Back to Journals » Cancer Management and Research » Volume 12
Resatorvid Relieves Breast Cancer Complicated with Depression by Inactivating Hippocampal Microglia Through TLR4/NF-κB/NLRP3 Signaling Pathway
Authors Luo W, Han Y, Meng P, Yang Q, Zhao H, Ling J, Wang Y
Received 1 September 2020
Accepted for publication 21 November 2020
Published 18 December 2020 Volume 2020:12 Pages 13003—13014
DOI https://doi.org/10.2147/CMAR.S279800
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alexandra R. Fernandes
Weixu Luo,1,* Yuanshan Han,2,* Pan Meng,1 Qin Yang,3 Hongqing Zhao,1 Jia Ling,1 Yuhong Wang1
1Institute of Innovation and Applied Research in Chinese Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, People’s Republic of China; 2Medical Experimental Innovation Center, The First Affiliated Hospital, Hunan University of Chinese Medicine, Changsha, Hunan, People’s Republic of China; 3Department of Pharmacy, Yueyang Hospital of Traditional Chinese Medicine, Yueyang, Hunan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuhong Wang
Institute of Innovation and Applied Research in Chinese Medicine, Hunan University of Chinese Medicine, Changsha, Hunan 410208, People’s Republic of China
Email [email protected]
Background: Breast cancer is one of the most common cancer with high risk in females all over the world. It is usually complicated with depression, which can further accelerate the development and progression of breast tumors. We aim to identify a new drug and identify its functional mechanism in the regulation of hippocampal microglia (MG) in breast cancer complicated with depression (BCCD).
Methods: The activation model of MG was established by treatments from corticosterone (CORT) or lipopolysaccharides (LPS). The inhibitory effects of resatorvid on MG were investigated by CCK-8, ROS, immunofluorescence, TUNEL, scratch test, ELISA, RT-qPCR and Western blot. BCCD animal model was established using 4T1 inflammatory breast cancer cells and CORT treatment in vitro. Open field experiment (OFE), tail suspension test (TST), ELISA, RT-qPCR and Western blot experiments were utilized to examine the effects of resatorvid on the animal model in vivo.
Results: The cell viability and migration ability of the BCCD model group were suppressed. The expressions of inflammatory factors, ROS, and the apoptotic rate of the BCCD model group were up-regulated, in contrast to the control group. The expressions related to the TLR4/NF-κB/NLRP3 signaling in the BCCD model group were also elevated. Resatorvid reversed the above changes, which showed good therapeutic effects in depression-related behavioral changes, tumor treatment, and blood–brain barrier function.
Conclusion: In summary, resatorvid inhibited the activation of hippocampal MG in BCCD by regulating TLR4/NF-κB/NLRP3 signaling pathway.
Keywords: resatorvid, breast cancer complicated with depression(BCCD), microglia(MG), TLR4/NF-κB/NLRP3 signaling pathway
Introduction
Breast cancer is regarded as one of the most common cancer types in females worldwide.1 Depression, characterized by long-term depression and cognitive impairment,2 greatly affects the social interaction and life quality of cancer patients.3 At present, a non-negligible amount of breast cancer patients are accompanied with depression.4–6 The clinical therapies of breast cancer complicated with depression (BCCD) usually rely on anti-depressant drugs after breast cancer surgery or chemotherapy.7,8 However, the psychological status of depressed patients reduces their compliance with drugs and increases the difficulties of treatment, which significantly limits the therapeutic effects. Therefore, it is valuable to discover more effective drugs to improve the outcome in BCCD patients.
Neuroimmunity is one of the most important pathological basis in the occurrence and development of BCCD.9 Hippocampal microglia (MG) are the main immune cells of the central nervous system, which can provide nutritional support for neurons.10 MG could defend itself, and play a crucial role in immune surveillance to keep a steady-state environment of the central nervous system.11 The activation of MG exerts important effects in depression, which urges us to identify a new drug to inactivate MG and examine its potential therapeutic outcome in BCCD.
The innate immune system includes several types of pattern recognition receptors (PRRs), including membrane-bound Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors.12 TLR4, a critical member of TLRs family widely distributed in the brain, is a key mediator of MG activation. It could affect the hosts’ immune response by activating downstream signaling pathways and mediating innate immune responses.13 It could also lead to changes in neurons and behavior through the generation of inflammatory factors and induction of depression and anxiety.14–18 Resatorvid (TAK-242), a small-molecule-specific inhibitor of TLR4 signal transduction, could inhibit the production of inflammatory mediators by binding to the intracellular domain of TLR4.19 TAK-242 could prevent tumor invasion in patients with breast cancer, with great potential to become a new promising target in cancer treatment strategies.20 However, its exact molecular function in BCCD has not been fully evaluated. Therefore, we plan to utilize resatorvid to block the TLR4 pathway and identify its role in BCCD.
NLRP3 inflammasomes acted as vital signaling molecule downstream of TLR4, which promotes the maturation of inflammatory cytokines. Fu et al have shown that tetramethylpyrazine could improve depression by inhibiting the TLR4-NLRP3 inflammatory signal pathway in mice.21 NF-κB also plays an important role in the treatment of neuroinflammation. Many drugs take the advantages of the inhibition of NF-κB activity to suppress neuroinflammation.22 In this research, the roles and relationship between resatorvid and TLR4/NF-κB/NLRP3 pathway were examined in BCCD. Our research may provide a new method for the treatment of BCCD and help breast cancer patients in the future.
Materials and Methods
Experimental Cells and Animals
4T1 cell (Catalog No. ZQ0201) and BV2 cell lines (Catalog No. ZQ0397) were purchased from Shanghai Zhongqiaoxinzhou Biotech (Shanghai, China). The cells were cultured according to the instructions. 80 SPF BALB/c female mice, about 4–6 weeks old, weighing 18–22 g, were purchased from Hunan Slack Jingda Experimental Animal Co., Ltd. Laboratory. This study was approved by the Hunan SJA Laboratory Animal Co., Ltd, with the license number of NO. IACUC-1,710,168. The animal license number and certificate number were SCXK (Hunan) 2013–0004 and 43004700024756, respectively. This study was conducted in strict accordance with the use of experimental animals. Animals were fed for 5 days from the date of purchase adaptively. They were maintained at a room temperature (25±2) °C, with relative humidity of about 55% and the 12h/12h light-dark cycle. They had free access to food and water.
Materials and Drugs
Resatorvid (Catalog No. 243,984–11-4) and Corticosterone (CORT, Catalog No. 50–22-6) were obtained from BOC Sciences, USA. Lipopolysaccharides (Catalog No. L118716) were purchased from Aladdin (Shanghai, China). Fluoxetine (Patheon France, approval number: 8742A, 20mg/capsule) was purchased from the First Hospital of Hunan University of Chinese Medicine. Paclitaxel (approval number: H20051076, specification 30mg/bottle) was purchased from Hunan Cancer Hospital. CCK-8 (Catalog No. HY-K0301) kit was obtained from MedChemExpress, USA. ELISA kits of IL-6 (96T, Catalog No. H007) and IL-10 (96T, Catalog No. H009) were obtained from Nanjing Jiancheng Bioengineering Institute. ROS kit (Catalog No. E004-1-1) was obtained from Nanjing Jiancheng Bioengineering Institute. TUNEL kit (40306ES50) was purchased from the Shanghai Yeasen Bio Technologies Co., Ltd (Shanghai, China).
BV2 Cell Activation Model and Drug Treatment
We divided the BV2 cells into four groups, including the BV2, the LPS+4T1 supernatant, the CORT, and the BCCD model. Among them, cells in the LPS+4T1 supernatant group were treated with 10μg/mL LPS to stimulate the 4T1 supernatant for 6 hours. The CORT group cells were treated with 200 μM CORT. The cells in the BCCD model group were treated with 10 μg/mL LPS stimulated 4T1 for 6 hours and 200 μM CORT at the same time. In the drug treatment part, we divided BV2 cells into 5 groups, including the control group, the DMSO group, the BCCD model group, the positive drug (paclitaxel + fluoxetine) group, and the resatorvid group. The supernatant of 4T1 was treated by 10 μg/mL LPS for 6 hours. The cells in the BCCD model group were treated with 200 μM CORT and the above supernatant. The cells in the positive drug group were treated with 200 μM fluoxetine and 200 μM paclitaxel based on the BCCD model group treatment. The cells in the resatorvid group were pretreated with 10 μg/mL resatorvid for 30 minutes, then 200μM CORT and the supernatant of 4T1 were added. The above groups were used for the detection of various indicators after 18 hours of drug intervention.
Construction of BCCD Animal Model and Drug Treatment
BALB/c mice were used for modeling. For this, 0.1 mL of 107 cells/mL 4T1 inflammatory breast cancer cells were inoculated in their armpits. Tumor formation was observed 7 days later. After successful completion of the mouse model of breast cancer, mice in the depression group and tumor-forming mice were injected with CORT suspension subcutaneously on the back at 30 mg/kg. The administration volume was 20 mL/kg. The drug was administered for 21 consecutive days, to further create a simple depression model and a BCCD mouse model. After 5 days of adaptive feeding, we divided the mice stochastically into eight groups on the basis of their consumption of sugar and water (n=10). The groups included the control group, the depression group, the breast cancer group, the BCCD group, the paclitaxel group, the fluoxetine hydrochloride group, the paclitaxel combined with fluoxetine hydrochloride (Pac+Flu) group, and the resatorvid group. Mice in the paclitaxel group were given paclitaxel liposomes for injection, intraperitoneally at a dose of 20 mg/kg once a week. Mice in the fluoxetine hydrochloride group were given fluoxetine hydrochloride, at a dose of 7.8 mg/kg once a day. The resatorvid group’s mice were injected with resatorvid intraperitoneally at the equivalent dose of 3 mg/kg mice once a day. The control group, the depression group, the breast cancer group and the BCCD group were all given equal volume of distilled water by gavage. Each group was tested for various indicators after 3 weeks of treatment.
Blood was taken from the orbit of all mice. The supernatants were taken according to the conventional method.23 The hippocampus tissue of all mice was taken out. They were quickly frozen in liquid nitrogen after aliquoting and transferred to ultra-low temperature storage.
CCK-8 Assay
The cells were inoculated in 96-well plates at a density of 5×103 cells/well, with 5 replicate wells in each group of 100 μL per well. After 24h, the cells were processed according to the above groups. Finally, the absorbance (OD) value was analyzed at 450 nm on the Bio-Tek microplate reader according to the CCK8 instruction. The average values were taken and the survival rate curves were drawn.
ELISA
The cells in the resatorvid group were pretreated with 10 μg/mL resatorvid for 30 minutes, then 200μM CORT and the supernatant of 4T1 were used to treat for 18 hours. Mice in the resatorvid group were injected with resatorvid intraperitoneally at the equivalent dose of 3 mg/kg once a day. After 3 weeks of treatment, blood samples were collected. The corresponding protein contents were detected according to the relevant instructions of the ELISA kit. ELISA kits of IL-6 (96T, Catalog No. H007) and IL-10 (96T, Catalog No. H009) were obtained from Nanjing Jiancheng Bioengineering Institute. The OD values were read of each well at 450 nm and 630 nm wavelengths. Then, the sample concentrations of each well were determined through the regression equation of the standard curve.
ROS Assay
The supernatant of 4T1 was treated by 10 μg/mL LPS for 6 hours. The cells in the BCCD model group were treated with 200 μM CORT and the above supernatant for 18 hours. The cells in the positive drug group were treated with 200 μM fluoxetine and 200 μM paclitaxel based on the BCCD model group treatment. The cells in the resatorvid group were pretreated with 10 μg/mL resatorvid for 30 minutes, then 200μM CORT and the supernatant of 4T1 were used to treat for 18 hours. According to the DHFC-DA probe kit instructions of ROS kit (Catalog No. E004-1-1, Nanjing Jiancheng), the cells of each group were processed, collected by trypsinization, and tested by flow cytometer.
Immunofluorescence Analysis
After the cell fixation of each group, we incubated the primary antibody, Iba1 (ab5076, abcam) and NLRP3 (19,771-1-AP, Proteintech), diluted in blocking buffer (1:50) overnight at 4°C. Secondary antibody of anti-goat (1:100, SA00003-3, Proteintech) and anti-rabbit-IgG (1:200, SA00013-8, Proteintech)-labeled fluorescein was applied the next day. Then, the nucleus of the cells was stained with DAPI solution. The slices were mounted with buffered glycerin, stored in the dark, and observed under a fluorescence microscope. The co-expression situations were analyzed by Image Pro-plus software.
TUNEL Staining
After the cell fixation of each group, the experiment was carried out strictly according to the TUNEL kit instructions. After the nucleus was stained, the slices were mounted with buffered glycerin, stored in the dark, and observed under a fluorescence microscope. The number of apoptotic cells were counted.
Wound-Healing Assay
BV2 cells were plated into 6-well plates. The supernatant of 4T1 was treated by 10 μg/mL LPS for 6 hours. The cells in the BCCD model group were treated with 200 μM CORT and the above supernatant for 18 hours. The cells in the positive drug group were treated with 200 μM fluoxetine and 200 μM paclitaxel based on the BCCD model group treatment. The cells in the resatorvid group were pretreated with 10 μg/mL resatorvid for 30 minutes, then 200μM CORT and the supernatant of 4T1 were used to treat for 18 hours. After the cells grew full in the culture dish, we scratched them with a pipette tip, and cultured them continuously. Finally, we took pictures and recorded at a predetermined time.
Behavioral Testing
Open field experiment (OFE) and tail suspension test (TST) were carried out, as previously described by the operation described by Zhang Junping.24 The experimental data were recorded.
Western Blot Assay
We extracted and denatured the total protein in each group of cells and tissues. After SDS-gelelectrophoresis, we transferred them to nitrocellulose membranes. Then, we incubated the different primary antibodies including TLR4 (1:500, ab13556, Abcam, UK), NLRP3 (1:1000, ab263899, Abcam), IL-1β (1:1000, ab234437, Abcam), IL-18 (1:4000, 60,070-1-Ig, Proteintech, USA), caspase-1 (1:1000, 22,915-1-AP, Proteintech), NF-κB (1:2000, 10,745-1-AP, Proteintech), ASC (1ug/mL, ab175449, Abcam), Pro-MMP9 (2ug/mL, MAB9111, R&D), Claudin5 (1:4000, ab131259, Abcam) and β-actin(1:5000, 66,009-1-Ig, Proteintech)overnight 4 °C.After washing the membranes three times, the blots were incubated with fluorescent-basedanti-rabbit(1:6000, SA00001-2, Proteintech), anti-mouse(1:5000, SA00001-1, Proteintech) or anti-goat(1:5000, SA00001-4, Proteintech) IgG secondary antibody at room temperature.We visualized the proteins by chemiluminescence detection reagent (Thermo Fisher, Waltham, MA, USA). We used Image J software to analyze the gray value of all bands quantitatively.
RT-qPCR Assay
RNA was extracted from tissues and cells by Trizol (Invitrogen). Then, we used SYBR Green and corresponding primers for RT-qPCR assay, according to a previous study.25 The PCR reaction system included 2μL template, 1μL 10μM primer R, 11μL ddH2O, and 15μL 2x SYBGREEN PCR Master Mix. The PCR amplification program were as follows: 10 minutes at −95 °C; 15 s at −95 °C, 30 s at −60 °C (40 cycles). The primer sequences were as follows (Table 1).
 |
Table 1 Primer Sequences |
Statistical Analysis
The SPSS 21.0 software was used for statistical analysis of the data, and the measurement data were expressed as mean ± standard deviation. One-way ANOVA analysis was used to compare multiple groups. P<0.05 indicated that there was a statistical difference between the results.
Results
Establishment of BV2 Cell Activation Model
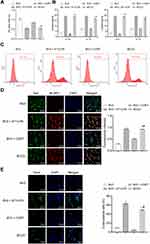
As shown in Figure 1A, the cell viability of the BV2+LPS/4T1 group and the BCCD model group was observably reduced compared with the BV2 group. ELISA results (Figure 1B) showed that the level of IL-1β, IL-18, IL-6 and IL-10 in the BV2+LPS/4T1 group and the BCCD group was significantly increased in contrast with the BV2 group. As shown in Figure 1C, the average fluorescence intensity of ROS in the BV2+LPS/4T1 group and the BCCD group increased in contrast to the BV2 and the BV2+CORT group. From the immunofluorescence images, the co-expression of NLRP3 and Iba-1 protein in the BV2+LPS/4T1 group was up-regulated compared to the control (Figure 1D). TUNEL staining (Figure 1E) results showed the BCCD group had obvious MG apoptosis. The positive rate of TUNEL increased, indicating that cells in the BV2+LPS/4T1 group and the BCCD model group were damaged to varying degrees. In summary, we successfully established a model of MG activation in a simulated BCCD environment. It can be used to further explore the mechanism of inflammatory response activated by MG in BCCD.
Resatorvid Reversed the Damage of BCCD to BV2 Cells
In Figure 2A, CCK-8 results demonstrated that the cell viability in the BCCD model group was decreased compared with the control group. In the resatorvid group and the positive drug group, the cell viability was improved compared with the BCCD model group. According to Figure 2B, the average fluorescence intensity of ROS in the BCCD group increased, different from the control group. However, the average fluorescence expression intensity of ROS in the resatorvid group and the positive drug group decreased visibly compared with the model group. Resatorvid could down-regulate the oxidative stress level under the simulated BCCD environment. From Figure 2C, the co-expression of NLRP3 and Iba-1 protein in the cells of the BCCD model group was overtly raised in contrast to the control. After treatment in resatorvid and the positive drug groups, the co-expression level of NLRP3 and Iba-1 alleviated significantly. TUNEL staining results suggested that the TUNEL positive rates in the BCCD model group were clearly elevated compared with the control group. In the resatorvid group and the positive drug group, TUNEL positive rates were markedly reduced (Figure 2D). It revealed that resatorvid had certain protective effects on MG. Its mechanism of drug action may be achieved by reducing MG apoptosis. The results of the scratch experiment showed that the healing speed of scratches in the BCCD model group was slower than the control (Figure 2E). The scratches healed faster in the resatorvid and positive drug groups than the BCCD model group. The cells of the BCCD model group suffered different degrees of damage compared to the control. However, resatorvid and the positive drug group could reverse this damage to a certain extent.
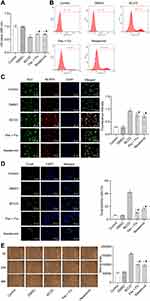
Resatorvid Inactivated Inflammatory Factors and TLR4/NF-κB/NLRP3 Signaling Pathway in BV2 Cells
In contrast to the control group, the expression levels of inflammatory factors IL-1β, IL-18, IL-6, and IL-10 in the cell supernatant of the BCCD model group were up-regulated. The positive drug and resatorvid group could reverse this transformation (Figure 3A). Compared to the control, the expression of TLR4, NLRP3, IL-1β, IL-18, caspase-1, NF-κB, ASC mRNA and protein in the BCCD model group increased (P<0.05). The expression level of the above genes and proteins in the positive drug and resatorvid group decreased visibly (Figure 3B and C). In summary, the cells in the BCCD model group were abnormally activated with more inflammatory factors. TLR4/NF-κB/NLRP3 signaling pathway–related proteins were activated accordingly. Resatorvid could reverse this change to a certain extent.
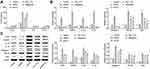
Resatorvid Alleviated the Symptom of BCCD Mice in vivo
The mice in all groups had tumor formation excluded from the control and the depression. Tumor size in the groups of the paclitaxel, fluoxetine hydrochloride, Pac+Flu and resatorvid were reduced compared with the model group (breast cancer and BCCD). Positive drug and resatorvid had certain therapeutic effects on mice of BCCD (Figure 4A). The number of crossing panels and the times of straighten in the depression group and the BCCD model group were obviously reduced compared with the control. After the corresponding treatments, the number of crossing panels and the times of straighten in the groups of paclitaxel, fluoxetine hydrochloride, Pac+Flu and resatorvid groups were up-regulated obviously in contrast to the BCCD model group (Figure 4B and C). Mice in BCCD had fewer activities, with less desire to explore. Their fear of new environment tension increased significantly in the new environment. Resatorvid relieved the above symptoms of BCCD mice. In contrast to the blank group, the total immobility times of mice in each group of model groups increased in the TST. The immobility times of the therapeutic groups, especially the resatorvid group, were markedly shorter than those in the BCCD group (Figure 4D). The above results suggested that BCCD mice had a state of desperate behavior. Resatorvid improved the desperate state of BCCD mice.
To detect the content of tumor markers, the models were evaluated and the intervention effects of different drugs on mice tumors were discussed. In contrast with the control group, the tumor markers, CEA and CA15-3, of the breast cancer group and the BCCD model group were clearly raised (Figure 4E). The expression of tumor markers in the paclitaxel, fluoxetine hydrochloride, Pac+Flu and resatorvid groups were down-regulated in contrast to the BCCD groups. MMP9 and Claudin5 are one of the important markers of the blood-brain barrier function structure. As shown in Figure 4F, the expressions of Pro-MMP9 and Claudin5 in the hippocampus of the depression group, breast cancer group and BCCD model group were all down-regulated compared with the control group. In contrast to the BCCD group, the expressions in the paclitaxel, fluoxetine hydrochloride, Pac+Flu and resatorvid increased markedly. Resatorvid improved the blood-brain barrier damage in BCCD mice by up-regulating the expression of Pro-MMP9 and Claudin5. Resatorvid could improve the damage to mice suffered from BCCD.
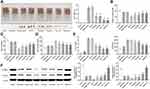
Resatorvid Inactivated Inflammatory Factors and TLR4/NF-κB/NLRP3 Signaling Pathway in vivo
According to Figure 5A, the serum level of IL-1β, IL-18, IL-6 and IL-10 in the depression group, the breast cancer group and the BCCD group increased obviously compared with the control group. However, the Pac+Flu and resatorvid groups could reverse the transformations. As shown in Figure 5B, the expression of TLR4, NLRP3, IL-1β, IL-18, caspase-1, NF-κB and ASC mRNA in the hippocampus of each group increased compared to the control. The mRNA of each index in the hippocampus of the mice in the Pac+Flu and resatorvid group were significantly attenuated, in contrast to the BCCD group. The expression of hippocampal TLR4, NLRP3, IL-1β, IL-18, caspase-1, NF-κB and ASC protein increased in the depression, the breast cancer group and the BCCD model group, while the resatorvid reversed the transformation, suggesting that resatorvid effectively reduced the level of inflammation in the brain (Figure 5C and D). Altogether, the results indicated that the therapeutic effects of resatorvid might be related to TLR4/NF-κB/NLRP3 signaling pathway.
Discussion
Darcet et al showed that subcutaneous injection of CORT could simulate the hippocampal function and structure damage caused by stress,26 avoiding the adaptive phenomenon of physical stress. Mice with breast cancer showed depression symptoms, changes in monoamine neurotransmitters, variations in breast cancer marker levels after CORT injection.27 The activation of MG in the brain was one of the most important pathogenesis of central nervous system inflammation. In our study, 4T1 cells/LPS and CORT were selected to simulate MG in the BCCD environment. The number of autonomous activities of BCCD mice decreased, and the time of tail suspension increased. CEA and CA15-3 of BCCD mice increased, indicating that the animal experimental model was successfully constructed. In the simulated BCCD environment, MG cells were activated. The inflammatory level was obviously increased, which demonstrated a significant immune activation.
Güney et al have shown that curcumin suppressed the metastasis of breast cancer by down-regulating TLR4.28 Wu et al found that the expression level of TLR4/MyD88 was strongly associated with breast cancer cell metastasis.29 Wang et al also showed that the extremely active TLR4-NF-κB signaling pathway and inflammatory cytokines were closely related to depression.30 In our study, the combination of paclitaxel and fluoxetine was used as the positive control groups. The effects of resatorvid were studied based on the behavior, anti-tumor effects, inflammatory factors, ROS, cell activity and the TLR4/NF-κB/NLRP3 signaling pathway in BCCD. Resatorvid could improve MG neuroinflammation in BCCD mice in a variety of aspects including morphology, structure, and function. It also showed that resatorvid regulated the expression of tight junction proteins Claudin5 and Pro-MMP9 on microvessels by improving the levels of immune inflammatory factors in the BCCD microenvironment.
Accumulative evidence suggests that the NF-κB signaling pathway is the main inflammation signaling pathway. NF-κB enters the nucleus after activation, thereby promoting the expression of inflammatory genes.31 In the process of inflammatory disorders in the central nervous system, the expression of TLR4 was a ligand for the body’s immune response.32 NLRP3 is mainly expressed on MG, acting as the basis of complement-mediated IL-1β activation in the posterior brain.33 NLRP3 inflammasome might affect the downstream inflammatory response through the NF-κB signaling pathway and participate in the occurrence and development of depression.34 The above studies all suggested the possible therapeutic effects of TLR4 inhibitors. Our experiments revealed the expression level of TLR4/NF-κB/NLRP3 signaling pathway. The expression of NF-κB in the resatorvid drug group was down-regulated. Resatorvid could reduce the inflammatory state of MG and improve the inflammatory environment in the brain. It could also effectively alleviate the immune inflammation of BCRD mice to achieve anti-inflammatory, anti-tumor and anti-depressive effects.
Furthermore, the treatment of sex steroid receptors has attracted great attention to breast cancer.35 Studies have shown that the regulation of estrogen and NF-κB p65/IKKα signaling pathway may become an effective way to treat breast cancer.36–39 Drugs that are effective in treating neuroinflammation may regulate the NF-κB p65/IKKα signaling pathway through androgen and estrogen receptors to inhibit neuroinflammation in hippocampus.37–39 In future research, we will further study the effects of resatorvid on steroid receptors and related proteins to discover more effective therapeutic methods to treat BCCD.
Conclusion
In this study, we simulated MG in BCCD environment and established BCCD animal models in vitro and in vivo successfully. We studied the possible mechanism of resatorvid interfering with the activation of hippocampal MG in BCCD based on TLR4/NF-κB/NLRP3 signaling. The experimental and modeling data demonstrated that resatorvid inhibited the abnormal activation of BCCD hippocampal MG by suppressing TLR4/NF-κB/NLRP3 signals.
Data Sharing Statement
The authors confirm that all data underlying the findings are available. All relevant data are within the paper.
Ethics Statement
This study was approved by the Hunan SJA Laboratory Animal Co., Ltd, with the license number of NO. IACUC-1,710,168. The animal license number and certificate number were SCXK (Hunan) 2013-0004 and 43004700024756, respectively. This study was complied with the ARRIVE guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Author Contributions
All authors made a significant contribution to the work reported, in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work. Co-first authors: Weixu Luo and Yuanshan Han. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Funding
This work was supported by the major science and technology project for “Significant New Drugs Creation” (2017ZX09309026), the National Natural Science Foundation of China Grants (81874464), the Key Projects of Hunan Science and Technology Department (2018DK2014), the Research and innovation project of graduate students in Hunan Province (CX2018B480) and the Subsidized Project of Top-Ranking Discipline Construction of Traditional Chinese Medicine in Hunan University of Chinese Medicine.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
2. Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. doi:10.1016/S0140-6736(18)31948-2
3. Valentine AD, Meyers CA. Cognitive and mood disturbance as causes and symptoms of fatigue in cancer patients. Cancer. 2001;92(6 Suppl):1694–1698.
4. Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54(3):269–282. doi:10.1016/S0006-3223(03)00566-3
5. Stommel M, Given BA, Given CW. Depression and functional status as predictors of death among cancer patients. Cancer. 2002;94(10):2719–2727. doi:10.1002/cncr.10533
6. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin North Am. 2017;101(6):1099–1113.
7. Andersen LT, Hansen MV, Rosenberg J, Ggenur I. Pharmacological treatment of depression in women with breast cancer: a systematic review. Breast Cancer Res Treat. 2013;141(3):325–330. doi:10.1007/s10549-013-2708-6
8. Sajadi Hezaveh M, Salehi B, Moshfeghi K. Comparison effect of drug therapy and drug-cognitive therapy on decreasing depression in women with breast cancer. Evidence for interpopulation differences in life history parameters of adult and F1 generation Lumbricus rubellus: the 7th International Symposium on Earthworm Ecology Cardiff Wales 2002 – Research Gate. Pedobiologia. 2008;47(5–6):535–541.
9. Han YS, Huang HZ, Meng P, et al. The effect of Xiaoyao Kangai Jieyu formula on expression of NMDAR related protein in hippocampus of mice with breast cancer related with depression. Chin J New Drugs. 2019.
10. Hanisch U-K. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–155. doi:10.1002/glia.10161
11. Lenz KM, Nelson LH. Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front Immunol. 2018;9:698. doi:10.3389/fimmu.2018.00698
12. Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol. 2016;12(1):13–26. doi:10.1038/nrneph.2015.175
13. Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32(1):4–16. doi:10.1097/SHK.0b013e318193e333
14. Nie X, Kitaoka S, Tanaka K, et al. The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron. 2018;99(3):464–79.e7. doi:10.1016/j.neuron.2018.06.035
15. Wang L, Chen J. Progress in studies on TLR4 signaling pathway and major depressive disorder. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42(6):725–729.
16. García Bueno B, Caso JR, Madrigal JL, Leza JC. Innate immune receptor toll-like receptor 4 signalling in neuropsychiatric diseases. Neurosci Biobehav Rev. 2016;64:134–147. doi:10.1016/j.neubiorev.2016.02.013
17. Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in toll-like receptor-mediated neuronal injury. Glia. 2010;58(3):253–263.
18. Lehnardt S, Schott E, Trimbuch T, et al. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28(10):2320–2331. doi:10.1523/JNEUROSCI.4760-07.2008
19. Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79(1):34–41. doi:10.1124/mol.110.068064
20. Zandi Z, Kashani B, Poursani EM, et al. TLR4 blockade using TAK-242 suppresses ovarian and breast cancer cells invasion through the inhibition of extracellular matrix degradation and epithelial-mesenchymal transition. Eur J Pharmacol. 2019;853:256–263. doi:10.1016/j.ejphar.2019.03.046
21. Fu S, Wang J, Hao C, Dang H, Jiang S. Tetramethylpyrazine ameliorates depression by inhibiting TLR4-NLRP3 inflammasome signal pathway in mice. Psychopharmacology (Berl). 2019;236(7):2173–2185. doi:10.1007/s00213-019-05210-6
22. Srinivasan M, Lahiri DK. Significance of NF-κB as a pivotal therapeutic target in the neurodegenerative pathologies of alzheimer’s disease and multiple sclerosis. Expert Opin Ther Targets. 2015;19(4):471–487. doi:10.1517/14728222.2014.989834
23. Nielsen S, Bassler N, Grzanka L, et al. Proton scanning and X-ray beam irradiation induce distinct regulation of inflammatory cytokines in a preclinical mouse model. Int J Radiat Biol. 2020;96(10):1238–1244.
24. Zhang JP, Zhang KY, Guo L, et al. Effects of 1.8 GHz radiofrequency fields on the emotional behavior and spatial memory of adolescent mice. Int J Environ Res Public Health. 2017;14:11. doi:10.3390/ijerph14111344
25. Feng X, Luo Q, Zhang H, et al. The role of NLRP3 inflammasome in 5-fluorouracil resistance of oral squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36(1):81. doi:10.1186/s13046-017-0553-x
26. Darcet F, Mendez-David I, Tritschler L, Gardier AM, Guilloux JP, David DJ. Learning and memory impairments in a neuroendocrine mouse model of anxiety/depression. Front Behav Neurosci. 2014;8:136. doi:10.3389/fnbeh.2014.00136
27. Qin Y, Pan M, Hui Y, et al. Correlation between damaged hippocampus induced by HPA axis disturbance and breast cancer complicated with depression in animal. Drug Eval Res. 2018.
28. Güney Eskiler G, Deveci Özkan A, Kaleli S, Bilir C. Inhibition of TLR4/TRIF/IRF3 signaling pathway by curcumin in breast cancer cells. J Pharm Pharm Sci. 2019;22(1):281–291. doi:10.18433/jpps30493
29. Wu K, Zhang H, Fu Y, et al. TLR4/MyD88 signaling determines the metastatic potential of breast cancer cells. Mol Med Rep. 2018;18(3):3411–3420.
30. Wang Y, Xu J, Liu Y, Li Z, Li X. TLR4-NF-κB signal involved in depressive-like behaviors and cytokine expression of frontal cortex and hippocampus in stressed C57BL/6 and ob/ob Mice. Neural Plast. 2018;2018:7254016. doi:10.1155/2018/7254016
31. Christiansen SH, Selige J, Dunkern T, Rassov A, Leist M. Combined anti-inflammatory effects of β2-adrenergic agonists and PDE4 inhibitors on astrocytes by upregulation of intracellular cAMP. Neurochem Int. 2011;59(6):837–846. doi:10.1016/j.neuint.2011.08.012
32. Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43(3):281–291. doi:10.1002/glia.10256
33. Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014;41:90–100. doi:10.1016/j.bbi.2014.04.007
34. Su WJ, Zhang Y, Chen Y, et al. NLRP3 gene knockout blocks NF-κB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav Brain Res. 2017;322(Pt A):1–8. doi:10.1016/j.bbr.2017.01.018
35. Kono M, Fujii T, Lim B, Karuturi MS, Tripathy D, Ueno NT. Androgen receptor function and androgen receptor-targeted therapies in breast cancer: a review. JAMA Oncol. 2017;3(9):1266–1273. doi:10.1001/jamaoncol.2016.4975
36. Perillo B, Di Santi A, Cernera G, Ombra MN, Castoria G, Migliaccio A. Phosphorylation of H3 serine 10 by IKKα governs cyclical production of ROS in estrogen-induced transcription and ensures DNA wholeness. Cell Death Differ. 2014;21(9):1503. doi:10.1038/cdd.2014.91
37. El-Bakoush A, Olajide OA. Formononetin inhibits neuroinflammation and increases estrogen receptor beta (ERβ) protein expression in BV2 microglia. Int Immunopharmacol. 2018;61:325–337. doi:10.1016/j.intimp.2018.06.016
38. Coyoy-Salgado A, Segura-Uribe JJ, Manuel Gallardo J, Estrada-Cruz NA, Camacho-Arroyo I, Guerra-Araiza C. Tibolone regulates systemic metabolism and the expression of sex hormone receptors in the central nervous system of ovariectomised rats fed with high-fat and high-fructose diet. Brain Res. 2020;1748:147096. doi:10.1016/j.brainres.2020.147096
39. Hidalgo-Lanussa O, Ávila-Rodriguez M, Baez-Jurado E, et al. Tibolone reduces oxidative damage and inflammation in microglia stimulated with palmitic acid through mechanisms involving estrogen receptor beta. Mol Neurobiol. 2018;55(7):5462–5477. doi:10.1007/s12035-017-0777-y
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.