Back to Journals » Journal of Inflammation Research » Volume 16
Research on Liver Damage Caused by the Treatment of Rheumatoid Arthritis with Novel Biological Agents or Targeted Agents
Authors Zhao X, Zhang C, An Y, Zhang Z, Zhao J, Zhang X, Yang Y, Cao W
Received 27 October 2022
Accepted for publication 24 December 2022
Published 3 February 2023 Volume 2023:16 Pages 443—452
DOI https://doi.org/10.2147/JIR.S395137
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Xin Zhao,1 Chenhao Zhang,2 Yi An,3 Zixuan Zhang,3 Jiahe Zhao,3 Xinwen Zhang,3 Yue Yang,4 Wei Cao4
1Department of Rheumatology, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Beijing, 100053, People’s Republic of China; 2Department of Emergency, Wangjing Hospital of China Academy of Chinese Medical Sciences, Beijing, 100102, People’s Republic of China; 3Department of School of Clinical Medicine, Beijing University of Chinese Medicine, Beijing, 100029, People’s Republic of China; 4Department of Wangjing Hospital of China Academy of Chinese Medical Sciences, Beijing, 100102, People’s Republic of China
Correspondence: Wei Cao, Department of Wangjing Hospital of China Academy of Chinese Medical Sciences, No. 6 Zhonghuan South Road, Chaoyang District, Beijing, 100102, People’s Republic of China, Tel +86 10-84739099, Email [email protected]
Abstract: Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by polyarticular, symmetric, and aggressive inflammation of the small joints in the hands and feet, resulting in dysfunction. With progress and development in medicine, treatment of RA is constantly evolving, making several drugs available for the treatment of RA. From the nonsteroidal anti-inflammatory drugs (NSAIDs) at the start of illness to glucocorticoids and then to conventional synthetic DMARDs (csDMARDs), biologic DMARDs (bDMARDs), and targeted synthetic DMARDs (tsDMARDs), therapeutic-use drugs for RA have been keeping pace with scientific research. However, various types of drugs have additional side effects when used over the long-term. New and emerging biological and targeted agents have been widely applied in recent years; however, the side effects have not been thoroughly investigated. In this paper, we review the research progress on liver damage caused by novel biological and targeted agents available for RA treatment. The aim is to provide a reference for rational clinical administration of such drugs.
Keywords: liver damage, novel biological agents, research progress, rheumatoid arthritis, targeted agents
Introduction
The etiology and pathogenesis of rheumatoid arthritis (RA) are unknown, but environmental factors, gene factors as well as environmental-genetic interaction are considered as major etiology of RA.Environmental factors especially cigarette smoking increase the risk of developing RA in those who have a hereditary disposition.1 And the prevailing pathogenic model of RA assumes an autoimmune mechanism.2 And the disease causes cartilage and bone destruction, resulting in degradation of joint functions, and even joint deformity and disability, seriously impairing the quality of life of patients.3
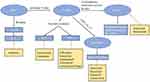
In recent years, novel biological and targeted agents have been increasingly used in the clinical treatment of RA, and compared to traditional anti-rheumatic drugs, significantly improve the clinical symptoms and slow down disease progression in patients. In the 2021 American College of Rheumatology (ACR) guidelines for the Treatment of Rheumatoid Arthritis, an adjustment was made with respect to bDMARDs and tsDMARDs as second-line therapy, especially tsDMARDs, which have a significantly improved therapeutic status compared to the previous guidelines, and reflected their efficacy and safety.4 Currently, there are a relatively large number of novel biological agents clinically used for RA, including tumor necrosis factor inhibitors (TNFi), interleukin (IL) receptor antagonists, B-cell depleting agents, T-cell targeting drugs, etc. Targeted DMARDs mainly include Janus kinase (JAKs) inhibitors. They belong to different drug categories and have different pathophysiological targets and mechanisms of action (refer to Figure 1 and Table 1).
 |
Table 1 Currently Approved Biological DMARDs and Targeted Agents |
RA rarely affects the liver by itself but liver damage has been a common adverse effect in RA patients over long-term or on irregular medication intake. Liver damage occurs in RA patients, mostly during NSAIDs and methotrexate (MTX) therapy.5 Methotrexate used for RA has been generally well tolerated, however, it has potential side effects like hepatotoxicity and cytopenia.6 In the application of leflunomide for RA, the elevation of liver enzymes usually occurs in the first six months of treatment and remains unresolved until follow-up.7 Leflunomide has been reported to increase the incidence of abnormal liver enzymes by 14%–22%.8 Hydroxychloroquine sulfate also bears certain side effects in the treatment of RA, including abnormal elevation of transaminases.9 Comparatively, liver damage is relatively uncommon with novel biological and targeted agents and may range from a slight increase in transaminases to severe forms of hepatotoxicity. In this paper, we summarize the mechanisms of action and possible manifestations of liver damage caused by novel biological and targeted agents in the treatment of RA in recent years, to provide guidance for clinical use.
TNF-α Inhibitor
Tumor necrosis factor alpha (TNF-α) plays an important role in the development of inflammation. Elevated levels of TNF-α have been observed in the synovium of RA patients, which is closely associated with the development of bone erosion.10 TNF-a inhibitors primarily include infliximab, etanercept, adalimumab, golimumab, and certolizumab. TNF-a inhibitors are potent therapeutic agents for RA, and can greatly alleviate the inflammatory state, while revolutionizing the management of RA by blocking cytokines characteristic of the innate immune system. Abnormalities in liver function tests may occur during anti-TNF-α therapy, including transient and self-limiting high aminotransferases, cholestatic disease, and hepatitis, which, in some cases may be severe or life-threatening.11
Infliximab
Infliximab (INF) is a monoclonal antibody targeting TNF-α that has been demonstrated to be effective in patients with RA. As observed by a multicenter study involving 6861 patients with RA, INF has the highest correlation with elevated liver function tests (LFTs) within the range of TNF-α inhibitors to the abnormal range.12 A randomized 1-year trial demonstrated a balanced incidence of adverse events regarding liver injury between the two groups using salazosulfapyridine and hydroxychloroquine or infliximab.13 It seems that infliximab-induced hepatitis is maintained by immune-mediated mechanisms simulating the features of type I autoimmune hepatitis14 with elevated antinuclear antibodies (ANA), anti-smooth muscle antibodies (ASMA), and anti-double-stranded DNA antibodies (anti-dsDNA). However, the exact pathogenesis of its induced autoimmune hepatitis is unknown. Available evidence suggests that the incidence of liver injury in RA patients on infliximab is significant but relatively rare, and often resolves spontaneously with a favorable prognosis after discontinuation of the drug.
Etanercept
Etanercept (ETN) is the first specific anti-cytokine drug approved for the treatment of RA. Adverse effects are rare in terms of elevated liver enzymes and autoimmune hepatitis.15 In terms of liver damage, ie, cirrhosis, a multicenter study revealed no correlation with the development of cirrhosis in RA patients.16 In terms of hepatitis virus infection, Brunasso et al published a systematic review17 which included 153 patients with HCV treated with anti-TNF-α drugs between 1999 and 2010, including 91 with RA, 7 with psoriatic arthritis, 8 with psoriasis, 8 with both psoriasis and psoriatic arthritis, 6 with CD, and 14 with other chronic inflammatory diseases. A total of 110 cases were treated with ETN, with only one clearly diagnosed case of worsening HCV hepatitis and five suspected cases. The liver showed improvement after discontinuation of ETN, indicating that it appears to have a favorable safety profile in terms of hepatitis virus infection.
Adalimumab
Adalimumab (ADA) is a human IgG1 monoclonal antibody binding soluble transmembrane TNF-α, which can block the interaction of cell surface TNF receptors, to achieve immunosuppressive effects.18 Multiple randomized controlled trials have demonstrated significant efficacy of ADA in patients with early and long-term RA.19,20 In studies assessing ADA for RA, asymptomatic alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations have been reported, however, the phenomenon is reversible with the discontinuation of ADA.21 In a 7-year clinical study, the association between ADA and elevated AST/ALT was identified to be modest compared to the control group on DMARDs.12 Furthermore, evidence suggests that there may be potential benefits of ADA in the treatment of specific liver diseases.22 In patients with concomitant HCV and HBV infection, data suggests that ADA does not alter viral load or liver enzyme levels or activate HBV and/or HCV. There is also a slight reduction in liver fibrosis scores before and after treatment, reflecting that the use of ADA in patients with combined HBV or HCV infection appears to have a favorable safety profile.23
Golimumab
Golimumab (GOL) is one of the TNF-α inhibitors approved for the treatment of RA. It is a human IgG1κ monoclonal antibody produced from a mouse hybridoma cell line with recombinant DNA technology. It mainly acts by targeting and neutralizing TNF-α to prevent inflammation, and cartilage and bone destruction. Compared to other anti-TNF drugs, GOL features a lower discontinuation rate and better tolerability.24 During a follow-up observation of 5154 Japanese RA patients treated with GOL, acceptable safety and efficacy were observed in all patients, and concomitant use of MTX was associated with increased efficacy, while persistence was associated with GOL.25 However, as a potent inhibitor of TNF-α, GOL may result in the relapse of chronic hepatitis B in susceptible patients.26
Certolizumab Pegol
Certolizumab Pegol (CZP) is a TNF-α inhibitor that can selectively neutralize the inflammation site by covalently binding polyethylene glycol (PEG) to the Fab fragment of recombinant human anti-TNF-α antibody, thereby binding with high affinity to human TNF-α. Nanau et al identified a low incidence of severe AEs in patients with CZP, with major AEs occurring in the neurological and respiratory systems.27 Currently, there is no evidence of adverse hepatic effects associated with pexelizumab treatment for RA.
To conclude, the administration of TNF-α receptor blockers in the autoimmune setting may result in liver injury, especially in the presence of pre-existing autoimmune serological signs. The incidence of liver injury resulting from anti-TNF-α therapy, though relatively low, is still significant. For patients with significantly elevated transaminases or clinical symptoms consistent with acute hepatitis, anti-TNF-α therapy should be discontinued prior to the onset of severe irreversible damage. The safety of anti-TNF-α drugs in the context of HCV infection appears to be acceptable, however, long-term and large controlled clinical trials are required for validation.
Interleukin Receptor Antagonists
Interleukin (IL) is an important pro-inflammatory cytokine in the pathogenesis of RA, and IL-1 and IL-6 are key cytokines in driving the inflammatory and destructive process in RA. They have an important role in the pathogenesis of RA.28 The inflammatory response in the synovium results in the production of cytokines such as IL-1 and IL-6, thereby activating osteoclasts and mediating articular cartilage and bone destruction.29 IL-17 is predominantly highly expressed in the synovial fluid of RA patients and is mainly associated with bone degradation.30 Presently, interleukin receptor antagonists are available for the treatment of rheumatoid arthritis, ankylosing spondylitis, arthritic psoriasis, etc.
IL-1 Receptor Antagonists
IL-1 is an important cytokine in the pathogenesis of RA. Natural IL-1 receptor antagonist (IL-1RN) is an anti-inflammatory protein and its combination with IL-1RN plays an important role in maintaining the immune homeostasis of the body.28 The expression of natural IL-1 receptor antagonists in the synovial tissue is reduced in patients with RA.31
Anakinra, a recombinant IL-1 receptor antagonist with anti-inflammatory and immunomodulatory effects, is the only IL-1 receptor antagonist currently used in RA. Studies have demonstrated that anabolic acid is a safe, effective, and a well-tolerated biological agent.32 Currently, no clear supporting data is available on liver damage due to anabolic agents for RA, Several randomized controlled and cohort trials have not demonstrated statistically significant differences in adverse events between anabolic agents and placebo in the treatment of RA.33,34
IL-6 Receptor Antagonists
Interleukin-6 is an important cytokine with pleiotropic and redundant functional activity. In RA, IL-6 plays an important role in the inflammatory processes, osteoclast-mediated bone resorption, and the development of vascular opacification, contributing to the development of IgM and IgG rheumatoid factors and antibodies to guanosine peptides, thus contributing to the characteristic increase in guanosine peptides.35 Some studies have identified that serum IL-6 levels were higher than normal in RA patients and that after treatment with DMARDs, serum IL-6 levels were significantly reduced, relieving the disease symptoms.36 Currently, IL-6 receptor antagonists like tocilizumab and sirukumab have been marketed and are in Phase 3 clinical trials.
Tocilizumab
Tocilizumab (TCZ) is a recombinant humanized anti-IL-6 receptor (IL-6R) monoclonal antibody inhibiting its binding to its receptor and reducing the pro-inflammatory activity of this cytokine, by competing with the soluble and membrane-bound forms of human IL-6 receptor 17.37 A 72-week randomized double-blind controlled trial identified that TCZ infusion add-on therapy has been highly effective and well tolerated in South Korean patients with active refractory RA compared to conventional DMARDs, with significant remission of DAS28. However, patients in the TCZ group had significantly more-frequent liver function abnormalities, mainly in the form of elevated mean AST and ALT levels, compared to the placebo group, but most of the liver function abnormalities were mild or moderate in intensity and asymptomatic in presentation.38 Amouzougan et al also demonstrated that TCZ alleviated RA symptoms, with liver enzyme levels one to two times the upper limit of normal without exhibiting any adverse effects, indicating that TCZ allows for adequate liver function and clinical safety.39
Sirukumab
Sirukumab (SRK) is an IgG1κ human anti-IL-6 monoclonal antibody binding to IL-6 to prevent IL-6-mediated downstream effects. SRK is an effective and well-tolerated tool for novel treatment of patients with active RA, and experts have recommended that SRK at 50 mg/4 weeks or 100 mg/2 weeks be used for the treatment of population with RA.40 The profile of adverse events appears to be like that of TCZ, most commonly with elevated liver enzymes, however, dose response to SRK has not been demonstrated. In a randomized controlled study, a mild increase in transaminases has been observed in patients with RA treated with SRK initially. It gradually resolved after 4 weeks of treatment, reflecting the relatively favorable safety profile.41 Results from another phase 3 clinical trial with Sirukumab in Japanese patients with refractory rheumatoid arthritis and poor results who were treated with TNFi, demonstrated that only a small number of patients treated with Sirukumab had ALT and AST levels above the normal range. This suggests that adverse effects in patients needed to be observed closely when administering the drug clinically.42
At the initial stage of administration, interleukin factor antagonists may result in elevated transaminases, in particular tocilizumab, which may be associated with disruption of fat metabolism and NAFLD. Therefore, it is necessary to monitor transaminases during the first 6 months of treatment.
B-Cell-Depleting Agents
B cells have an important role in the development of RA, and the mechanisms of action mainly include the production of antibodies, such as rheumatoid factor, the production of inflammatory cytokines, such as IL-6 and IL-17, and the activation of T cells in the pathogenesis of RA.43 Currently, the main B-cell-depleting agents already available for clinical treatment of RA include rituximab and belimumab.
Rituximab
Rituximab (RTX) is a monoclonal antibody targeting the CD20 molecule on B lymphocytes and serves as the standard of care for RA. A randomized, double-blind, multicenter trial involving 517 patients with RA demonstrated that some patients rapidly developed acute liver failure and jaundice, often referred to as acute hepatic necrosis, after initiation of rituximab, which may be associated with toxicity or ischemic injury.44 Nevertheless, a retrospective clinical study with a duration of 9.5 years demonstrated no significant difference in the incidence of adverse events between rituximab and placebo.45
Belimumab
Belimumab is a fully human IgG1 monoclonal antibody targeting B-cell activation factors, thereby inhibiting B-cell activation. Belimumab has been proven to be a safe and effective drug in the treatment of SLE and of developmental value in the treatment of RA.46 Currently, no clear supporting data on liver damage due to belimumab for RA is available, and some randomized controlled trials have demonstrated no statistically significant differences in adverse events between belimumab and placebo in the treatment of RA.47
The risks of liver damage resulting from B-cell-depleting agents are relatively low, and rituximab is a relatively safe option for patients with RA not combined with viral hepatitis. However, compared to TNF-α inhibitors, B-cell-depleting agents are relatively infrequently used in the clinical treatment of RA, and further clinical trials are still required to provide more data on the association with liver damage.
T-Cell-Targeted Drugs
T cells modulate the course of disease in RA at multiple levels and are a logical option for anti-inflammatory therapy. Therapeutic interventions against T cells may target antigen recognition or interfere with co-stimulatory processes. The most widely recognized co-stimulatory routes involve CD28, cytotoxic T lymphocyte-associated protein 4 (CTLA-4), CD80, and CD86. The major T-cell-targeting drug currently available for clinical application in RA is abatacept.
Abatacept is a designed protein and blocks the connection of CD28 to CD80 and CD86, thereby preventing the activation and proliferation of CD4+ T cells. Reynolds and Zen et al identified the possibility of hepatitis in patients receiving CTLA-4 inhibitors.48,49 A study conducted between January 1, 2006, and June 30, 2021 collected a total of 77,669 adverse events with abatacept and 2889 reports of hepatitis B virus reactivation (HBVr) with other drugs,50 based on the use of the FDA Adverse Event Reporting System (FAERS) database. All 55 cases taking abatacept reported serious adverse events, including 6 hospitalizations and 4 deaths.
JAK Inhibitor
Janus kinases (JAKs) are non-receptor tyrosine kinases in the cells and play a key role in the signaling pathway of cytokines. The dysregulation of the signal transducer and activator of transcription (STAT) pathway of JAKs has been associated with various immune disorders. In recent years, the choice of drugs targeting components of the JAK-STAT pathway has been widely noticed as a potential new treatment for RA, demonstrating promising efficacy in improving the clinical symptoms of RA. Currently, four oral JAK inhibitors have been approved for the treatment of RA, including tofacitinib, baricitinib, upadacitinib, and filgotinib.
Tofacitinib
Tofacitinib (TOF) is a small synthetic molecule targeting JAK1 and JAK3 as an inhibitor. Studies have demonstrated that TOF may significantly inhibit IL-17 and IFNc production by synovial and peripheral blood CD4+T cells, reduce IL-6 production in synovial fibroblasts, and further inhibit the progression of structural damage in arthritic joints, while improving health-related quality of life (HR-QOL) by reducing RANKL production.51 Tofacitinib is typically well tolerated, with most adverse events being mild or moderately severe. Elevated serum creatinine level is the most common adverse effect of TOF.52 A prospective study revealed elevated ALT or AST in patients exposed to TOF compared to placebo,53 however, only two studies in a review describing efficiency, retention, and safety have reported elevated liver enzymes. The current evidence indicates that oral TOF is an effective and safer option in the treatment of patients with RA, although large long-term follow-up studies are required for further confirmation.
Baricitinib
Baricitinib is an orally available reversible inhibitor of Janus kinases JAK1 and JAK2, often administered in patients with moderately or severely active RA. Findings from a 52-week phase 3 clinical trial have demonstrated a significant inhibition of radiographic progression of joint injury and a higher ACR20 response rate in patients in the baricitinib group compared to the placebo and adalimumab groups. Clinical symptoms significantly improved in patients,54 but were accompanied by a higher incidence of adverse events in the baricitinib group. Baricitinib has been reported to be associated with elevated liver enzyme levels, and in the control study, elevated ALT and AST have been observed in patients treated with baricitinib at week 16, with most cases of elevated liver transaminases being asymptomatic and transient.55 The RA-BEGIN study concluded that baricitinib requires no dose adjustments in patients with mild or moderate hepatic impairment, however, baricitinib is not recommended in patients with severe hepatic impairment.56
Filgotinib
In clinical drug trials of upadacitinib and filgotinib, transient elevations in Grade 1 or 2 AST and ALT levels have been reported without any drug-induced hepatocellular injury, and the incidences of Grade 3 or 4 AST/ALT elevations have been lower than that of MTX.57 In a long-term, open-label extension study, the safety and efficacy of filgotinib, with or without MTX, has been evaluated in patients with RA. Excellent tolerability and safety were demonstrated for filgotinib + MTX and filgotinib monotherapy groups.58
Upadacitinib
Studies have demonstrated that the manifestations of liver damage in RA patients treated with upadacitinib are mainly elevated ALT or AST levels that are not severe.59 In a 24-week, phase 3, double-blind, controlled trial,60 patients were randomly assigned to receive oral upadacitinib or intravenous abciximab in a ratio of 1:1. Each drug was administered in conjunction with a stable synthetic DMARD—liver transaminase levels were higher in the upadacitinib group than in the abatacept group. Around 2.6% of patients treated with upadacitinib experienced Grade 3 ALT and AST level elevations and 0.3% reported Grade 4 elevations, however, none of the patients met Hay’s Law criteria suggestive of drug-related liver injury. In a UPA pharmacokinetic study of 18 patients with cirrhosis, using Child-Pugh Class A and Class B, as well as matched healthy controls, the baseline AST, ALT, and bilirubin had no effect on upadacitinib exposure over a very short period.61
Currently, JAK inhibitors are novel targeted drugs for RA treatment. Recent studies have demonstrated that four JAK inhibitors have better safety profiles but are limited by the short duration of clinical application. More recent study data is required to further illustrate the safety of JAK inhibitors.
Others
There are also some biosimilars among the latest drugs available for the treatment of RA. Infliximab biosimilars CT-P13 and SB2 were approved in 2016 and 2017, respectively, and both have similar therapeutic efficacy and safety profiles compared to infliximab and induced comparable liver injury compared to infliximab.62 Other biologics such as levilimab (targeting interleukins or the receptors), satralizumab and cytokine or chemokine-targeted biologics such as mavrilimumab and otilimab are still used in clinical trials. Although different biosimilars have demonstrated efficacy and safety compared to the reference product, serious adverse reactions may not have been fully identified and confidence in their use is still lacking.
Conclusion
TNF-α inhibitors are first-line biologics for the treatment of RA and the first biologics used in RA. The incidence of liver damage may be low, however, acute liver injury and significant increases in transaminases are required. It should be noted that Chinese patients with RA using TNF-α inhibitors should be highly alert to the risk of hepatitis B virus replication and tuberculosis relapse. Interleukin factor antagonists may result in elevated transaminases during the initial administration, and monitoring of liver function is essential during dosing. B-cell-depleting agents are frequently administered as second-line agents in RA, mostly in patients who fail to respond to treatment with TNF-α inhibitors or interleukin antagonists. Attention should be paid to whether the patient has viral hepatitis when administering B-cell antagonists; rituximab may result in viral reactivation. The safety of B-cell antagonists is more reliable in patients who do not have viral hepatitis. T-cell-targeted drugs are second-line biological agents for RA, which have a relatively high potential to cause adverse events such as liver damage. Regular monitoring of liver function is recommended during drug administration, and it is not indicated for patients with abnormal liver function or viral hepatitis. The incidence of tumors and infections resulting from JAK inhibitors is comparable to that of TNF-α inhibitors, and both hepatitis B and tuberculosis screening should be completed prior to treatment.
The effects of biologics on the liver represents a particularly important part along with the therapeutic effects. The long-term effects of TNF-α inhibitors and JAK inhibitors on the liver are expected to be of major focus in future research. The increasing incidence of liver disease emphasizes the necessity to be more cautious when prescribing anti-rheumatic drugs due to their hepatotoxicity. According to study data, all drugs may result in elevated liver enzymes early in their administration, and the monitoring of changes in transaminase levels is critical for all patients with RA, especially those treated with combined methotrexate and leflunomide. Furthermore, prior to initiating treatment with bDMARDs, RA patients should be screened for chronic HCV and closely monitored for liver enzymes as well as HCV viral load. In patients with chronic HCV, ACR recommends that etanercept be administered, while in patients with acute or chronic HCV with severe liver damage (Child-Pugh classification B or C), biological agents should be avoided. In patients with RA who are infected with HBV, concomitant anti-hepatitis B virus medication should be administered, while liver function and nucleic acid levels should be monitored for viral replication.
The clinical application of new biological and targeted agents provides new approaches and ideas for the treatment of RA. With the publication of further data on clinical efficacy and safety, an increasing number of biological and targeted agents with different targets of action will be approved for the clinical treatment of RA, and may play an important role in alleviating the clinical symptoms in RA patients, thus preventing inflammation from worsening and ultimately improving patients’ quality of life.
Funding
National Key R&D Program of China (2018YFC1705502).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Giannini D, Antonucci M, Petrelli F, Bilia S, Alunno A, Puxeddu I. One year in review 2020: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2020;38(3):387–397.
2. Hanan A, Walaa G, Ahmed M, et al. Investigating the balance between Th17/Treg cells in rheumatoid arthritis and its association with disease activity. J Child Sci. 2019;09(1):e75–e83. doi:10.1055/s-0039-1693158
3. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
4. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021;73(7):1108–1123. doi:10.1002/art.41752
5. Selmi C, De Santis M, Gershwin ME. Liver involvement in subjects with rheumatic disease. Arthritis Res Ther. 2011;13(3):226. doi:10.1186/ar3319
6. García-González CM, Baker J. Treatment of early rheumatoid arthritis: methotrexate and beyond. Curr Opin Pharmacol. 2022;64:102227. doi:10.1016/j.coph.2022.102227
7. van Roon EN, Jansen TL, Houtman NM, Spoelstra P, Brouwers JR. Leflunomide for the treatment of rheumatoid arthritis in clinical practice: incidence and severity of hepatotoxicity. Drug Saf. 2004;27(5):345–352. doi:10.2165/00002018-200427050-00006
8. Curtis JR, Beukelman T, Onofrei A, et al. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann Rheum Dis. 2010;69(1):43–47. doi:10.1136/ard.2008.101378
9. Nazir AM, Koganti B, Gupta K, et al. Evaluating the use of hydroxychloroquine in treating patients with rheumatoid arthritis. Cureus. 2021;13(11):e19308. doi:10.7759/cureus.19308
10. Radner H, Aletaha D. Anti-TNF in rheumatoid arthritis: an overview. Wien Med Wochenschr. 2015;165(1–2):3–9. doi:10.1007/s10354-015-0344-y
11. Ghabril M, Bonkovsky HL, Kum C, et al. Liver injury from tumor necrosis factor-α antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol. 2013;11(5):558–564.e3. doi:10.1016/j.cgh.2012.12.025
12. Sokolove J, Strand V, Greenberg JD, et al. Risk of elevated liver enzymes associated with TNF inhibitor utilisation in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69(9):1612–1617. doi:10.1136/ard.2009.112136
13. van Vollenhoven RF, Ernestam S, Geborek P, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet. 2009;374(9688):459–466. doi:10.1016/S0140-6736(09)60944-2
14. Kluger N, Girard C, Guillot B, Bessis D. Efficiency and safety of etanercept after acute hepatitis induced by infliximab for psoriasis. Acta Derm Venereol. 2009;89(3):332–334. doi:10.2340/00015555-0619
15. Zhao S, Mysler E, Moots RJ. Etanercept for the treatment of rheumatoid arthritis. Immunotherapy. 2018;10(6):433–445. doi:10.2217/imt-2017-0155
16. Chen DY, Lin CH, Chen HH, Tang KT. Association of tumor necrosis factor-α inhibitors and liver cirrhosis in patients with rheumatoid arthritis: a nationwide population-based nested case-control study. Int J Rheum Dis. 2022;25(3):327–334. doi:10.1111/1756-185X.14272
17. Brunasso AM, Puntoni M, Gulia A, Massone C. Safety of anti-tumour necrosis factor agents in patients with chronic hepatitis C infection: a systematic review. Rheumatology. 2011;50(9):1700–1711. doi:10.1093/rheumatology/ker190
18. Zhao S, Chadwick L, Mysler E, Moots RJ. Review of biosimilar trials and data on adalimumab in rheumatoid arthritis. Curr Rheumatol Rep. 2018;20(10):57. doi:10.1007/s11926-018-0769-6
19. Furst DE, Schiff MH, Fleischmann RM, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). J Rheumatol. 2003;30(12):2563–2571.
20. van de Putte LB, Atkins C, Malaise M, et al. Efficacy and safety of Adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63(5):508–516. doi:10.1136/ard.2003.013052
21. Hartmann U, Schmitt S, Reuss-Borst M. Leberwerterhöhung bei rheumatoider Arthritis: differenzialdiagnostische Uberlegungen an einem Fallbeispiel [Elevated liver enzymes in rheumatoid arthritis: differential diagnostic considerations based on a case report]. Z Rheumatol. 2008;67(5):440–444. doi:10.1007/s00393-008-0288-3
22. Lopetuso LR, Mocci G, Marzo M, et al. Harmful effects and potential benefits of anti-tumor necrosis factor (TNF)-α on the liver. Int J Mol Sci. 2018;19(8):2199. doi:10.3390/ijms19082199
23. Piaserico S, Dapavo P, Conti A, Gisondi P, Russo FP. Adalimumab is a safe option for psoriasis patients with concomitant hepatitis B or C infection: a multicentre cohort study of 37 patients and review of the literature. J Eur Acad Dermatol Venereol. 2017;31(11):1853–1859. doi:10.1111/jdv.14146
24. Pelechas E, Voulgari PV, Drosos AA. Golimumab for rheumatoid arthritis. J Clin Med. 2019;8(3):387. doi:10.3390/jcm8030387
25. Kanbori M, Suzuka H, Yajima T, et al. Postmarketing surveillance evaluating the safety and effectiveness of golimumab in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2018;28(1):66–75. doi:10.1080/14397595.2017.1325058
26. Antagonists, Tumor Necrosis Factor. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
27. Nanau RM, Neuman MG. Safety of anti-tumor necrosis factor therapies in arthritis patients. J Pharm Pharm Sci. 2014;17(3):324–361. doi:10.18433/J3WP4F
28. Magyari L, Varszegi D, Kovesdi E, et al. Interleukins and interleukin receptors in rheumatoid arthritis: research, diagnostics and clinical implications. World J Orthop. 2014;5(4):516–536. doi:10.5312/wjo.v5.i4.516
29. Fardellone P, Salawati E, Le Monnier L, Goëb V. Bone loss, osteoporosis, and fractures in patients with rheumatoid arthritis: a review. J Clin Med. 2020;9(10):3361. doi:10.3390/jcm9103361
30. Azizi G, Jadidi-Niaragh F, Mirshafiey A. Th17 cells in immunopathogenesis and treatment of rheumatoid arthritis. Int J Rheum Dis. 2013;16(3):243–253. doi:10.1111/1756-185X.12132
31. Abbasi M, Mousavi MJ, Jamalzehi S, et al. Strategies toward rheumatoid arthritis therapy; the old and the new. J Cell Physiol. 2019;234(7):10018–10031. doi:10.1002/jcp.27860
32. Bedaiwi MK, Almaghlouth I, Omair MA. Effectiveness and adverse effects of anakinra in treatment of rheumatoid arthritis: a systematic review. Eur Rev Med Pharmacol Sci. 2021;25(24):7833–7839. doi:10.26355/eurrev_202112_27630
33. den Broeder AA, de Jong E, Franssen MJ, Jeurissen ME, Flendrie M, van den Hoogen FH. Observational study on efficacy, safety, and drug survival of anakinra in rheumatoid arthritis patients in clinical practice. Ann Rheum Dis. 2006;65(6):760–762. doi:10.1136/ard.2004.033662
34. Cohen SB, Moreland LW, Cush JJ, et al. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis. 2004;63(9):1062–1068. doi:10.1136/ard.2003.016014
35. Pandolfi F, Franza L, Carusi V, Altamura S, Andriollo G, Nucera E. Interleukin-6 in Rheumatoid Arthritis. Int J Mol Sci. 2020;21(15):5238. doi:10.3390/ijms21155238
36. Li Y, Zhang W. IL-6: TNF-α 之后的类风湿关节炎治疗关键靶点 [IL-6: the next key target for rheumatoid arthritis after TNF-α]. Sheng Wu Gong Cheng Xue Bao. 2017;33(1):36–43. Chinese. doi:10.13345/j.cjb.160241
37. Scott LJ. Tocilizumab: a Review in Rheumatoid Arthritis. Drugs. 2017;77(17):1865–1879. doi:10.1007/s40265-017-0829-7
38. Baek HJ, Lim MJ, Park W, et al. Efficacy and safety of tocilizumab in Korean patients with active rheumatoid arthritis. Korean J Intern Med. 2019;34(4):917–931. doi:10.3904/kjim.2017.159
39. Amouzougan A, Dénarié D, Marotte H, Roblin X, Thomas T. Rheumatoid arthritis treated with tocilizumab in two patients with complicated chronic liver disease with portal hypertension. Joint Bone Spine. 2018;85(1):115–117. doi:10.1016/j.jbspin.2017.07.004
40. Bartoli F, Bae S, Cometi L, Matucci Cerinic M, Furst DE. Sirukumab for the treatment of rheumatoid arthritis: update on sirukumab, 2018. Expert Rev Clin Immunol. 2018;14(7):539–547. doi:10.1080/1744666X.2018.1487291
41. Lazzerini PE, Capecchi PL, Guidelli GM, Selvi E, Acampa M, Laghi-Pasini F. Spotlight on sirukumab for the treatment of rheumatoid arthritis: the evidence to date. Drug Des Devel Ther. 2016;10:3083–3098. doi:10.2147/DDDT.S99898
42. Tanaka Y, Takeuchi T, Harigai M, et al. Efficacy and safety of sirukumab in Japanese patients with active rheumatoid arthritis who were refractory or intolerant to anti-tumor necrosis factor therapy: subgroup analysis of a randomized, double-blind, multicenter, phase 3 study (SIRROUND-T). Mod Rheumatol. 2019;29(2):306–313. doi:10.1080/14397595.2018.1452345
43. Moura RA, Fonseca JE. JAK inhibitors and modulation of B cell immune responses in rheumatoid arthritis. Front Med. 2021;7:607725. doi:10.3389/fmed.2020.607725
44. Keystone EC, Cohen SB, Emery P, et al. Multiple courses of rituximab produce sustained clinical and radiographic efficacy and safety in patients with rheumatoid arthritis and an inadequate response to 1 or more tumor necrosis factor inhibitors: 5-year data from the REFLEX study. J Rheumatol. 2012;39(12):2238–2246. doi:10.3899/jrheum.120573
45. van Vollenhoven RF, Emery P, Bingham CO, et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis. 2013;72(9):1496–1502. doi:10.1136/annrheumdis-2012-201956
46. Kaegi C, Steiner UC, Wuest B, Crowley C, Boyman O. Systematic review of safety and efficacy of belimumab in treating immune-mediated disorders. Allergy. 2021;76(9):2673–2683. doi:10.1111/all.14704
47. Stohl W, Merrill JT, McKay JD, et al. Efficacy and safety of belimumab in patients with rheumatoid arthritis: a Phase II, randomized, double-blind, placebo-controlled, dose-ranging Study. J Rheumatol. 2013;40(5):579–589. doi:10.3899/jrheum.120886
48. Reynolds K, Thomas M, Dougan M. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991–997. doi:10.1634/theoncologist.2018-0174
49. Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol. 2018;31(6):965–973. doi:10.1038/s41379-018-0013-y
50. Wang J, Zhang X, Geng X, et al. Risk of hepatitis B virus reactivation following treatment with Abatacept: a retrospective study of international pharmacovigilance databases. EClinicalMedicine. 2022;48:101425. doi:10.1016/j.eclinm.2022.101425
51. Maeshima K, Yamaoka K, Kubo S, et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-γ and interleukin-17 production by human CD4+ T cells. Arthritis Rheum. 2012;64(6):1790–1798. doi:10.1002/art.34329
52. Morinobu A. JAK inhibitors for the treatment of rheumatoid arthritis. Immunol Med. 2020;43(4):148–155. doi:10.1080/25785826.2020.1770948
53. van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or Adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508–519. doi:10.1056/NEJMoa1112072
54. Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662. doi:10.1056/NEJMoa1608345
55. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374(13):1243–1252. doi:10.1056/NEJMoa1507247
56. Lopez-Romero P, de la Torre I, Haladyj E, Aletaha D, Smolen JS. Baricitinib further enhances disease-modifying effects by uncoupling the link between disease activity and joint structural progression in patients with rheumatoid arthritis. Ann Rheum Dis. 2022;81(5):622–631. doi:10.1136/annrheumdis-2021-221323
57. Clarke B, Yates M, Adas M, Bechman K, Galloway J. The safety of JAK-1 inhibitors. Rheumatology. 2021;60(Suppl2):ii24–ii30. doi:10.1093/rheumatology/keaa895
58. Kavanaugh A, Westhovens RR, Winthrop KL, et al. Safety and efficacy of filgotinib: up to 4-year results from an open-label extension study of Phase II rheumatoid arthritis programs. J Rheumatol. 2021;48(8):1230–1238. doi:10.3899/jrheum.201183
59. Wang F, Sun L, Wang S, et al. Efficacy and safety of tofacitinib, baricitinib, and upadacitinib for rheumatoid arthritis: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(7):1404–1419. doi:10.1016/j.mayocp.2020.01.039
60. Rubbert-Roth A, Enejosa J, Pangan AL, et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. N Engl J Med. 2020;383(16):1511–1521. doi:10.1056/NEJMoa2008250
61. Trueman S, Mohamed MF, Feng T, Lacerda AP, Marbury T, Othman AA. Characterization of the effect of hepatic impairment on upadacitinib pharmacokinetics. J Clin Pharmacol. 2019;59(9):1188–1194. doi:10.1002/jcph.1414
62. Sagami S, Nishikawa K, Yamada F, Suzuki Y, Watanabe M, Hibi T. Post-marketing analysis for biosimilar CT-P13 in inflammatory bowel disease compared with external data of originator infliximab in Japan. J Gastroenterol Hepatol. 2021;36(8):2091–2100. doi:10.1111/jgh.15399
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.