
Incidence rates and residual risk of transmission of HIV, HBV, and HCV by blood transfusion remain high in our country. It seems necessary to check risk behavior for HIV, HBV, and HCV in seroconverted blood donors, in order to undertake adequate changes in medical predonation criteria and strengthen the skills of blood-service staff for rigorous medical donor selection. In addition, we recommend the prevention of HBV infection by vaccination in repeated donors. Finally, the introduction of nucleic acid testing in donation screening will contribute to reducing the residual risk of transmission of HIV, HBV, and HCV by blood transfusion in Burkina Faso.
Abbreviations
HBV, hepatitis B virus; HCV, hepatitis C virus; PY, person year; NBTC, National Blood Transfusion Center; RBTC, Regional Blood Transfusion Center.
Acknowledgments
We thank all the staff of the Regional Blood Transfusion Center for their participation in the blood drives and the biological qualification of donations. We especially thank Cheick Ali Lingani and Kisito Kienou for management of the database (CTS server Inlog, France) and data extraction for this study.
Author contributions
APY, SS, KN, and JS oversaw the conception and design of the study. APY, SS, STS, AKO, and BD obtained data and drafted the article. AS, YDY, AGS, BED, SZ, APS, RSN, and HD performed the statistical analysis and interpretation. DOY, JS, and HD approved the final version of the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Kleinman S, Busch MP, Korelitz JJ, Schreiber GB. The incidence/window period model and its use to assess the risk of transfusion-transmitted human immunodeficiency virus and hepatitis C virus infection. Transfus Med Rev. 1997;11(3):155–172. | ||
UNAIDS. Global Report on AIDS Epidemic: Treatment and care; 2008. Available from: www.unaids.org/sites/default/files/media_asset/jc1510_2008globalreport_en_0.pdf. Accessed 09/04/2018. | ||
Jayaraman S, Chalabi Z, Perel P, Guerriero C, Roberts I. The risk of transfusion-transmitted infections in sub-Saharan Africa. Transfusion. 2010;50(2):433–442. | ||
Fasola F, Otegbayo I. Post-transfusion viral hepatitis in sickle cell anaemia: retrospective-prospective analysis. Niger J Clin Pract. 2002;5(1):16–19. | ||
Yooda AP, Soubeiga ST, Nebie KY, et al. Impact of multiplex PCR in reducing the risk of residual transfusion-transmitted human immunodeficiency and hepatitis B and C viruses in Burkina Faso. Mediterr J Hematol Infect Dis. 2018;10(1):e2018041. | ||
Nagalo MB, Sanou M, Bisseye C, et al. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis among blood donors in Koudougou (Burkina Faso) in 2009. Blood Transfus. 2011;9(4):419–424. | ||
Diarra B, Yonli AT, Ouattara AK, et al. World hepatitis day in Burkina Faso, 2017: seroprevalence and vaccination against hepatitis B virus to achieve the 2030 elimination goal. Virol J. 2018;15(1):121. | ||
Nébié KY, Olinger CM, Kafando E, et al. [Lack of knowledge among blood donors in Burkina Faso (West Africa); potential obstacle to transfusion security]. Transfus Clin Biol. 2007;14(5):446–452. French. | ||
World Health Organisation (2010). Geneva, Screening Donated Blood for Transfusion. Available from: http://www.who.int/bloodsafety/ScreeningDonatedBloodforTransfusion.pdf. Accessed 9 February 2016. | ||
WHO. Blood Safety and Blood Supply. Checklist. N° 279. Geneva, Switzerland: WHO; 2013. | ||
Schreiber G. The risk of transfusion-transmitted viral infections. The Epidemiology Donor Study. N Engl J Med. 1996;334(26):1734–1735. | ||
Jackson BR, Busch MP, Stramer SL, AuBuchon JP. The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations. Transfusion. 2003;43(6):721–729. | ||
Couroucé AM, Pillonel J. Estimation du risque de transmission des virus des hépatites B et C et des rétrovirus humains par transfusion de dérivés sanguins labiles. [Risk of transfusion-transmitted viral infections]. Transfus Clin Biol. 1996;3(1):13–18. | ||
Kania D, Sangaré L, Sakandé J, et al. A new strategy to improve the cost-effectiveness of human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and syphilis testing of blood donations in sub-Saharan Africa: a pilot study in Burkina Faso. Transfusion. 2009;49(10):2237–2240. | ||
Collenberg E, Ouedraogo T, Ganamé J, et al. Seroprevalence of six different viruses among pregnant women and blood donors in rural and urban Burkina Faso: a comparative analysis. J Med Virol. 2006;78(5):683–692. | ||
Nagalo BM, Bisseye C, Sanou M, et al. Seroprevalence and incidence of transfusion-transmitted infectious diseases among blood donors from regional blood transfusion centres in Burkina Faso, West Africa. Trop Med Int Health. 2012;17(2):247–253. | ||
CNLS/IST. Rapport d’activité sur la riposte au SIDA au Burkina Faso; 2016. Available from: http://www.unaids.org/en/file/110917/download?token=6aVHZQzY. Accessed 09/04/2018. | ||
Tao I, Compaoré TR, Diarra B, et al. Seroepidemiology of hepatitis B and C viruses in the general population of Burkina Faso. Hepat Res Treat. 2014;2014:781843. | ||
Meda N, Tuaillon E, Kania D, et al. Hepatitis B and C virus seroprevalence, Burkina Faso: a cross-sectional study. Bull World Health Organ. 2018;96(11):750–759. | ||
Zeba MT, Sanou M, Bisseye C, et al. Characterisation of hepatitis C virus genotype among blood donors at the regional blood transfusion centre of Ouagadougou, Burkina Faso. Blood Transfus. 2014;12(Suppl 1): s54. | ||
Kafando E, Nébié Y SS, Kienou K, Dahourou H, Simpore J. Risk behavior among ineligible blood donors in a blood transfusion center (Burkina Faso). J Hematol Blood Transfus Disord. 2017;4:015. | ||
Koura M, Hema A, Coulibaly A. Incidence et risque résiduel de transmission des virus de l’hépatite B et C par transfusion sanguine Bobo-Dioulasso (Burkina Faso): Étude de cohorte. [Incidence and residual risk of transmission of hepatitis B and C by blood transfusion in Bobo-Dioulasso (Burkina Faso): cohort study]. Science et technique, Sciences de la santé. 2017;40(2):25–33. | ||
Stramer SL, Dodd RY, Brodsky JP. The value of screening signal-to-cutoff ratios for hepatitis C virus antibody confirmation. Transfusion. 2013;53(7):1497–1500. | ||
Pillonel J, Legrand D, Sommen C, Laperche S. Surveillance épidémiologique des donneurs de sang et risque résiduel de transmission du VIH, de l’HTLV, du VHC et du VHB par transfusion en France entre 2008 et 2010. [Epidemiological surveillance of blood donors and residual risk of transfusion transmission of HIV, HTLV, HCV and HBV in France, 2008 to 2010]. Bull Epidemiol Hebd. 2012;39–40:438–442. | ||
Zou S, Stramer SL, Dodd RY. Donor testing and risk: current prevalence, incidence, and residual risk of transfusion-transmissible agents in US allogeneic donations. Transfus Med Rev. 2012;26(2):119–128. | ||
Ouattara H, Siransy-Bogui L, Fretz C, et al. Residual risk of HIV, HVB and HCV transmission by blood transfusion between 2002 and 2004 at the Abidjan National Blood Transfusion Center. Transfus Clin Biol. 2006;13(4):242–245. | ||
Touré-Fall AO, Dièye TN, Sall A, et al. [Residual risk of transmission of HIV and HBV, in Senegalese national blood bank from 2003 to 2005]. Transfus Clin Biol. 2009;16(5–6):439–443. French. | ||
Rerambiah LK, Rerambiah LE, Bengone C, Djoba Siawaya JF, Siawaya JFD. The risk of transfusion-transmitted viral infections at the Gabonese National Blood Transfusion Centre. Blood Transfus. 2014;12(3):330. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.