Back to Journals » International Journal of Nanomedicine » Volume 17
Research Progress on Improving the Efficiency of CDT by Exacerbating Tumor Acidification
Authors Chen W, Liu J, Zheng C, Bai Q, Gao Q, Zhang Y, Dong K, Lu T
Received 12 March 2022
Accepted for publication 16 May 2022
Published 10 June 2022 Volume 2022:17 Pages 2611—2628
DOI https://doi.org/10.2147/IJN.S366187
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Wenting Chen,1 Jinxi Liu,1 Caiyun Zheng,1 Que Bai,1 Qian Gao,1 Yanni Zhang,1 Kai Dong,2 Tingli Lu1
1Key Laboratory for Space Biosciences and Biotechnology, School of Life Sciences, Northwestern Polytechnical University, Xi’an, 710072, People’s Republic of China; 2School of Pharmacy, Xi’an Jiaotong University, Xi’an, 710072, People’s Republic of China
Correspondence: Tingli Lu, Key Laboratory for Space Biosciences and Biotechnology, School of Life Sciences, Northwestern Polytechnical University, Xi’an, 710072, People’s Republic of China, Tel/Fax +86 29 8846 0332, Email [email protected] Kai Dong, School of Pharmacy, Xi’an Jiaotong University, Xi’an, 710072, People’s Republic of China, Tel/Fax +86 29-82655139, Email [email protected]
Abstract: In recent years, chemodynamic therapy (CDT) has received extensive attention as a novel means of cancer treatment. The CDT agents can exert Fenton and Fenton-like reactions in the acidic tumor microenvironment (TME), converting hydrogen peroxide (H2O2) into highly toxic hydroxyl radicals (·OH). However, the pH of TME, as an essential factor in the Fenton reaction, does not catalyze the reaction effectively, hindering its efficiency, which poses a significant challenge for the future clinical application of CDT. Therefore, this paper reviews various strategies to enhance the antitumor properties of nanomaterials by modulating tumor acidity. Ultimately, the performance of CDT can be further improved by inducing strong oxidative stress to produce sufficient ·OH. In this paper, the various acidification pathways and proton pumps with potential acidification functions are mainly discussed, such as catalytic enzymes, exogenous acids, CAIX, MCT, NHE, NBCn1, etc. The problems, opportunities, and challenges of CDT in the cancer field are also discussed, thereby providing new insights for the design of nanomaterials and laying the foundation for their future clinical applications.
Keywords: chemodynamic therapy, Fenton/Fenton-like reactions, tumor microenvironment, reactive oxygen specie
Graphical Abstract:
Introduction
Chemodynamic therapy (CDT) has been novel method in tumor treatment in recent years.1,2 CDT utilizes the characteristics of relatively low pH and high endogenous H2O2 in the tumor microenvironment (TME),3 and some transition metal ions, such as Fe2+, Mn2+, can act as catalysts to accelerate the Fenton-like reaction of H2O2 to produce the ·OH, which will kill tumor cells by oxidizing the lipids, nucleic acids, proteins and other biological molecules (Figure 1).4–8 Its excellent therapeutic effect on tumors has been verified in laboratory studies.9 Chemotherapy and radiotherapy, as treatments for cancer, have serious side effects, including toxicity and immunosuppression, which limit their anti-tumor efficacy and increase the risk of infection.10,11 Compared to them, CDT agents are less prone to serious side effects and drug resistance. Simultaneously, there is no need to be limited by oxygen and near-infrared light (NIR) as that of photothermal therapy (PTT) or photodynamic therapy (PDT). It can use the unique microenvironment in the tumor to produce oxidative stress reaction, realize the specificity and efficiency of tumor treatment, and reduce the damage to normal cells or tissues.12 As a new way of cancer treatment, CDT can solve some drawbacks of traditional cancer therapy to a certain extent.
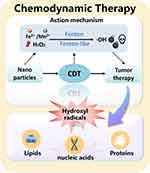 |
Figure 1 Schematic diagram of catalytic mechanism for cancer treatment of CDT. |
Although CDT has excellent prospects, there are still some challenges in the therapeutic effect of CDT agents.13 The main influencing factors are as follows. First, endogenous H2O2 (50–100 μM) in tumor cells is difficult to support the continuous production of ·OH, which is insufficient to achieve numerous killing effects on tumor cells.14,15 Second, the concentration of glutathione (GSH) in normal cells is about 1–2 mM, but its concentration can reach 10 mM in cancer cells.16,17 So, a large amount of reduced GSH produced in TME will remove ·OH and reduce oxidative stress, resulting in the obstacles to CDT.18,19 CDT is highly dependent on Fenton reaction, which has high requirements for the biological microenvironment, a solid acidic with pH 2–4.20 pH responsive nanoparticles can effectively release iron ions from nanocarriers to a certain extent in the acidic microenvironment of tumors with low pH.21 Subsequently, iron ions are effectively converted into highly active •OH by Fenton reaction for CDT. Whereas the extracellular pH (pHe) of TME is only about 6.8 and the intracellular (pHi) is approximately 7.2, which is higher than that of the Fenton reaction (pH 2–4).22–25 Therefore, due to the heterogeneity of tumor histopathology and physiology, it is expected to develop more active strategies of tissue microenvironment reprogramming through nanotechnology to improve the performance of CDT, which provides the compelling impetus for the Fenton reaction.26
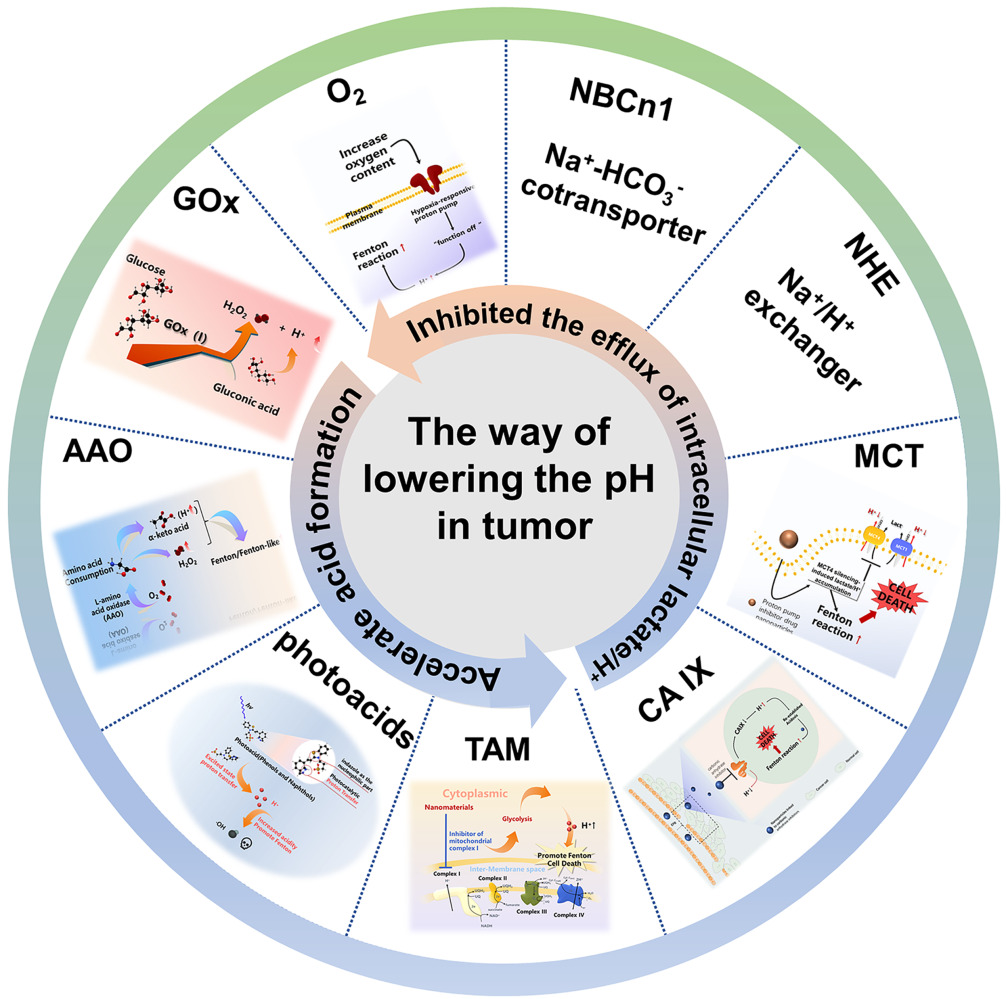
Increasing intracellular acidification is an effective way to enhance the Fenton reaction and efficacy of CDT. Tumor microenvironment reprogramming is essential for improving the responsiveness and performance of nanomedicines. Accelerating the formation of acid and inhibiting the efflux of intracellular lactate/H+ all can reduce the pH value of tumor. For instance, producing acid by enzyme catalysis, introducing photoacid in tumor, and production of H+ by mitochondrial respiratory complex enzyme inhibitor, all can accelerate acid formation. According to inhibiting the efflux of intracellular lactate/H+, pHe was indirectly reduced by inhibiting various proton pumps on the surface of the cell membrane and blocking the efflux of lactic acid. In this review, we focus on two perspectives to reduce intracellular pH, accelerating acid formation and inhibiting the efflux of intracellular lactate/H+. We also discuss the inhibitors of different proton pumps, which provide a new perspective for the design of subsequent nanoparticles to realize the acidification function.
Accelerate Acid Formation
Use of Catalytic Enzymes
A Low level of pH value is considered to be the critical factor for the efficacy of CDT in tumors.27 It is believed that there are more glucose and amino acids in tumor tissue for its rapid proliferation, and gluconic acid and α-keto acid are their oxidation products, which are conducive to reducing pH value.28,29 The catalytic mechanism is shown in Figure 2.
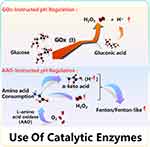 |
Figure 2 Mechanism diagram of promoting pH reduction by using of catalytic enzymes. |
GOx-Instructed pH Regulation
Glucose is an indispensable energy source in cells.30 Compared with normal cells, rapid proliferating cancer cells will consume more glucose to produce more energy, so they are highly sensitive to changes in the level of glucose.31–33 Glucose in TME will convert to gluconic acid and H2O2 by introducing exogenous glucose oxidase (GOx), which is often used as one of the ways of tumor starvation therapy.34,35 Among them, gluconic acid can provide appropriate acidic conditions for the Fenton reaction. Similarly, acidification can also further decompose the nano-delivery system and promote the cascade reaction.36 Thus, it is worth noting that the Fenton reaction induced by glucose acidification is a promising strategy of cancer therapy.
Glucose acid is widely considered as a promoter to improve Fenton reaction efficiency. A nanoscale Co-ferrocene metal-organic framework (Co-Fc NMOF) with high Fenton activity was synthesized by research group of Fang, and then combined with glucose oxidase (GOx) to construct a cascade enzymatic/Fenton catalytic platform (Co-Fc@GOx) for strengthening tumor treatment.37 In this system, Co-Fc-NMOF acted as an effective carrier of GOx and could produce highly toxic ·OH with a good Fenton effect. When this system reached the tumor microenvironment, GOx would catalyze the endogenous glucose to produce gluconic acid and H2O2. Both the increase of intracellular acidity and H2O2 content in situ were conducive to the Fenton reaction of Co-Fc-NMOF, which would further promote the generation of ROS in the local tumor site. In 4T1 tumor-bearing mice, it was found that the tumor volume in the treated group was smaller than that in the control, showing the tumor inhibition by Co-Fc-NMOF introduced. It was noteworthy that the most significant antitumor effect could be obtained with Co-Fc@GOx treated through the cascade enzyme/Fenton reaction. The authors also found that GOx alone had a limited inhibitory effect on tumor growth, which could be attributed to its diffusion and degradation in tumor tissues. Therefore, the appropriate nanocarriers were essential for the load and protection of GOx, which also could greatly avoid the damage of GOx to the normal tissues. Additionally, a kind of block copolymers was prepared by research group of Li, which was composed of poly(ethylene glycol) (PEG) and copolymerized monomers of camptothecin (CPT) and piperidine-modified methacrylate [P(CPTMA-co-PEMA)]. It could self-assemble into polymer vesicles in aqueous solution for encapsulation of GOx.38 Vesicle structure had good integrity and stability in the process of intracellular action, so as to protect GOx from harsh environment and maintain long-term activity.39,40 Therefore, it was concluded that GOx could provide a potential therapeutic platform for tumor collaborative therapy and effectively regulate the tumor microenvironment.
The efficiency of GOx catalytic reaction is strongly dependent on oxygen concentration, but the hypoxia condition in TME leads to low activity of GOx.41 In order to overcome this problem, a dual-catalytic nanoreactor with oxygen-containing support was prepared by research group of Zhang. Hollow mesoporous silica nanoparticles (HMSNs) were used to load Fe3O4 nanoparticles as the Fenton reaction catalyst and GOx as the glucose oxidation catalyst on the HMSN surface. The oxygen-carrying perfluorohexane (PFC), acting as an oxygen carrier, was encapsulated in the pores of HMSNs. Finally, the cancer cell membrane was coated on the nanoreactor to construct the final GOx-Fe3O4-HMSNs-PFC/O2@C. The results showed that GOx effectively consumed glucose in tumor and increased the generation of H2O2, which was further reacted with Fe3O4. Based on this, the Fenton reaction was enhanced and led to the increased production of highly toxic ·OH and the apoptosis of cancer cells in the end. For the hypoxia in the tumor microenvironment, PFC, containing an extensive amount of O2, could improve the catalytic efficiency of GOx, increase the production of glucuronic acid and H2O2, and provide a suitable condition for the activation of CDT by Fenton reaction in the next step. In addition, treated with GOx-Fe3O4-HMSNs-PFC/O2@C obtained higher tumor inhibition effect compared with other treatment groups in vivo. Take the life span of mice for example, the mice treated with Fe3O4-HMSNs-PFC/O2@C was survived only for 13 days, greatly reduced about 17 days of that in control. These results might be attributed to the synergistic inhibitory effect of Fe3O4 nanoparticles, GOx, and O2 against tumor, as well as the significant efficacy of GOx in Fenton-mediated CDT.13
In situ decomposition of H2O2 into O2 in tumor cells by catalase-active nanozymes is also an important strategy for reoxygenation.35 A multi-functional nanoreactor Fe–MIL-88B–NH2@PFC-1-GOx (MPG), based on MOFs (Fe-MIL-88B-NH2), hydrogen-bonded organic frameworks (PFC-1), and GOx, was reported by Hu. Here, the function of GOx was like the previous report.42 Thanks to its excellent catalase-like (CAT) activity, O2 was generated for the reaction of MPG with H2O2, which could alleviate the hypoxia in TME, further accelerate the glucose oxidation process, and improve the efficiency of CDT.15
It has been proved that gluconic acid can not only provide an appropriate pH for the Fenton reaction but also promote the decomposition and release of the acid sensitive nano-delivery system. The mesoporous iron oxide nanoparticles (IONP) were firstly modified by GOx, and then the artemisinin (ART) was loaded to design a cascade catalytic nanoplatform IONP-GOx@ART. Glucose oxidation was catalyzed by GOx and gluconic acid and H2O2 were produced, resulted in the tumor starvation and the activity of IONP-mediated Fenton reaction. The more acidic TME based on the gluconic acid generated was helpful for the release of Fe2+ and Fe3+ ions, which could generate ·OH through the Fenton reaction. In addition, the presence of Fe2+ would lead to destroy of the endoperoxide bridge in ART molecule, which caused the high level of ROS. All of the above results were benefit to CDT. This effect was again proved in 4T1 tumor-bearing mice, the tumor growth of treated by IONP-GOx@ART was significantly inhibited compared with that of IONP@ART,43 demonstrating the great potential of GOx in anti-tumor therapy.
In short, GOx has been widely used in CDT, which provides a bright prospect for the anti-tumor treatment of CDT. The unique chemical reaction of GOx in tumor and its diversified applications make it a priority to regulate the acidic microenvironment of tumor. GOx can produce gluconic acid and abundant H2O2, which provides sufficient reactants for the Fenton reaction. So, GOx can regulate TME and provide a new idea for the treatment of malignant tumors. However, free GOx tends to overflow before reaching the tumor site and get the nonspecific accumulation in normal tissues, which inevitably limited the efficiency of CDT and enhanced its cytotoxicity. Therefore, the selection of nanocarriers is very important, which can be supported by MOF, mesoporous silica, or modified by chemical bonds. Since the catalytic reaction of GOx only works in the presence of O2, it is imperative to provide sufficient O2 to alleviate the hypoxic environment of the tumor, as well as to improve the catalytic efficiency of GOx.
L-Amino Acid Oxidase-Instructed pH Regulation
Amino acid (AA), a monomer for protein synthesis, is an important nutrient for the proliferation of cells, and it is also necessary for the survival of cells.44,45 In addition, AA is also an essential substance in the biosynthesis of lipids, nucleotides, and other substances. Therefore, the proliferation of tumor cells needs more AAs in response to meet the needs of the increased cell anabolism and rapid growth.46 However, L-amino acid oxidase (AAO) can catalyze the oxidation of amino acids to produce α-keto acids, releasing hydrogen peroxide and ammonia.47 Among them, α-keto acid is helpful to adjust the pH value of tumor and promote Fenton or Fenton-like reaction, and H2O2 can also continuously provide the substrates for Fenton reaction.
Research groups of Chu grafted L-Amino acid oxidase (AAO) on the surface of the hollow Fe3+/tannic acid nano capsules (HFe-TA), and covered 4T1 cancer cell membrane to form M@AAO@HFe-TA. In this system, AAO significantly consumed amino acids after entering cancer cells and produced α-keto acids and H2O2 to promote the formation of ·OH in HFe-TA. The cancer cell membrane on the surface of nanocapsules played a protective role in preventing AAO exposure and its potential cytotoxicity. Meanwhile, nanoparticles with certain immune escape and tumor targeting abilities could be got by the surface modification with the cancer cell membrane. Hence, this study might provide a new method to treat tumors and improve its biosafety and efficiency.48
Photoacid-Instructed pH Regulation
Photoacid can produce H+ and reduce pH value under light stimulation.49 Most of photoacid are the aromatic organic molecules and the weak acid in the ground electronic state. While in the first excited electronic state, they will generate a larger order of magnitude of H+.50 The irradiated photoacid is expected to realize the remote space and time control of proton dissociation, and also realize the conversion of light energy into other types of energy.51 It has been confirmed that some phenolic derivatives, such as phenols and naphthols, can significantly increase acidity under light irradiation.52 At present, photoacid has been used as part of nanomaterials to improve the efficiency of CDT (Figure 3).
 |
Figure 3 Mechanism diagram of promoting pH reduction by carrying photoacid acid. |
Research groups of Chen designed a near infrared light (NIR)-controlled nano-proton supplier, whose upconversion nanoparticles (UCNPs) were used as the core of the MIL-88B coating for internal photoacids (PA) loading (UCNP @ MIL-88B @ PA, abbreviated as UMP). It was verified that the emission light of UCNPs under 980 nm laser irradiation could activate PA molecules cyclization to release H+. When UMP penetrated the cytoplasm of tumor cells, rapid dissociation of protons led to the decrease of the intracellular pH value. Under increasingly strong acidic conditions, the catalytic active sites Fe in the MIL-88B shell could effectively react with H2O2 to enhance the therapeutic effect of CDT on tumors. Therefore, all reliable evidence shows that PA can release H+ under the control of light and play an effective role in CDT.53
In summary, PA can specifically increase the H+ concentration in TME under the control of specific light, but have almost no effect on the normal cells, with good biocompatibility. Therefore, the introduction of PA can significantly enhance the efficiency of Fenton/Fenton-like reactions and tumor treatment.
Inhibitor of Mitochondrial Complex I-Instructed pH Regulation
Mitochondrial respiration is the basis of normal metabolism in most mammalian cells, which provides a central mechanism for coupling fuel and oxygen consumption with ATP synthesis.54 According to previous studies, mitochondrial dysfunction will affect tumorigenesis.55 Mitochondrial complex I. is called a reduced nicotinamide adenine dinucleotide (NADH)-ubiquinone oxidoreductase (Q reductase), which can catalyze the NADH oxidation in the electron transport chain.56 Complex I has a great impact on cell respiration and metabolic reprogramming and oxidative stress in a variety of malignant tumors (Figure 4). However, inhibitors of mitochondria complex I can reduce the mitochondrial respiration and ATP production, and promote cancer cells to adopt glycolysis resulting in higher lactate levels.57 Therefore, complex I inhibitors are important in oncology research.58
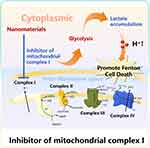 |
Figure 4 Mechanism diagram of promoting pH reduction by Inhibitor of mitochondrial complex I. |
The research group of Shi developed a nanoplatform (FePt@FeOx@TAM-PEG) that could achieve efficient and specific anti-cancer effect through a dual pathway of cyclic amplification strategy. Tamoxifen (TAM), an inhibitor of mitochondrial complex I, could enhance glycolysis and lactate content, leading to the intracellular H+ accumulation and overcoming the limitation of TME. Owing to the continuous cyclic release process, more FePt@FeOx were activated via a dual pathway of positive feedback loop, which would induce the strong ROS accumulation within cancer cells and lead to the significant increase of oxidative stress and apoptosis. Notably, the pH-responsive characteristics of TAM allowed the FePt@FeOx to be “turn on” in acidic TME, but keep “turn off” under neutral conditions. Therefore, it is of great significance to further strengthen tumor therapy by reprogramming TME.59
Inhibitors of mitochondrial complex I play an essential role in enhancing the effect of CDT. So, it is crucial to find new inhibitors of mitochondrial complex I. The research group of Ju confirmed that the anti-cancer drug carboxyamidotriazole (CAI) could inhibit mitochondrial respiration in cancer cells and enhance its anti-cancer activity by further regulating energy metabolism. When it was incubated with cancer cells, it could stimulate glucose uptake and lactate production, and inhibit oxidative phosphorylation (OXPHOS) in cancer cells, resulting in a decrease in the activity of the respiratory chain complex I. This result could lead to the lack of ATP production in mitochondria and force tumor cells to up-regulate glycolysis, leading to the increase of lactic acid.60 In addition, META-IOD-BENZYLGUANIDINE (MIBG) could also inhibit the complex I of the mitochondrial respiratory chain. Related experiments showed that a progressive increase of the lactate was observed after incubation of the cells with glucose and rising concentration of MIBG.61
In conclusion, TAM, CAI and MIBG can influence mitochondrial respiration, up-regulate glycolysis and increase lactic acid content. Thus, they are expected to combine with CDT to efficiently treat cancer.
Inhibited the Efflux of Intracellular Lactate/H+
The acidic microenvironment is an important condition for promoting treatment of CDT.62 At present, the main proton pumps and proteins involved in tumor pH regulation are carbonic anhydrase IX (CAIX),63 monocarboxylate transporters (MCT),64 Na+/H+ exchanger (NHE),65 Na+-HCO3−cotransporter (NBCn1) and so on,66 and its regulation mechanism is showed in Figure 5. These inhibitors can regulate the activity of these proton pumps and proteins to inhibit the efflux of intracellular lactate/H+ and acidify the TME, which is more conducive to the occurrence of Fenton reaction. So far, some proton pumps and protein inhibitors have been widely reported and achieve the excellent therapeutic effects in inhibiting H+ efflux (Table 1).
 |
Table 1 The Inhibitors for Proton Pumps |
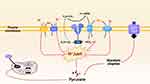 |
Figure 5 Mechanism of H+ transport by various proton pumps. |
CA IX-Instructed pH Regulation
Carbonic anhydrase is widely distributed in mammalian cells, mainly in the cytoplasm (CA I, CA II, CA III, CA VII and CA XIII), mitochondria (CA VA and CA VB), membrane (CA IV, CA IX, CA XII, CA XIV and CA XV). Some carbonic anhydrase isoenzymes (CA II, CA IX and CA XII) are closely related to tumorigenesis, especially CA IX.110 Carbonic anhydrase IX (CAIX) is a zinc-containing transmembrane metalloenzyme. Carbon dioxide is also a key source of acid in tumors. CAIX can catalyze the conversion of CO2 into bicarbonate and H+ (CO2 + H2O ⇋ HCO3−+ H+), which will stabilize intracellular pH value and enhance the extracellular matrix decomposition and cell invasion.111,112 The structure of carbonic anhydrase has been widely studied. For instance, the CA IX protein (called MN or G250) detected in HeLa cells, contains an N-terminal proteoglycan-like domain, a CA domain, a transmembrane anchor, and a C-terminal cytoplasmic tail.113 It is upregulated in tumor cells under hypoxic conditions. The accumulation of hypoxia-inducible factor-1α induces the expression of CAIX and causes a variety of downstream effects, including acidification of the extracellular, loss of cellular adhesion, and increased tumor cell migration.114 Therefore, CA IX has been recognized as a valuable target for cancer diagnosis and treatment because of its unique property in hypoxic TME.115
Fortunately, these special physiological processes can be reverted by inhibiting the activity of the CA IX enzyme through carbonic anhydrase IX inhibitor (CAI). Chen proposed a self-enhanced CDT to inhibit tumor occurrence and metastasis by constructing a tumor acidosis model AFeNPs@CAI. AFeNPs@CAI nanocomposites were composed of unique amorphous iron nanoparticles (AFeNPs) loaded with CAI, which facilitated the Fenton reaction and enlarged the oxidative damage to cells (Figure 6). At the same time, the over-expression of CAIX in cancer cells was inhibited by CAI, and the possibility of tumor invasion and metastasis was effectively inhibited by re-established tumor acidosis. The decrease of pHi effectively increased productivity of ·OH by the Fenton reaction based on AFeNPs, and aggravated the oxidative stress in tumor cells and induced cells apoptosis.116 Thus, CAI not only potentiates the application of CDT in tumor therapy, but also provides a new anticancer idea of re-establishing TME for a better therapeutic effect, showing promising effective treatment of tumors.
 |
Figure 6 Schematic diagram of self-enhanced CDT via CAI. |
In addition, research group of Angeli evaluated a series of telluride-containing compounds bearing the benzenesulfonamide group in vitro and found they could act as an effective inhibitor of carbonic anhydrase IX. These compounds exhibited inhibitory activity against tumor-associated CA IX at low concentrations (KI 2.2–2.9 nM), providing the possibility of treating the MDA-MB-231 breast cancer. In this case, the organotellurium derivatives as CAI inhibitors have opened up new avenues for novel antitumor agents.74
Furthermore, it was found that the loss of CAIX expression in 4T1 mouse metastatic breast cancer cells mediated by shRNA led to the regression in orthotopic mammary tumors and inhibited spontaneous lung metastasis. Meanwhile, the stable depletion of CAIX in MDA-MB-231 human breast cancer xenografts also resulted in the weakening of primary tumor growth. What’s more, a novel CAIX-specific small molecule inhibitor was used to treat CAIX-positive 4T1 breast tumors in mice. The inhibitor mimicked the effect of CAIX deficiency in vitro and significantly inhibited the tumor growth and metastasis in both spontaneous and experimental metastasis models but had no inhibitory effect on CAIX-negative tumors. The similar inhibitory effects were observed on primary tumor growth in orthotopic tumor mice bearing lung metastatic MDA-MB-231 LM2-4Luc cells.68 Therefore, targeting CAIX activity with specific drug inhibitors may help inhibit disease progression.
Some carbonic anhydrase-related proteins also affect the expression of carbonic anhydrase. Cullin-associated NEDD8-dissociation protein 1 (CAND1), a nuclear protein involved in gene transcription and SCF ubiquitin ligase complex assembly, which could interact with CAIX. In fact, this interaction was identified, and the low levels of CA IX were observed in cells with reduced CAND1 expression through shRNA-mediated interference. Due to the role of CAND1 in stabilizing CAIX, these molecules might have potent to reduce the amount of CAIX in hypoxic cancer cells. It might be a new prospect for the design of anticancer drugs targeting CAIX.117 In summary, the combination of CAI and CDT can significantly amplify the oxidative stress in tumor sites, effectively kill the tumor, as well as reduce its side effects on the normal tissues.
Monocarboxylate Transporters (MCT)
The Monocarboxylate transporters (MCTs), are a family of 14 members and products of the SLC16 gene family, among which MCT1–4 can transport one monocarboxylic acid molecule across the cell membrane such as L-lactate and pyruvate and so on.118 Different from other MCTs, MCT4 is activated by HIF-1α and will exhibit 3–5 fold mRNA expression during hypoxia, showing significantly higher tissue expression in hypoxic region of tumor site.119,120 MCT-4 is also a low-affinity and high-capacity lactate transporter, which exists in cells with increased glycolytic activity and participates in the release of lactate in cell glycolysis.121 MCT4 is highly expressed in various types of solid tumors, for instance, the breast cancer.122 Previous studies showed that the cells expressing MCT-4 showed stronger invasion behavior than those without MCT-4 expression.123 Therefore, silencing MCT-4 gene and using MCT4 inhibitors are expected to reduce pHi and improve the efficiency of CDT.
An innovative amorphous iron oxide (AIO) RNAi NP platform was constructed by research group of Liu. RNAi NP platform could regulate the glycolysis pathway by silencing MCT-4 to block the outflow of intracellular lactate/H+, optimizing the catalytic efficiency of Fenton/Fenton-like reactions and upgrading the therapeutic performance of CDT. It was worth noting that blocking intracellular lactate efflux by MCT-4 silencing also could further stimulate more H2O2 production to amplify the Fenton-like reaction and oxidative damage to tumor cells, and result in the effective combination therapy (Figure 7).86 In short, MCT-4 inhibitors are of great significance for the upregulation of total ROS by CDT, which ultimately improves the anti-cancer efficiency of CDT.
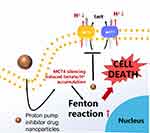 |
Figure 7 Anticancer mechanism of MCT 4. |
Na+/H+ Exchanger (NHE1)
NHE1, an H+-regulated membranous transport protein, is encoded by human solute carrier family 9A1 (SLC9A1) gene and can maintain an acidic extracellular pH in cancer cells.65,94 NHE1 drives H+ efflux to exchange Na+ influx to maintain pHi, which is an important driving force for glycolytic metabolism.124 The transmembrane domain of NHE1 is necessary for ion transport, and its ∼300-residue long, regulatory C-terminal cytosolic tail controls the pHi set point of the transporter and is also required for allosteric NHE1 regulation.125 So far, 10 isoforms have been identified in the human NHE family.126 In addition, the hypoxic and serum-depleted tumor microenvironment further promote excessive activation of NHE1.127 Researches show that the activation of NHE1 enhances the migratory capability and invasiveness of human melanoma cells and breast carcinoma cells.128 Thus, it is important for inhibiting H+ efflux by choosing a specific NHE1 inhibitor.
NHE1 inhibitors partially inhibit NHE1 by competing with Na+ at the transport site.129 At present, NHE1 inhibitor has not yet been combined with CDT, but their advantages in tumor treatment have been proved. The stable shRNA-mediated NHE1 gene knockdown (KD) in the MDA-MB-231 triple-negative breast cancer (TNBC) cell line significantly lowered pH(i) and capacity for pH(i) recovery after an acid load.130 KR-33028 (4-cyano (benzo[b]thiophene-2-carbonyl) guanidine), an effective and selective NHE1 inhibitor, could inhibit metastatic of TNBC cells and reduce cell invasion through the extracellular matrix. Meanwhile, the effect of KR-33028 on MDA-MB-231 cells lacking NHE1 expression (231koNHE1) was also evaluated. It was found that there was no difference between untreated control cells and 231koNHE1 cells treated with KR-33028. Thus, KR-33028-mediated inhibition of NHE1 has implications for limiting cell metastasis in vivo.96
The three-dimensional (3D) spheroids with MDA-MB-231 and MCF-7 cell were constructed and treated with pyrazinoylguanidine-type NHE1 inhibitors for 2–7 days, followed by analyses of the viability and death-associated signaling. It was found that this type of NHE1 inhibitor could reduce the viability of breast cancer spheres in a dose-dependent manner.97 In conclusion, the introduction of NHE1 inhibitor in the nanoparticle is helpful to prevent H+ efflux and effectively prevents the invasion of the TNBC. Therefore, by inhibiting NHE1 activity not only contributes to CDT optimization, but also provides an opportunity to develop more specific approaches to regulate pH of the TME.
Na+-HCO3− Cotransporter NBCn1
Na+-HCO3− cotransporter NBCn1 constitutes the majority of the acid extrusion capacity in human breast carcinomas.131 Most evidence indicates that the protein expression of cotransporter NBCn1 is increased in primary breast carcinomas and lymph node metastases. Compared to the matched normal breast tissue, the expression of NBCn1 protein in human breast cancer is about twofold to threefold of that in normal one.132 Driven by the Na+ concentration gradient, NBCn1 normally can move Na+ and HCO3− into cells.133 The upregulated cellular net acid extrusion in breast cancer depends on NBCn1-mediated HCO3− uptake.130,134 However, Na+-HCO3− cotransporter NBCn1 inhibitor can block HCO3− uptake and its transformation to carbon dioxide driven by CAIX, and finally inhibiting H+ emission, maintaining pH values in cell, and thereby limiting cell proliferation and breast cancer occurrence. Thus, NBCn1 is widely expressed and likely to play an important physiological role in pHi regulation in numerous tumors.135
The functional consequences of NBCn1 knockout (KO) were tested during the breast cancer development. The results showed that NBCn1 gene mutation delayed the development of breast cancer. Compared with wild-type (WT) mice, the tumor growth rate was about 65% decreased in NBCn1 KO mice, while the incubation period of breast tumors was prolonged. The cell proliferation rate of NBCn1KO cancer mice was about 60% lower than that of WT by Ki-67 and phosphohistone H-3 staining. It was also found that CO2, HCO3− dependent net acid extrusion was suppressed and the steady-state pHi decreased in breast cancer tissue of NBCn1 KO mice.136 The disruption of NBCn1 expression also delayed the growth of the ErbB2-induced breast carcinogenesis. Take the survival period for example, it was about 9.5 months for a median tumor-free in wild-type mice, but prolonged to 12 months in NBCn1-knockout mice.137 Thus, these findings demonstrated that NBCn1 might act as a target for anti-cancer therapy, even combined with CDT to improve the efficiency of cancer treatment.
Conclusion
Compared with traditional cancer therapy, CDT is a new cancer treatment method.138 Because of its high specificity and sensitivity, researchers have paid extensive attention to converting H2O2 into highly toxic ·OH by initiating Fenton and Fenton-like reactions.139 Although the TME is weakly acidic, its pH value is unsuitable for Fenton reaction occurrence.140
In order to overcome the limitation, strategies to improve CDT efficiency in view of acidification is summarized in this review. Increasing the concentration of H+ in tumor cells is the first way to be studied. For example, by introducing the catalytic enzymes and exogenous acids, such as GOx, AAO, PA, is able to reduce the pH value in tumors and improve the efficiency of CDT against cancer. The mitochondrial complex I inhibitor is used to block the oxidative phosphorylation of cell respiration, resulting in forcing cells to produce lactic acid by anaerobic glycolysis and increasing the intracellular acidity. Similarly, the proton pump protein inhibitors, such as CA IX, MCT-4, NHE1, NBCn1, are introduced to inhibit intracellular lactate/H+ efflux and reduce intracellular pH. These catalytic enzymes and inhibitors will serve as potential cancer adjuvants for CDT. Except for NBCn1, other proton pumps are mainly activated under anoxic conditions. Therefore, we can also inhibit the function of proton pump from the perspective of increasing tumor oxygen concentration, so as to improve the acidity of TME and the efficiency of CDT.
Many research works have proved that acidification of TME does have effect in promoting the action of CDT, but there are still some challenges. For GOx, it is critical to ensure that its internal substance, GOx, is not released before reaching the tumor site. GOx catalysis can consume glucose and O2, which will damage the normal cells if the leakage occurs during transportation.141 Therefore, a variety of fine therapeutic nanocarriers were developed, including cell membrane (CM),142,143 metal polyphenol networks,144 metal-organic frameworks,2 zeolitic imidazolate framework.145 All of these nanocarriers were confirmed to maximize its safety and improve the therapeutic effects and reduce side effects.
In addition, acidification can combine with other factors to affect Fenton reaction. Firstly, by strengthening the conversion rate of Fe3+ to Fe2+, the Fe3+ local electron density can be adjusted to increase the electron density. At this time, electrons will move the atoms from the non-reaction center to the reaction area and the reaction dynamic process is accelerated.146,147 Secondly, regulating the TME to enhance CDT performance, such as increasing H2O2 concentration in tumor,148,149 and reducing the excessive intracellular antioxidant GSH,150 can improve efficiency of CDT.151 In addition, CDT-based combination therapy can be developed, such as CDT-PDT,152 CDT-PTT,153,154 CDT-chemotherapy,155 CDT-immunotherapy,156 CDT-RT,157 CDT-SDT,158 CDT-starvation therapy,159 which can produce significant synergistic effects and reduce the side effects of CDT agents. However, in order to address the current barriers of CDT for clinical applications, we should try to avoid large doses, complex synthesis processes and cumbersome auxiliary devices when designing nanoplatforms.160 It is important to develop a simple and efficient CDT nanoplatform for the field of cancer nanomedicine.
At present, different from the classic pH-dependent Fenton/Fenton-like reaction, some researchers have developed a pH-independent reaction to increasing the production of ·OH, which is also confirmed the certain potential in CDT treatment, but its mechanism is still needed to be explored.161
In summary, CDT shows a broad application prospect in cancer treatment and is worth further exploration in different tumors.162 The acidic TME is crucial for Fenton and Fenton-like reactions and plays a decisive role in the anti-tumor effect of CDT.163 So, it is the unremitting goal to design nano-platforms with simpleness, good biocompatibility, low toxic, and high efficiency to CDT, as well as the perspective therapy to achieve early clinical application.
Acknowledgments
We acknowledge funding support by Key Research and Development Program of Shaanxi (Program No. 2021SF-106), the Innovation Capability Support Program of Shaanxi (Program No.2020TD-042), the joint funding from Department of Science and Technology of Shaanxi Province and Northwestern Polytechnical University (No. 2020GXLH-Z-021) and the innovation foundation for doctor dissertation of Northwestern Polytechnical University (No. CX2021099).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jia CY, Guo YX, Wu FG. Chemodynamic therapy via fenton and fenton-like nanomaterials: strategies and recent advances. Small. 2022;18(6):2103868. doi:10.1002/smll.202103868
2. Bai Y, Zhao J, Zhang L, et al. A smart near-infrared carbon dot-metal organic framework assemblies for tumor microenvironment-activated cancer imaging and chemodynamic-photothermal combined therapy. Adv Healthcare Mater;2022. e2102759–e2102759. doi:10.1002/adhm.202102759
3. Gao FL, Wu J, Gao HQ, et al. Hypoxia-tropic nanozymes as oxygen generators for tumor-favoring theranostics. Biomaterials. 2020;230:119635. doi:10.1016/j.biomaterials.2019.119635
4. Zhang SC, Cao CY, Lv XY, et al. A H2O2 self-sufficient nanoplatform with domino effects for thermal-responsive enhanced chemodynamic therapy. Chem Sci. 2020;11(7):1926–1934. doi:10.1039/C9SC05506A
5. Li Y, Jia R, Lin HM, Sun XL, Qu FY. Synthesis of MoSe2/CoSe2 nanosheets for NIR-enhanced chemodynamic therapy via synergistic in-situ H2O2 production and activation. Appl Organomet Chem. 2020;31(8):2008420.
6. Lin J, He T, Yuan Y, et al. Light-triggered transformable ferrous ion delivery system for photothermal primed chemodynamic therapy. Angewandte Chemie. 2020;60(11):6047–6054.
7. Jia T, Wang Z, Sun QQ, et al. Intelligent Fe-Mn layered double hydroxides nanosheets anchored with upconversion nanoparticles for oxygen-elevated synergetic therapy and bioimaging. Small. 2020;16(46):2001343. doi:10.1002/smll.202001343
8. Nie X, Xia L, Wang HL, et al. Photothermal therapy nanomaterials boosting transformation of Fe(III) into Fe(II) in tumor cells for highly improving chemodynamic therapy. Acs Appl Mater Inter. 2019;11(35):31735–31742. doi:10.1021/acsami.9b11291
9. He Z, Zhang H, Li H, et al. Preparation, biosafety, and cytotoxicity studies of a newly tumor-microenvironment-responsive biodegradable mesoporous silica nanosystem based on multimodal and synergistic treatment. Oxid Med Cell Longev. 2020;2020:7152173. doi:10.1155/2020/7152173
10. Li SS, Huang J, Guo Y, et al. PAC1 receptor mediates electroacupuncture-induced neuro and immune protection during cisplatin chemotherapy. Front Immunol. 2021;12:714244. doi:10.3389/fimmu.2021.714244
11. Zheng NN, Wang Q, Li CL, et al. Responsive degradable theranostic agents enable controlled selenium delivery to enhance photothermal radiotherapy and reduce side effects. Adv Healthc Mater. 2021;10(10):2002024. doi:10.1002/adhm.202002024
12. Tang ZM, Liu YY, He MY, Bu WB. Chemodynamic therapy: tumour microenvironment-mediated fenton and fenton-like reactions. Angewandte Chemie. 2019;58(4):946–956. doi:10.1002/anie.201805664
13. Zhang HW, Lu F, Pan W, et al. A dual-catalytic nanoreactor for synergistic chemodynamic-starvation therapy toward tumor metastasis suppression. Biomater Sci-Uk. 2021;9(10):3814–3820. doi:10.1039/D1BM00240F
14. He YL, Jin XY, Guo SW, Zhao HX, Liu Y, Ju HX. Conjugated polymer-ferrocence nanoparticle as an NIR-II light powered nanoamplifier to enhance chemodynamic therapy. Acs Appl Mater Inter. 2021;13(27):31452–31461. doi:10.1021/acsami.1c06613
15. Hu CL, Wang JZ, Liu SN, et al. Urchin-shaped metal organic/hydrogen-bonded framework nanocomposite as a multifunctional nanoreactor for catalysis-enhanced synergetic therapy. Acs Appl Mater Inter. 2021;13(4):4825–4834. doi:10.1021/acsami.0c19584
16. Fu LH, Wan YL, Qi C, et al. Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv Mater. 2021;33(7):2006892. doi:10.1002/adma.202006892
17. He H, Yang QY, Li HM, et al. Hollow mesoporous MnO2-carbon nanodot-based nanoplatform for GSH depletion enhanced chemodynamic therapy, chemotherapy, and normal/cancer cell differentiation. Microchim Acta. 2021;188(4):141. doi:10.1007/s00604-021-04801-5
18. Yang GB, Ji JS, Liu Z. Multifunctional MnO2 nanoparticles for tumor microenvironment modulation and cancer therapy. Wires Nanomed Nanobi. 2021;13(6):1720. doi:10.1002/wnan.1720
19. Bao YW, Hua XW, Zeng J, Wu FG. Bacterial template synthesis of multifunctional nanospindles for glutathione detection and enhanced cancer-specific chemo-chemodynamic therapy. Research-China. 2020;2020:9301215.
20. Kang YW, Hwang KY. Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res. 2000;34(10):2786–2790. doi:10.1016/S0043-1354(99)00388-7
21. Ke WD, Li JJ, Mohammed F, et al. Therapeutic polymersome nanoreactors with tumor-specific activable cascade reactions for cooperative cancer therapy. Acs Nano. 2019;13(2):2357–2369. doi:10.1021/acsnano.8b09082
22. Dong SM, Dong YS, Jia T, et al. GSH-depleted nanozymes with hyperthermia-enhanced dual enzyme-mimic activities for tumor nanocatalytic therapy. Adv Mater. 2020;32(42):2002439. doi:10.1002/adma.202002439
23. Zhang Y, Eltayeb O, Meng YT, et al. Tumor microenvironment responsive mesoporous silica nanoparticles for dual delivery of doxorubicin and chemodynamic therapy (CDT) agent. New J Chem. 2020;44(6):2578–2586. doi:10.1039/C9NJ05427H
24. Tang W, Gao HB, Ni DL, et al. Bovine serum albumin-templated nanoplatform for magnetic resonance imaging-guided chemodynamic therapy. J Nanobiotechnol. 2019;17:68. doi:10.1186/s12951-019-0501-3
25. Yang SD, Wang Y, Ren ZX, Chen MT, Chen WL, Zhang XN. Stepwise pH/reduction-responsive polymeric conjugates for enhanced drug delivery to tumor. Mat Sci Eng C-Mater. 2018;82:234–243. doi:10.1016/j.msec.2017.08.079
26. Li JJ, Kataoka K. Chemo-physical strategies to advance the in vivo functionality of targeted nanomedicine: the next generation. J Am Chem Soc. 2021;143(2):538–559. doi:10.1021/jacs.0c09029
27. Zhou H, Li XW, Niu DC, et al. Ultrasensitive chemodynamic therapy: bimetallic peroxide triggers high pH-activated, synergistic effect/H2O2 self-supply-mediated cascade fenton chemistry. Adv Healthc Mater. 2021;10(9):2002126. doi:10.1002/adhm.202002126
28. Naser FJ, Jackstadt MM, Fowle-Grider R, et al. Isotope tracing in adult zebrafish reveals alanine cycling between melanoma and liver. Cell Metab. 2021;33(7):1493. doi:10.1016/j.cmet.2021.04.014
29. Butler M, van der Meer LT, Van Leeuwen FN. Amino acid depletion therapies: starving cancer cells to death. Trends Endocrin Met. 2021;32(6):367–381. doi:10.1016/j.tem.2021.03.003
30. Yang C, Gao M, Zhao H, et al. A dual-functional biomimetic-mineralized nanoplatform for glucose detection and therapy with cancer cells in vitro. J Mater Chem B. 2021;9(18):3885–3891. doi:10.1039/D1TB00324K
31. Ming J, Zhu TB, Yang WH, et al. Pd@Pt-GOx/HA as a novel enzymatic cascade nanoreactor for high-efficiency starving-enhanced chemodynamic cancer therapy. Acs Appl Mater Inter. 2020;12(46):51249–51262. doi:10.1021/acsami.0c15211
32. Deng FA, Fan GL, Yuan P, et al. A self-accelerated biocatalyst for glucose-initiated tumor starvation and chemodynamic therapy. Chem Commun. 2020;56(93):14633–14636. doi:10.1039/D0CC06483A
33. Yang GB, Wang DD, Phua SZF, et al. Albumin-based therapeutics capable of glutathione consumption and hydrogen peroxide generation for synergetic chemodynamic and chemotherapy of cancer. Acs Nano. 2022;16(2):2319–2329. doi:10.1021/acsnano.1c08536
34. Wang M, Wang DM, Chen Q, Li CX, Li ZQ, Lin J. Recent advances in glucose-oxidase-based nanocomposites for tumor therapy. Small. 2019;15(51):1903895. doi:10.1002/smll.201903895
35. Wang Q, Niu DG, Shi JS, Wang LL. A three-in-one ZIFs-derived CuCo(O)/GOx@PCNs hybrid cascade nanozyme for immunotherapy/enhanced starvation/photothermal therapy. Acs Appl Mater Inter. 2021;13(10):11683–11695. doi:10.1021/acsami.1c01006
36. Liu XP, Liu ZW, Dong K, et al. Tumor-activatable ultrasmall nanozyme generator for enhanced penetration and deep catalytic therapy. Biomaterials. 2020;258:120263. doi:10.1016/j.biomaterials.2020.120263
37. Fang C, Deng Z, Cao GD, et al. Co-Ferrocene MOF/Glucose oxidase as cascade nanozyme for effective tumor therapy. Adv Funct Mater. 2020;30(16):1910085. doi:10.1002/adfm.201910085
38. Li JJ, Li YF, Wang YH, et al. Polymer prodrug-based nanoreactors activated by tumor acidity for orchestrated oxidation/chemotherapy. Nano Lett. 2017;17(11):6983–6990. doi:10.1021/acs.nanolett.7b03531
39. Li JJ, Anraku Y, Kataoka K. Self-boosting catalytic nanoreactors integrated with triggerable crosslinking membrane networks for initiation of immunogenic cell death by pyroptosis. Angewandte Chemie. 2020;59(32):13526–13530. doi:10.1002/anie.202004180
40. Li JJ, Dirisala A, Ge ZS, et al. Therapeutic vesicular nanoreactors with tumor-specific activation and self-destruction for synergistic tumor ablation. Angewandte Chemie. 2017;56(45):14025–14030. doi:10.1002/anie.201706964
41. Yang X, Yang Y, Gao F, Wei JJ, Qian CG, Sun MJ. Biomimetic hybrid nanozymes with self-supplied H(+) and accelerated O2 generation for enhanced starvation and photodynamic therapy against hypoxic tumors. Nano Lett. 2019;19(7):4334–4342. doi:10.1021/acs.nanolett.9b00934
42. Zhang L, Wang ZZ, Zhang Y, et al. Erythrocyte membrane cloaked metal-organic framework nanoparticle as biomimetic nanoreactor for starvation-activated colon cancer therapy. Acs Nano. 2018;12(10):10201–10211. doi:10.1021/acsnano.8b05200
43. Shao YJ, Wang ZY, Hao YT, et al. Cascade catalytic nanoplatform based on “butterfly effect” for enhanced immunotherapy. Adv Healthc Mater. 2021;10(8):2002171. doi:10.1002/adhm.202002171
44. Zhang YW, Hu HR, Liu WW, et al. Amino acids and RagD potentiate mTORC1 activation in CD8(+) T cells to confer antitumor immunity. J Immunother Cancer. 2021;9(4):e002137. doi:10.1136/jitc-2020-002137
45. Mazzoni A, Capone M, Ramazzotti M, et al. IL4I1 is expressed by head-neck cancer-derived mesenchymal stromal cells and contributes to suppress T cell proliferation. J Clin Med. 2021;10(10):2111. doi:10.3390/jcm10102111
46. Cormerais Y, Vucetic M, Pouyssegur J. Targeting amino acids transporters (SLCs) to starve cancer cells to death. Biochem Bioph Res Co. 2019;520(4):691–693. doi:10.1016/j.bbrc.2019.10.173
47. Zhang L, Wu WT. Isolation and characterization of ACTX-6: a cytotoxic L-amino acid oxidase from Agkistrodon acutus snake venom. Nat Prod Res. 2008;22(6):554–563. doi:10.1080/14786410701592679
48. Chu Q, Zhu H, Liu B, et al. Delivery of amino acid oxidase via catalytic nanocapsules to enable effective tumor inhibition. J Mater Chem B. 2020;8(37):8546–8557. doi:10.1039/D0TB01425G
49. Sanborn CD, Chacko JV, Digman M, Ardo S. Interfacial and nanoconfinement effects decrease the excited-state acidity of polymer-bound photoacids. Chem-Us. 2019;5(6):1648–1670. doi:10.1016/j.chempr.2019.04.022
50. Peretz-Soroka H, Pevzner A, Davidi G, et al. Manipulating and monitoring on-surface biological reactions by light-triggered local pH alterations. Nano Lett. 2015;15(7):4758–4768. doi:10.1021/acs.nanolett.5b01578
51. Liao Y. Design and applications of metastable-state photoacids. Accounts Chem Res. 2017;50(8):1956–1964.
52. Yan DM, Chen JR, Xiao WJ. New roles for photoexcited Eosin Y in photochemical reactions. Angewandte Chemie. 2019;58(2):378–380. doi:10.1002/anie.201811102
53. Chen X, Chen Y, Wang C, et al. NIR-triggered intracellular H+ transients for lamellipodia-collapsed antimetastasis and enhanced chemodynamic therapy. Angew Chem Int Ed Engl. 2021;60(40):21905–21910. doi:10.1002/anie.202107588
54. McKenzie M, Liolitsa D, Akinshina N, et al. Mitochondrial ND5 gene variation associated with encephalomyopathy and mitochondrial ATP consumption. J Biol Chem. 2007;282(51):36845–36852. doi:10.1074/jbc.M704158200
55. Park JS, Sharma LK, Li HZ, et al. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum Mol Genet. 2009;18(9):1578–1589. doi:10.1093/hmg/ddp069
56. Chen Z, Wei XY, Wang XY, et al. NDUFA4L2 promotes glioblastoma progression, is associated with poor survival, and can be effectively targeted by apatinib. Cell Death Dis. 2021;12(4):377. doi:10.1038/s41419-021-03646-3
57. Chaube B, Malvi P, Singh SV, Mohammad N, Meena AS, Bhat MK. Targeting metabolic flexibility by simultaneously inhibiting respiratory complex I and lactate generation retards melanoma progression. Oncotarget. 2015;6(35):37281–37299. doi:10.18632/oncotarget.6134
58. Heinz S, Freyberger A, Lawrenz B, Schladt L, Schmuck G, Ellinger-Ziegelbauer H. Mechanistic investigations of the mitochondrial complex i inhibitor rotenone in the context of pharmacological and safety evaluation. Sci Rep-Uk. 2017;7:45465. doi:10.1038/srep45465
59. Shi LN, Wang YJ, Zhang C, et al. An acidity-unlocked magnetic nanoplatform enables self-boosting ROS generation through upregulation of lactate for imaging-guided highly specific chemodynamic therapy. Angewandte Chemie. 2021;60(17):9562–9572. doi:10.1002/anie.202014415
60. Ju R, Guo L, Li J, et al. Carboxyamidotriazole inhibits oxidative phosphorylation in cancer cells and exerts synergistic anti-cancer effect with glycolysis inhibition. Cancer Lett. 2016;370(2):232–241. doi:10.1016/j.canlet.2015.10.025
61. Cornelissen J, Wanders RJA, Vangennip AH, Vandenbogert C, Voute PA, Vankuilenburg ABP. Metaiodobenzylguanidine inhibits Complex-I and complex-iii of the respiratory-chain in the human cell-line Molt-4. Biochem Pharmacol. 1995;49(4):471–477. doi:10.1016/0006-2952(94)00450-Z
62. Tian H, Zhang M, Jin G, Jiang Y, Luan Y. Cu-MOF chemodynamic nanoplatform via modulating glutathione and H2O2 in tumor microenvironment for amplified cancer therapy. J Colloid Interface Sci. 2021;587:358–366. doi:10.1016/j.jcis.2020.12.028
63. Xu P, Zhang Y, Ge F, Zhang F, He X, Gao X. Modulation of tumor microenvironment to enhance radiotherapy efficacy in esophageal squamous cell carcinoma by inhibiting carbonic anhydrase IX. Front Oncol. 2021;11:637252. doi:10.3389/fonc.2021.637252
64. Papakonstantinou E, Vlachakis D, Thireou T, Vlachoyiannopoulos PG, Eliopoulos E. A holistic evolutionary and 3D pharmacophore modelling study provides insights into the metabolism, function, and substrate selectivity of the human monocarboxylate transporter 4 (hMCT4). Int J Mol Sci. 2021;22(6):2918. doi:10.3390/ijms22062918
65. Hou KY, Liu J, Du JY, et al. Dihydroartemisinin prompts amplification of photodynamic therapy-induced reactive oxygen species to exhaust Na/H exchanger 1-mediated glioma cells invasion and migration. J Photoch Photobio B. 2021;219:112192. doi:10.1016/j.jphotobiol.2021.112192
66. Toft NJ, Axelsen TV, Pedersen HL, et al. Acid-base transporters and pH dynamics in human breast carcinomas predict proliferative activity, metastasis, and survival. Elife. 2021;10:e68447. doi:10.7554/eLife.68447
67. Bozdag M, Ferraroni M, Ward C, et al. Carbonic anhydrase inhibitors based on sorafenib scaffold: design, synthesis, crystallographic investigation and effects on primary breast cancer cells. Eur J Med Chem. 2019;182:111600. doi:10.1016/j.ejmech.2019.111600
68. Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71(13):4733.
69. Williams KJ, Gieling RG. Preclinical evaluation of ureidosulfamate carbonic anhydrase IX/XII inhibitors in the treatment of cancers. Int J Mol Sci. 2019;20:23. doi:10.3390/ijms20236080
70. Kurt BZ, Sonmez F, Ozturk D, Akdemir A, Angeli A, Supuran CT. Synthesis of coumarin-sulfonamide derivatives and determination of their cytotoxicity, carbonic anhydrase inhibitory and molecular docking studies. Eur J Med Chem. 2019;183. doi:10.1016/j.ejmech.2018.11.064
71. Ni K, Lan G, Song Y, Hao Z, Lin W. Biomimetic nanoscale metal-organic framework harnesses hypoxia for effective cancer radiotherapy and immunotherapy. Chem Sci. 2020;11(29):7641–7653. doi:10.1039/D0SC01949F
72. Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71(9):3364–3376. doi:10.1158/0008-5472.CAN-10-4261
73. Sarnella A, D’Avino G, Hill BS, et al. A novel inhibitor of carbonic anhydrases prevents hypoxia-induced TNBC cell plasticity. Int J Mol Sci. 2020;21(21):8405. doi:10.3390/ijms21218405
74. Angeli A, Pinteala M, Maier SS, et al. Tellurides bearing benzensulfonamide as carbonic anhydrase inhibitors with potent antitumor activity. Bioorg Med Chem Lett. 2021;45:128147. doi:10.1016/j.bmcl.2021.128147
75. Kugler M, Holub J, Brynda J, et al. The structural basis for the selectivity of sulfonamido dicarbaboranes toward cancer-associated carbonic anhydrase IX. J Enzym Inhib Med Ch. 2020;35(1):1800–1810. doi:10.1080/14756366.2020.1816996
76. Zhang ZP, Zhong Y, Han ZB, et al. Synthesis, molecular docking analysis and biological evaluations of saccharide-modified thiadiazole sulfonamide derivatives. Int J Mol Sci. 2021;22(11). doi:10.3390/ijms222413610
77. Petrenko M, Guttler A, Funtan A, et al. Combined 3-O-acetylbetulin treatment and carbonic anhydrase IX inhibition results in additive effects on human breast cancer cells. Chem Biol Interact. 2021;4:333.
78. Bua S, Lomelino C, Murray AB, et al. “A sweet combination”: developing saccharin and acesulfame K structures for selectively targeting the tumor-associated carbonic anhydrases IX and XII. J Med Chem. 2020;63(1):321–333. doi:10.1021/acs.jmedchem.9b01669
79. Mboge MY, Combs J, Singh S, et al. Inhibition of carbonic anhydrase using SLC-149: support for a noncatalytic function of CAIX in breast cancer. J Med Chem. 2021;64(3):1713–1724. doi:10.1021/acs.jmedchem.0c02077
80. D’Ascenzio M, Secci D, Carradori S, et al. 1,3-Dipolar Cycloaddition, HPLC enantioseparation, and docking studies of saccharin/isoxazole and saccharin/isoxazoline derivatives as selective carbonic anhydrase IX and XII inhibitors. J Med Chem. 2020;63(5):2470–2488. doi:10.1021/acs.jmedchem.9b01434
81. Grandane A, Nocentini A, Domraceva I, Zalubovskis R, Supuran CT. Development of oxathiino[6,5-b]pyridine 2,2-dioxide derivatives as selective inhibitors of tumor-related carbonic anhydrases IX and XII. Eur J Med Chem. 2020;45:200.
82. Malebari AM, Ibrahim TS, Salem IM, et al. The anticancer activity for the bumetanide-based analogs via targeting the tumor-associated membrane-bound human carbonic anhydrase-IX enzyme. Pharmaceuticals-Base. 2020;13(9):e34.
83. Hanson DJ, Nakamura S, Amachi R, et al. Effective impairment of myeloma cells and their progenitors by blockade of monocarboxylate transportation. Oncotarget. 2015;6(32):33568–33586. doi:10.18632/oncotarget.5598
84. Beloueche-Babari M, Wantuch S, Galobart TC, et al. MCT1 inhibitor AZD3965 increases mitochondrial metabolism, facilitating combination therapy and noninvasive magnetic resonance spectroscopy. Cancer Res. 2017;77(21):5913–5924. doi:10.1158/0008-5472.CAN-16-2686
85. Guan X, Rodriguez-Cruz V, Morris ME. Cellular uptake of MCT1 inhibitors AR-C155858 and AZD3965 and their effects on MCT-mediated transport of l-lactate in murine 4T1 breast tumor cancer cells. Aaps J. 2019;21(2). doi:10.1208/s12248-018-0279-5
86. Liu YL, Ji XY, Tong WWL, et al. Engineering multifunctional RNAi nanomedicine to concurrently target cancer hallmarks for combinatorial therapy. Angewandte Chemie. 2018;57(6):1510–1513. doi:10.1002/anie.201710144
87. Puri S, Juvale K. Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: a review with structure-activity relationship insights. Eur J Med Chem. 2020;45:199.
88. Renner K, Bruss C, Schnell A, et al. Restricting glycolysis preserves T cell effector functions and augments checkpoint therapy. Cell Rep. 2019;29(1):135. doi:10.1016/j.celrep.2019.08.068
89. Sadeghzadeh M, Moldovan RP, Teodoro R, Brust P, Wenzel B. One-step radiosynthesis of the MCTs imaging agent F-18 FACH by aliphatic F-18-labelling of a methylsulfonate precursor containing an unprotected carboxylic acid group. Sci Rep-Uk. 2019;9:45.
90. Yang XK, Wang DS, Dong W, Song ZS, Dou KF. Expression and modulation of Na+/H+ exchanger 1 gene in hepatocellular carcinoma: a potential therapeutic target. J Gastroenterol Hepatol. 2011;26(2):364–370. doi:10.1111/j.1440-1746.2010.06382.x
91. Yang X, Wang D, Dong W, Song Z, Dou K. Suppression of Na+/H+ exchanger 1 by RNA interference or amiloride inhibits human hepatoma cell line SMMC-7721 cell invasion. Med Oncol. 2011;28(1):385–390. doi:10.1007/s12032-010-9447-x
92. Yang X, Wang D, Dong W, Song Z, Dou K. Inhibition of Na(+)/H(+) exchanger 1 by 5-(N-ethyl-N-isopropyl) amiloride reduces hypoxia-induced hepatocellular carcinoma invasion and motility. Cancer Lett. 2010;295(2):198–204. doi:10.1016/j.canlet.2010.03.001
93. Wang J, Xu H, Wang Q, et al. CIAPIN1 targets Na+/H+ exchanger 1 to mediate MDA-MB-231 cells’ metastasis through regulation of MMPs via ERK1/2 signaling pathway. Exp Cell Res. 2015;333(1):60–72. doi:10.1016/j.yexcr.2015.02.012
94. Guan XD, Luo LX, Begum G, et al. Elevated Na/H exchanger 1 (SLC9A1) emerges as a marker for tumorigenesis and prognosis in gliomas. J Exp Clin Canc Res. 2018;37:456.
95. Zhu W, Carney KE, Pigott VM, et al. Glioma-mediated microglial activation promotes glioma proliferation and migration: roles of Na+/H+ exchanger isoform 1. Carcinogenesis. 2016;37(9):839–851. doi:10.1093/carcin/bgw068
96. Amith SR, Wilkinson JM, Fliegel L. KR-33028, a potent inhibitor of the Na+/H+ exchanger NHE1, suppresses metastatic potential of triple-negative breast cancer cells. Biochem Pharmacol. 2016;118:31–39. doi:10.1016/j.bcp.2016.08.010
97. Rolver MG, Elingaard-Larsen LO, Andersen AP, Counillon L, Pedersen SF. Pyrazine ring-based Na+/H+ exchanger (NHE) inhibitors potently inhibit cancer cell growth in 3D culture, independent of NHE1. Sci Rep-Uk. 2020;10(1):e65.
98. Marala RB, Brown JA, Kong JX, et al. Zoniporide: a potent and highly selective inhibitor of human Na+/H+ exchanger-1. Eur J Pharmacol. 2002;451(1):37–41. doi:10.1016/S0014-2999(02)02193-3
99. Wu DM, Doods H, Stassen JM. Inhibition of human pulmonary artery smooth muscle cell proliferation and migration by sabiporide, a new specific NHE-1 inhibitor. J Cardiovasc Pharm. 2006;48(2):34–40. doi:10.1097/01.fjc.0000239691.69346.6a
100. Wang D, Balkovetz DF, Warnock DG. Mutational analysis of transmembrane histidines in the amiloride-sensitive Na+/H+ exchanger. Am J Physiol. 1995;269(2 Pt 1):C392–402. doi:10.1152/ajpcell.1995.269.2.C392
101. Kim MJ, Moon CH, Kim MY, et al. KR-32570, a novel Na+/H+ exchanger-1 inhibitor, attenuates hypoxia-induced cell death through inhibition of intracellular Ca2+ overload and mitochondrial death. pathway in H9c2 cells. Eur J Pharmacol. 2005;525(1–3):1–7. doi:10.1016/j.ejphar.2005.09.043
102. Kawamoto T, Kimura H, Kusumoto K, et al. Potent and selective inhibition of the human Na+/H+ exchanger isoform NHE1 by a novel aminoguanidine derivative T-162559. Eur J Pharmacol. 2001;420(1):1–8. doi:10.1016/S0014-2999(01)00991-8
103. Lorrain J, Briand V, Favennec E, et al. Pharmacological profile of SL 59.1227, a novel inhibitor of the sodium/hydrogen exchanger. Brit J Pharmacol. 2000;131(6):1188–1194. doi:10.1038/sj.bjp.0703671
104. He BY, Deng CS, Zhang M, Zou DD, Xu M. Reduction of intracellular pH inhibits the expression of VEGF in K562 cells after targeted inhibition of the Na+/H+ exchanger. Leukemia Res. 2007;31(4):507–514. doi:10.1016/j.leukres.2006.06.015
105. Andersen AP, Flinck M, Oernbo EK, Pedersen NB, Viuff BM, Pedersen SF. Roles of acid-extruding ion transporters in regulation of breast cancer cell growth in a 3-dimensional microenvironment. Mol Cancer. 2016;15. doi:10.1186/s12943-016-0500-z
106. Lee SP, Chao SC, Huang SF, Chen YL, Tsai YT, Loh SH. Expressional and functional characterization of intracellular ph regulators and effects of ethanol in human oral epidermoid carcinoma cells. Cell Physiol Biochem. 2018;47(5):2056. doi:10.1159/000491473
107. Giambelluca MS, Ciancio MC, Orlowski A, Gende OA, Pouliot M, Aiello EA. Characterization of the Na+/HCO3−cotransport in human neutrophils. Cell Physiol Biochem. 2014;33(4):982–990. doi:10.1159/000358669
108. Orlowski A, Vargas LA, Aiello EA, Alvarez BV. Elevated carbon dioxide upregulates NBCn1 Na+/HCO3− cotransporter in human embryonic kidney cells. Am J Physiol-Renal. 2013;305(12):F1765–F1774. doi:10.1152/ajprenal.00096.2013
109. Nordstrom T, Andersson LC, Akerman KEO. Regulation of intracellular pH by electrogenic Na+/HCO3−co-transporters in embryonic neural stem cell-derived radial glia-like cells. Bba-Biomembranes. 2019;1861(6):1037–1048. doi:10.1016/j.bbamem.2019.03.007
110. Radvak P, Repic M, Svastova E, et al. Suppression of carbonic anhydrase IX leads to aberrant focal adhesion and decreased invasion of tumor cells. Oncol Rep. 2013;29(3):1147–1153. doi:10.3892/or.2013.2226
111. Juhasz M, Chen J, Lendeckel U, et al. Expression of carbonic anhydrase IX in human pancreatic cancer. Aliment Pharm Ther. 2003;18(8):837–846. doi:10.1046/j.1365-2036.2003.01738.x
112. Stock C, Schwab A. Protons make tumor cells move like clockwork. Pflug Arch Eur J Phy. 2009;458(5):981–992. doi:10.1007/s00424-009-0677-8
113. Pastorek J, Pastorekova S, Callebaut I, et al. Cloning and characterization of Mn, a human tumor-associated protein with a domain homologous to carbonic-anhydrase and a putative helix-loop-helix DNA-binding segment. Oncogene. 1994;9(10):2877–2888.
114. Aldera AP, Govender D. Carbonic anhydrase IX: a regulator of pH and participant in carcinogenesis. J Clin Pathol. 2021;74(6):350–354. doi:10.1136/jclinpath-2020-207073
115. Zhang SY, Yang CM, Lu WQ, et al. A highly selective space-folded photo-induced electron transfer fluorescent probe for carbonic anhydrase isozymes IX and its applications for biological imaging. Chem Commun. 2011;47(29):8301–8303. doi:10.1039/c1cc12386f
116. Chen XY, Zhang HL, Zhang M, et al. Amorphous Fe-based nanoagents for self-enhanced chemodynamic therapy by re-establishing tumor acidosis. Adv Funct Mater. 2020;30(6):1908365. doi:10.1002/adfm.201908365
117. Buonanno M, Langella E, Zambrano N, et al. Disclosing the interaction of carbonic anhydrase IX with cullin-associated NEDD8-dissociated protein 1 by molecular modeling and integrated binding measurements. ACS Chem Biol. 2017;12(6):1460–1465. doi:10.1021/acschembio.7b00055
118. Chandel V, Maru S, Kumar A, et al. Role of monocarboxylate transporters in head and neck squamous cell carcinoma. Life Sci. 2021;279:119709. doi:10.1016/j.lfs.2021.119709
119. Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1 alpha-dependent mechanism. J Biol Chem. 2006;281(14):9030–9037. doi:10.1074/jbc.M511397200
120. Kiran D, Basaraba RJ. Lactate metabolism and signaling in tuberculosis and cancer: a comparative review. Front Cell Infect Microbiol. 2021;11:624607. doi:10.3389/fcimb.2021.624607
121. Luo F, Zou Z, Liu X, et al. Enhanced glycolysis, regulated by HIF-1alpha via MCT-4, promotes inflammation in arsenite-induced carcinogenesis. Carcinogenesis. 2017;38(6):615–626. doi:10.1093/carcin/bgx034
122. Hu X, Liu Z, Duan X, et al. Blocking MCT4 SUMOylation inhibits the growth of breast cancer cells. Mol Carcinog. 2021;60(10):702–714. doi:10.1002/mc.23336
123. Ruan Y, Zeng F, Cheng Z, Zhao X, Fu P, Chen H. High expression of monocarboxylate transporter 4 predicts poor prognosis in patients with lung adenocarcinoma. Oncol Lett. 2017;14(5):5727–5734. doi:10.3892/ol.2017.6964
124. Stock C, Pedersen SF. Roles of pH and the Na+/H+ exchanger NHE1 in cancer: from cell biology and animal models to an emerging translational perspective? Semin Cancer Biol. 2017;43:5–16. doi:10.1016/j.semcancer.2016.12.001
125. Hendus-Altenburger R, Vogensen J, Pedersen ES, et al. The intracellular lipid-binding domain of human Na+/H+ exchanger 1 forms a lipid-protein co-structure essential for activity. Commun Biol. 2020;3(1):731. doi:10.1038/s42003-020-01455-6
126. Ariyoshi Y, Shiozaki A, Ichikawa D, et al. Na+/H+ exchanger 1 has tumor suppressive activity and prognostic value in esophageal squamous cell carcinoma. Oncotarget. 2017;8(2):2209–2223. doi:10.18632/oncotarget.13645
127. Reshkin SJ, Cardone RA, Harguindey S. Na+-H+ exchanger, pH regulation and cancer. Recent Pat Anti-Canc. 2013;8(1):85–99.
128. Chiang YH, Chou CY, Hsu KF, Huang YF, Shen MR. EGF upregulates Na+/H+ exchanger NHE1 by post-translational regulation that is important for cervical cancer cell invasiveness. J Cell Physiol. 2008;214(3):810–819. doi:10.1002/jcp.21277
129. Buckley BJ, Kumar A, Aboelela A, et al. Screening of 5-and 6-substituted amiloride libraries identifies dual-uPA/NHE1 active and single target-selective inhibitors. Int J Mol Sci. 2021;22(6):2999. doi:10.3390/ijms22062999
130. Andersen AP, Samsoe-Petersen J, Oernbo EK, et al. The net acid extruders NHE1, NBCn1 and MCT4 promote mammary tumor growth through distinct but overlapping mechanisms. Int J Cancer. 2018;142(12):2529–2542. doi:10.1002/ijc.31276
131. Gorbatenko A, Olesen CW, Morup N, et al. ErbB2 upregulates the Na+,HCO3 (-)-cotransporter NBCn1/SLC4A7 in human breast cancer cells via Akt, ERK, Src, and Kruppel-like factor 4. FASEB J. 2014;28(1):350–363. doi:10.1096/fj.13-233288
132. Boedtkjer E. Na+,HCO3− cotransporter NBCn1 accelerates breast carcinogenesis. Cancer Metast Rev. 2019;38(1–2):165–178. doi:10.1007/s10555-019-09784-7
133. Yang HS, Cooper DS, Rajbhandari I, Park HJ, Lee S, Choi I. Inhibition of rat Na+-HCO3− cotransporter (NBCn1) function and expression by the alternative splice domain. Exp Physiol. 2009;94(11):1114–1123. doi:10.1113/expphysiol.2009.048603
134. Lee S, Mele M, Vahl P, Christiansen PM, Jensen VED, Boedtkjer E. Na+,HCO3 (-)-cotransport is functionally upregulated during human breast carcinogenesis and required for the inverted pH gradient across the plasma membrane. Pflug Arch Eur J Phy. 2015;467(2):367–377. doi:10.1007/s00424-014-1524-0
135. Boedtkjer E, Bunch L, Pedersen SF. Physiology, pharmacology and pathophysiology of the pH regulatory transport proteins NHE1 and NBCn1: similarities, differences, and implications for cancer therapy. Curr Pharm Des. 2012;18(10):1345–1371. doi:10.2174/138161212799504830
136. Lee S, Axelsen TV, Andersen AP, Vahl P, Pedersen SF, Boedtkjer E. Disrupting Na+, HCO3–cotransporter NBCn1 (Slc4a7) delays murine breast cancer development. Oncogene. 2016;35(16):2112–2122. doi:10.1038/onc.2015.273
137. Lee S, Axelsen TV, Jesse N, Pedersen SF, Vahi P, Boedtkjer E. Na+,HCO3–cotransporter NBCn1 (Slc4a7) accelerates ErbB2-induced breast cancer development and tumor growth in mice. Oncogene. 2018;37(41):5569–5584. doi:10.1038/s41388-018-0353-6
138. Lin L, Wang S, Deng H, et al. Endogenous labile iron pool-mediated free radical generation for cancer chemodynamic therapy. J Am Chem Soc. 2020;142(36):15320–15330. doi:10.1021/jacs.0c05604
139. Liu Y, Zhen WY, Wang YH, et al. One-dimensional Fe2P acts as a fenton agent in response to NIR II light and ultrasound for deep tumor synergetic theranostics. Angewandte Chemie. 2019;58(8):2407–2412. doi:10.1002/anie.201813702
140. Liu X, Liu Y, Wang J, Wei T, Dai Z. Mild hyperthermia-enhanced enzyme-mediated tumor cell chemodynamic therapy. ACS Appl Mater Interfaces. 2019;11(26):23065–23071. doi:10.1021/acsami.9b08257
141. Wu H, Chen F, Gu D, You C, Sun B. A pH-activated autocatalytic nanoreactor for self-boosting Fenton-like chemodynamic therapy. Nanoscale. 2020;12(33):17319–17331. doi:10.1039/D0NR03135F
142. Xu X, Zhang R, Yang X, et al. A honeycomb-like bismuth/manganese oxide nanoparticle with mutual reinforcement of internal and external response for triple-negative breast cancer targeted therapy. Adv Healthc Mater. 2021;10:e2100518. doi:10.1002/adhm.202100518
143. Su JJ, Lu S, Wei Z, et al. Biocompatible inorganic nanoagent for efficient synergistic tumor treatment with augmented antitumor immunity. Small. 2022;18(16):2200897.
144. Guo Y, Jia HR, Zhang X, et al. A glucose/oxygen-exhausting nanoreactor for starvation- and hypoxia-activated sustainable and cascade chemo-chemodynamic therapy. Small. 2020;16(31):e2000897. doi:10.1002/smll.202000897
145. Zhang L, Yang Z, He W, Ren J, Wong CY. One-pot synthesis of a self-reinforcing cascade bioreactor for combined photodynamic/chemodynamic/starvation therapy. J Colloid Interface Sci. 2021;599:543–555. doi:10.1016/j.jcis.2021.03.173
146. Zhao PR, Tang ZM, Chen XY, et al. Ferrous-cysteine-phosphotungstate nanoagent with neutral pH fenton reaction activity for enhanced cancer chemodynamic therapy. Mater Horiz. 2019;6(2):369–374. doi:10.1039/C8MH01176A
147. Zhang HL, Li JJ, Chen Y, et al. Magneto-electrically enhanced intracellular catalysis of FePt-FeC heterostructures for chemodynamic therapy. Adv Mater. 2021;33(17):2100472. doi:10.1002/adma.202100472
148. Meng X, Chen L, Lv R, Liu M, He N, Wang Z. A metal-phenolic network-based multifunctional nanocomposite with pH-responsive ROS generation and drug release for synergistic chemodynamic/photothermal/chemo-therapy. J Mater Chem B. 2020;8(10):2177–2188. doi:10.1039/D0TB00008F
149. Liu Y, Chi SY, Cao Y, Liu ZH. Glutathione-responsive biodegradable core-shell nanoparticles that self-generate H2O2 and deliver doxorubicin for chemo-chemodynamic therapy. Acs Appl Nano Mater. 2022;5(2):2592–2602. doi:10.1021/acsanm.1c04277
150. Wang Y, Song M. pH-responsive cascaded nanocatalyst for synergistic like-starvation and chemodynamic therapy. Colloids Surf B Biointerfaces. 2020;192:111029. doi:10.1016/j.colsurfb.2020.111029
151. Chen J, Wang X, Zhang Y, et al. A redox-triggered C-centered free radicals nanogenerator for self-enhanced magnetic resonance imaging and chemodynamic therapy. Biomaterials. 2021;266:120457. doi:10.1016/j.biomaterials.2020.120457
152. Cui Y, Chen X, Cheng Y, et al. CuWO4 nanodots for NIR-induced photodynamic and chemodynamic synergistic therapy. ACS Appl Mater Interfaces. 2021;13(19):22150–22158. doi:10.1021/acsami.1c00970
153. Hu T, Yan L, Wang Z, et al. A pH-responsive ultrathin Cu-based nanoplatform for specific photothermal and chemodynamic synergistic therapy. Chem Sci. 2021;12(7):2594–2603. doi:10.1039/D0SC06742C
154. Tao Q, He GH, Ye S, et al. Mn doped Prussian blue nanoparticles for T-1/T-2 MR imaging, PA imaging and Fenton reaction enhanced mild temperature photothermal therapy of tumor. J Nanobiotechnol. 2022;20(1). doi:10.1186/s12951-021-01235-2
155. Peng H, Qin YT, Feng YS, He XW, Li WY, Zhang YK. Phosphate-degradable nanoparticles based on metal-organic frameworks for chemo-starvation-chemodynamic synergistic antitumor therapy. ACS Appl Mater Interfaces. 2021;13(31):37713–37723. doi:10.1021/acsami.1c10816
156. Zheng RX, Cheng Y, Qi F, et al. Biodegradable copper-based nanoparticles augmented chemodynamic therapy through deep penetration and suppressing antioxidant activity in tumors. Adv Healthc Mater. 2021;10(14):2100412. doi:10.1002/adhm.202100412
157. Lin XH, Zhu R, Hong ZZ, et al. GSH-responsive radiosensitizers with deep penetration ability for multimodal imaging-guided synergistic radio-chemodynamic cancer therapy. Adv Funct Mater. 2021;31(24):2101278. doi:10.1002/adfm.202101278
158. Fu S, Yang R, Ren J, et al. Catalytically active CoFe2O4 nanoflowers for augmented sonodynamic and chemodynamic combination therapy with elicitation of robust immune response. Acs Nano. 2021;15(7):11953–11969. doi:10.1021/acsnano.1c03128
159. Zhang X, He C, Chen Y, et al. Cyclic reactions-mediated self-supply of H2O2 and O2 for cooperative chemodynamic/starvation cancer therapy. Biomaterials. 2021;275:120987. doi:10.1016/j.biomaterials.2021.120987
160. Yang BC, Liu QY, Yao XX, et al. FePt@MnO-based nanotheranostic platform with acidity-triggered dual-ions release for enhanced MR imaging-guided ferroptosis chemodynamic therapy. Acs Appl Mater Inter. 2019;11(42):38395–38404. doi:10.1021/acsami.9b11353
161. Wang YN, Song D, Zhang WS, Xu ZR. Enhanced chemodynamic therapy at weak acidic pH based on g-C3N4-supported hemin/Au nanoplatform and cell apoptosis monitoring during treatment. Colloid Surface B. 2021;197:111437. doi:10.1016/j.colsurfb.2020.111437
162. Li T, He F, Liu B, et al. In situ synthesis of FeOCl in hollow dendritic mesoporous organosilicon for ascorbic acid-enhanced and MR imaging-guided chemodynamic therapy in neutral pH conditions. ACS Appl Mater Interfaces. 2020;12(51):56886–56897. doi:10.1021/acsami.0c19330
163. Dong SM, Dong YS, Jia T, et al. Sequential catalytic, magnetic targeting nanoplatform for synergistic photothermal and NIR-enhanced chemodynamic therapy. Chem Mater. 2020;32(23):9868–9881. doi:10.1021/acs.chemmater.9b05170
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
