Back to Journals » OncoTargets and Therapy » Volume 12
Resection as first-line therapy for large hepatic sclerosing hemangioma: a case report
Authors Xu L, Yang X , Ke S, Ding X, Wang S , Gao J , Sun W
Received 29 May 2019
Accepted for publication 30 July 2019
Published 22 August 2019 Volume 2019:12 Pages 6839—6842
DOI https://doi.org/10.2147/OTT.S217528
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Li Xu, Xu Yang, Shan Ke, Xue-mei Ding, Shao-hong Wang, Jun Gao, Wen-bing Sun
Department of Hepatobiliary Surgery, Beijing Chao-yang Hospital Affiliated with Capital Medical University, Beijing 100043, People’s Republic of China
Correspondence: Jun Gao; Wen-bing Sun
Department of Hepatobiliary Surgery, Beijing Chao-yang Hospital Affiliated with Capital Medical University, No. 5 Jingyuan Street, Beijing 100043, People’s Republic of China
Fax +86 105 171 8017
Email [email protected]
[email protected]
Abstract: Hepatic sclerosing hemangioma is a rare benign disease that occurs in association with hepatic cavernous hemangioma degeneration and sclerosis. Recent studies have shown that radiofrequency (RF) ablation is an alternative treatment for hepatic cavernous hemangiomas, even for large hemangiomas (≥10 cm). However, RF ablation might not be suitable to treat large sclerosing hemangiomas. We herein report the successful surgical removal of a large hepatic sclerosing hemangioma after RF ablation treatment failure in a 65-year-old man. In conclusion, we suggest that resection should be chosen as a first-line therapy for the disease.
Keywords: resection, radiofrequency ablation, hepatic cavernous hemangiomas, hepatic sclerosing hemangioma
Introduction
Hepatic cavernous hemangioma is one of the most frequently encountered benign hepatic neoplasms, but hepatic sclerosed hemangioma is very rare.1–8 Hemangioma degeneration can occur through an increase in the degree of fibrosis and thrombosis of its vascular channels, a condition that is known as sclerosing and/or hyalinizing hemangioma. This can then lead to the end stage, known as the involution stage, in which the hemangioma becomes completely sclerosed and/or hyalinized.1–3 The frequency of cavernous hemangioma acquiring sclerosis does not seem to be high, and lesion formation requires many years.4
Most accidentally identified and asymptomatic hepatic hemangiomas do not require medical intervention. When hemangiomas are larger than 5.0 cm and cause abdominal symptoms, or increase in size during follow-up, radical interventions need to be considered.9–15 Traditionally, surgery is the most commonly used treatment of choice, including hemangioma enucleation, lobectomy (segmentectomy) or partial hepatectomy, and tumor suture or ligation. However, surgery is rather invasive and is associated with relatively high risks of perioperative morbidity (27%) and mortality (3%), and also with long hospitalization.9,10 In recent years, radiofrequency (RF) ablation has been increasingly used to treat hepatic cavernous hemangiomas because of its unique advantages, such as minimal invasiveness, efficacy, high safety, fast recovery, and wide applicability.9,11–15 Recent studies have shown that RF ablation is an alternative treatment for hepatic cavernous hemangiomas, even large hemangiomas (≥10 cm).13–15 However, it might not be suitable for the treatment of large sclerosing hemangiomas.
We herein report the case of a patient with successful surgical removal of large hepatic sclerosing hemangioma after RF ablation treatment failure. We also discuss the best therapy course for the disease.
Case report
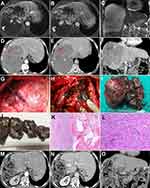
A 65-year-old Chinese man was admitted to our hospital with an enlarging hepatic hemangioma following regular follow-up imaging over 16 years and complaints of intermittent abdominal pain for 2 years. Sixteen years ago, a tumor measuring 4.5 cm was accidentally identified in the right lobe of liver. A fine-needle biopsy was performed, and hepatic cavernous hemangioma was confirmed by histology. The tumor mass was found to be increasing in size upon regular imaging follow-up and it was not palpable by physical examination. Contrast-enhanced MRI showed a large hepatic cavernous hemangioma in the right lobe (16.7×15.9 cm) (Figure 1A–C). Laboratory examinations including routine blood tests, biochemistry tests for liver, renal and coagulation function, and tumor markers did not show any abnormalities. Based on our accumulated experience of treating large hepatic cavernous hemangiomas with RF ablation [9], we treated the patient with RF ablation via a laparoscopic approach as the first-line treatment. We used a Cool-tip ACTC2025 electrode (an internally cooled cluster electrode) and an RF generator (Covidien Healthcare, Ireland) for tumor coagulation. With a 2.5-cm exposed tip, the Cool-tip electrode can produce ablation zones of 3.0 cm after one ablation session at the maximum power of 200 W within approximately 3–5 min. Visualization via laparoscopy showed a 16.0 cm tumor on the right lobe of the liver and the texture of the tumor was tough, unlike that for cavernous hemangioma. Laparoscopic biopsy of the liver lesion before ablation was performed to aid in making the diagnosis. RF treatment was terminated when hemoglobinuria occurred after 90 min of coagulation time. The lesion did not shrink as expected and only one-third of the tumor was ablated. Pathological examination confirmed that it was a hepatic cavernous hemangioma and no sclerosis was observed. The patient was discharged 9 days after surgery.
Two months post-surgery, contrast-enhanced computed tomography (CT) confirmed that only one-third of the tumor was ablated, and the ablated tumor tissue had not decreased in size (Figure 1D–F). Moreover, the patient’s abdominal pain symptoms persisted. Because the minimally invasive therapy with RF ablation had failed, RF-assisted right hepatectomy was performed (Figure 1G–H). The surgical time and the intra-operative blood loss were 140 min and 1800 mL, respectively. On pathological examination, the resected liver tumor was found to be composed of solid grey-white masses, which indicated sclerosing hemangiomas (30%), and dark red sponge-like masses, which indicated cavernous hemangiomas (70%) (Figure 1I and J). Histopathological evaluation of tissue sections revealed that the tumor mainly comprised hyalinized tissue and collagen fibers with thin-walled and variably cavernous vascular channels that were lined by endothelial cells (Figure 1K and L). Upon histological examination, it was diagnosed as a hepatic sclerosing hemangioma. Our patient’s post-operative course was uneventful, and he was discharged from hospital 15 days after surgery. The abdominal pain disappeared after surgery. Postoperative contrast-enhanced CT follow-up confirmed that the hemangioma was completely dissected without any residual tissue one month after the surgery (Figure 1M–O). No late complications were observed 5 months after surgery.
Discussion
Cavernous hemangioma is the most common benign tumor of the liver, with a frequency of 0.4–20% in autopsy studies.1–8 However, sclerosing and sclerosed hemangioma is a rare condition found in only two of 1000 autopsy cases.1–3 Hepatic sclerosing hemangiomas are caused by degenerative changes such as recent hemorrhages, hemosiderin deposits, thrombus formation, necrosis, and scar formation in hepatic cavernous hemangiomas.1 This can then lead to the end stage, in which the hemangioma becomes completely sclerosed and/or hyalinized. Sclerosing hemangioma accompanies cavernous hemangioma or preexisting cavernous hemangioma changes to sclerosed hemangioma over a period of many years.4 Characteristically, in the present case, both sclerosing hemangioma and cavernous hemangiomas were synchronously found inside the tumor, which was valuable in providing insight into the development process from cavernous hemangioma to sclerosing hemangioma.
Hepatic sclerosing hemangiomas are caused by degenerative changes such as thrombus formation, necrosis, and scar formation within liver cavernous hemangioma, and such variations in the pathological characteristics make precise radiological diagnosis very difficult.5 Sclerosing hemangioma is frequently misdiagnosed as an intrahepatic malignancy or metastatic lesions, for which a hepatectomy is performed.5–8 In the present case, the preoperative contrast MRI captured a large hepatic cavernous hemangioma and the sclerosing hemangioma could not be detected, because most of the tumor was composed of cavernous hemangiomas. Additionally, based on our accumulated experience of treating large hepatic cavernous hemangiomas with RF ablation,9 we selected RF ablation as a first-line strategy to manage the tumor.
RF ablation for hepatic cavernous hemangiomas is performed using RF-induced thermal energy to damage the endothelial cell-lined vascular structures resulting from thrombosis promotion, to induce necrotic coagulation, and to destroy erythrocytes and cause vascular smooth muscle cell disappearance and fibrosis in the ablated zone.9 One well-performed ablation session for a hepatic cavernous hemangioma can lead to a clear collapse of the tumor tissue around the ablation zone, making RF ablation for hepatic cavernous hemangiomas more efficient and easier to perform compared with malignant hepatic lesions.12 Meanwhile, the collapse of the tumor is also the basis for the relief of abdominal symptoms after ablation treatment. However, sclerosing hemangiomas are characterized by extensive fibrosis with subsequent hyalinization and marked narrowing or obliteration of the vascular spaces, which makes the texture of the tumor very tough, just like intrahepatic malignancies or metastatic lesions. Thus, only one-third of the tumor was ablated in the present case, and the ablated tumor did not shrink even after prolonged ablation. Therefore, when the texture of hepatic cavernous hemangiomas is found to be tough during surgery, accompanied sclerosing hemangioma should be considered, and hepatic resection should be the first-line therapy.
In conclusion, hepatic sclerosing hemangioma is a rare benign disease, and occurs in association with the degeneration and sclerosis of cavernous hemangiomas of the liver. We suggest that resection should be an option for first-line therapy of the disease.
Ethics approval
We confirm that written informed consent was provided by the patient to have their case details and any accompanying images published. Institutional approval was not required to publish the case details.
Acknowledgment
We thank H. Nikki March, PhD and Jodi Smith, PhD, from Liwen Bianji, Edanz Editing China, for editing a draft of this manuscript. This study was supported by the Program for High-level Technical Talents in the Beijing Health System (2015-03-025).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Makhlouf HR, Ishak KG. Sclerosed hemangioma and sclerosing cavernous hemangioma of the liver: a comparative clinicopathologic and immunohistochemical study with emphasis on the role of mast cells in their histogenesis. Liver. 2002;22(1):70–78. doi:10.1046/j.0106-9543.2001.01604.x
2. Mori H, Ikegami T, Imura S, et al. Sclerosed hemangioma of the liver: report of a case and review of the literature. Hepatol Res. 2008;38(5):529–533. doi:10.1111/j.1872-034X.2007.00306.x
3. Yamada S, Shimada M, Utsunomiya T, et al. Hepatic screlosed hemangioma which was misdiagnosed as metastasis of gastric cancer: report of a case. J Med Invest. 2012;59(3–4):270–274. doi:10.2152/jmi.59.270
4. Shimada Y, Takahashi Y, Iguchi H, et al. A hepatic sclerosed hemangioma with significant morphological change over a period of 10 years: a case report. J Med Case Rep. 2013;7:139. doi:10.1186/1752-1947-7-139
5. Behbahani S, Hoffmann JC, Stonebridge R, Mahboob S. Clinical case report: sclerosing hemangioma of the liver, a rare but great mimicker. Radiol Case Rep. 2016;11(2):58–61. doi:10.1016/j.radcr.2016.02.015
6. Navale P, Habib M, Stueck A, Fiel MI. Hepatic sclerosing hemangioma simulating gallbladder carcinoma: a rare case. J Clin Exp Hepatol. 2018;8(4):474–477. doi:10.1016/j.jceh.2018.07.003
7. Sugo H, Sekine Y, Miyano S, et al. Hepatic sclerosing hemangioma with predominance of the sclerosed area mimicking a biliary cystadenocarcinoma. Case Reports Hepatol. 2018;2018:7353170. doi:10.1155/2018/7353170
8. Koyama R, Minagawa N, Maeda Y, Shinohara T, Hamada T. A hepatic sclerosing hemangioma emerged in the postoperative course of multiple gastric carcinoid tumors masquerading as metachronous liver metastasis. Int J Surg Case Rep. 2019;58:1–5. doi:10.1016/j.ijscr.2019.03.018
9. Gao J, Fan RF, Yang JY, et al. Radiofrequency ablation for hepatic hemangiomas: a consensus from a Chinese panel of experts. World J Gastroenterol. 2017;23(39):7077–7086. doi:10.3748/wjg.v23.i39.7077
10. Miura JT, Amini A, Schmocker R, et al. Surgical management of hepatic hemangiomas: a multi-institutional experience. HPB (Oxford). 2014;16(10):924–928. doi:10.1111/hpb.12291
11. Park SY, Tak WY, Jung MK, et al. Symptomatic-enlarging hepatic hemangiomas are effectively treated by percutaneous ultrasonography-guided radiofrequency ablation. J Hepatol. 2011;54(3):559–565. doi:10.1016/j.jhep.2010.07.024
12. Gao J, Ke S, Ding XM, et al. Radiofrequency ablation for large hepatic hemangiomas: initial experience and lessons. Surgery. 2012;153(1):78–85. doi:10.1016/j.surg.2012.06.004
13. Gao J, Ding X, Ke S, et al. Radiofrequency ablation in treatment of huge hepatic hemangiomas: a comparison of multi-tined and internally cooled electrodes. J Clin Gastrol. 2014;48(6):540–547. doi:10.1097/MCG.0b013e31829ef037
14. Gao J, Kong J, Ding XM, et al. Laparoscopic vs computerized tomography-guided radiofrequency ablation for large hepatichemangiomas abutting the diaphragm. World J Gastroenterol. 2015;21(19):5941–5949. doi:10.3748/wjg.v21.i19.5941
15. Gao J, Ji JS, Ding XM, et al. Laparoscopic radiofrequency ablation for large subcapsular hepatic hemangiomas: technical and clinical outcomes. PLoS One. 2016;11(2):e0149755. doi:10.1371/journal.pone.0149755
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.