Back to Journals » Clinical Epidemiology » Volume 13
Reproductive Factors, Use of Exogenous Hormones, and Pancreatic Cancer Incidence: The Norwegian Women and Cancer Study
Authors Alvarez A , Benjaminsen Borch K , Rylander C
Received 19 June 2020
Accepted for publication 7 December 2020
Published 5 February 2021 Volume 2021:13 Pages 67—80
DOI https://doi.org/10.2147/CLEP.S268556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Vera Ehrenstein
Antoine Alvarez,1 Kristin Benjaminsen Borch,2 Charlotta Rylander2
1Faculty of Medicine, Paris-Sud University, Paris, France; 2Department of Community Medicine, Faculty of Health Sciences, UiT The Arctic University of Norway, Tromsø, Norway
Correspondence: Charlotta Rylander
Department of Community Medicine, Faculty of Health Sciences, UiT The Arctic University of Norway, Tromsø, 9037, Norway
Tel +47-77-644-831
Email [email protected]
Introduction: The incidence of pancreatic cancer is increasing worldwide and characterized by a particularly low survival rate. Studies have reported weak and inconsistent evidence for associations among reproductive factors, use of exogenous hormones, and pancreatic cancer incidence in women.
Purpose: To investigate relationships between reproductive factors, exogenous hormones, and the rate of pancreatic cancer incidence in a large population-based prospective cohort of women in Norway.
Methods: We used data from the Norwegian Women and Cancer study on 588 incident cases of pancreatic cancer diagnosed among 165,419 women, with mean follow-up of 18.7 years. Cox proportional-hazard models were used to estimate HRs and 95% CIs for associations of interest.
Results: Cumulative breastfeeding duration > 24 months was associated with 63% decreased incidence of pancreatic cancer compared to no breastfeeding. We observed an inverse linear dose–response trend between cumulative breastfeeding duration and pancreatic cancer incidence, which was confirmed in parous women and ever-smokers. Higher age at first birth and menopause were inversely associated with pancreatic cancer incidence, though with less precise effect estimates. Current use of oral contraceptives was associated with a doubling of pancreatic cancer incidence, but the analysis was hampered by a small number of cases. There was no evidence of any associations between age at menarche, parity or use of menopausal hormone therapy, and incidence of pancreatic cancer.
Conclusion: Our results suggest a potential protective effect of breastfeeding duration against pancreatic cancer incidence. Inconsistent results for the other reproductive factors suggested no important role of estrogens in pancreatic cancer etiology.
Keywords: pancreatic neoplasms, incidence, women, prospective study, breastfeeding, age at first birth
Introduction
Pancreatic cancer accounts for <4% of all cancer prevalence in Norway; however, it is the fourth-leading cause of cancer-related death.1 This important paradox is explained by the extremely low survival rate of patients: in 2019, the 5-year survival rate among women was 13.8% and 13.1% in men, the lowest rates of all cancers in Norway. Over the last 60 years, pancreatic cancer incidence has increased from 7.4 per 100,000 women in 1959 to 12.6 per 100,000 women in 2019, and for men it has increased from 11.5 to 18.3.1 The trend of increasing incidence has also been reported from other countries.2,3 Few risk factors have been identified. Modifiable risk factors for pancreatic cancer include tobacco smoking, overweight/obesity, red- and processed-meat intake, alcohol consumption, and intake of saturated fat and beverages containing fructose; however modifiable risk factors are supported by various level of evidence.4 Other risk factors include age, height, diabetes, chronic pancreatitis, and some inherited genetic factors.5,6 As pancreatic cancer is rarely detected at an early stage and the prognosis of the disease is poor, identification of new risk factors is highly relevant in order to improve the understanding of pancreatic cancer etiology and the identification of high-risk populations for prevention.7
The male:female ratio of pancreatic cancer incidence in Norway has declined from 1.4 30 years ago to 1.2 in 2019.1 Despite the observed increase in the cancer incidence among women, the lower incidence in women compared to males may suggest a protective effect of female hormones. Studies that have assessed the relationship between pancreatic cancer risk and reproductive factors, such as age at menarche, age at menopause, menopausal status, parity, abortions, age at first birth, breastfeeding, total menstruation duration, and exogenous hormone use, have reported inconsistent results.8–22
In 2012, a large European cohort study assessed the association between reproductive factors, hormone use, and risk of pancreatic cancer.21 No significant association was identified. Thereafter, two meta-analyses reported that parity was inversely associated with pancreatic cancer risk.23,24 Since then, three recent cohort studies observed no association or an inverse association between parity and pancreatic cancer risk.8,12,25 A meta-analysis published in 2015 reported no associations between age at menarche, age at menopause, use of menopausal hormone therapy (MHT) or oral contraceptives (OCs), hysterectomy, oophorectomy, and risk of pancreatic cancer.26 More recent prospective studies have mainly supported the finding of no association with use of OCs.12,27 For MHT use, controversies exist, and inverse relationships between MHT use and pancreatic cancer risk have recently been reported.8,19,22 Furthermore, one study has identified increased risk with increasing age at menarche.8 Likewise, higher age at first birth was identified as a risk factor for pancreatic cancer in a meta-analysis published in 2016, and a more recent cohort study supported this finding.12,28 Others have reported no association.8,25 Increased duration of breastfeeding was inversely associated with pancreatic cancer incidence in one cohort study, whereas other prospective studies have reported nonsignificant results.8,10,12,29,30 Overall, these results suggest that some reproductive factors and exogenous hormones may be related to pancreatic cancer incidence. However, comparisons across studies are complicated by the fact that different reproductive factors are addressed in the various studies, and that exogenous hormone use and reproductive factors vary across layers of social, economic, and environmental factors.31–34 There has been no consensus on categorization of included exposure either, which makes comparisons across studies challenging. For instance, some studies only assessed the association between ever/never breastfeeding.12,15 At the same time, others explored cumulative breastfeeding duration.8,10,35 Study design and number of cases also vary across studies, which may affect the results considerably. Prospective studies exist; however, there are few that have included both reproductive factors and exogenous hormones with detailed information on these variables and in a large population with several cases of pancreatic cancer. As such, large and prospective studies are warranted to improve our understanding of the association between reproductive factors, exogenous hormones, and pancreatic cancer. The aim of this etiologic study was to investigate relationships between parity, age at first birth, age at menarche and menopause, cumulative breastfeeding duration, use of OCs or MHT, and pancreatic cancer incidence in a large population-based prospective cohort of women in Norway.
Methods
Study Design and Participants
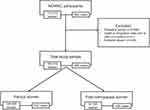
The Norwegian Women and Cancer (NOWAC) study is a nationally representative and prospective cohort that was initiated in 1991 with the aim of investigating the etiology of cancer among women in Norway. Details on the design, materials, and procedures of the NOWAC study have been described previously.36 Briefly, women aged 30–70 years were randomly sampled from the National Registry and invited to participate in the study. The response rate varied between 48% and 57% at enrollment, representing 172,472 women, included between 1991 and 2007. Women included completed up to two follow-up questionnaires, with approximately 7 years between questionnaires. The unique personal identity number assigned to every resident of Norway allowed for linkage to national registers. External validity in the NOWAC study is considered high, as the distribution of exposure is independent of the response rate and the cumulative incidence of cancers does not differ from national figures from the Cancer Registry of Norway.37 The NOWAC study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate, and all participants enrolled in the study gave written informed consent. Women with prevalent cancer (n=6,696), death, emigration prior to date of inclusion (n=21), or those that reported extreme values in either questionnaire (n=336) were excluded from the analyses. Extreme values were defined for weight (<30 or >240 kg), height (<100 or >230 cm), age at menopause (<25 or >60 years), age at menarche (<8 or >20 years), and age at first birth (<12 or >50 years). After exclusions, the total study sample comprised 165,419 women, of which 149,095 were parous and 81,020 postmenopausal at baseline (Figure 1).
Outcome Assessment
The NOWAC study receives annual updates from the Cancer Registry of Norway, in order to identify study participants diagnosed with cancer during the preceding year. In the present study, women diagnosed with a first primary invasive malignant neoplasm of the pancreas were identified from the International Classification of Diseases and Related Health Problems (tenth revision, code C25). Information about deaths and emigration was extracted from the Causes of Death Registry and the National Registry.
Reproductive Factors, Exogenous Hormones, and Covariates
For all participants included, we extracted information on body type at age 7 years (very thin, thin/normal/fat, or very fat), age at menarche (≤12, 13–14, ≥15 years), age at menopause (≤47, 48–49, 50–51, ≥52 years), age at first birth (≤19, 20–24, 25–29, ≥30 years), parity (0, 1, 2, 3, 4, ≥5 live and stillborn children), cumulative breastfeeding duration from all pregnancies (0, 1–6, 7–12, 13–18, 19–24, ≥25 months), OC use (never/former/current), and MHT use (never/former/current) from the baseline and follow-up questionnaires. We also included information about education (<10 years/10–12 years/>12 years, corresponding to primary and intermediate school/high school/higher education), current physical activity level (low/moderate/high), current smoking status (never/former/current), smoking duration and intensity, measured as pack-years (0/>0–10/11–20/>20 years), current diabetes status (yes/no), current body-mass index (BMI; <25, 25–<30, ≥30 kg/m2), current alcohol intake (abstinent/≤10 g per day/>10 g per day), and height (continuous metric). The accuracy of self-reported information on diabetes, physical activity, parity, and education has been validated previously and found to be satisfactory.37–40
Statistical Analysis
HRs and 95% CIs for associations between reproductive factors, use of exogenous hormones, and pancreatic cancer incidence were calculated using Cox proportional-hazard regression. Entry time was age at answering the first questionnaire and exit time age at death, emigration, cancer diagnosis, or end of follow-up (December 31, 2018), whichever occurred first. We updated information on main exposure and covariates during follow-up for women that answered several questionnaires. The proportional-hazard assumption was assessed using Schoenfeld residual plots. To model the relationship between cumulative breastfeeding duration and pancreatic cancer incidence as a continuous exposure metric and to allow for nonlinear effects, we fitted regression models with restricted cubic spline transformations (four knots) of the exposure variable. Knots were placed at equally spaced percentiles. We evaluated nonlinearity by testing the null hypothesis, ie, the second and third spline coefficients jointly equaling zero.
As reproductive factors and exogenous hormone exposure refer to different life stages and conditions, analyses were carried out in different subsamples. We studied associations between cumulative breastfeeding duration, OC use, parity, age at menarche and pancreatic cancer incidence in the total study sample. In parous women, we assessed the effect of age at first birth and cumulative breastfeeding duration on pancreatic cancer incidence, whereas MHT use and age at menopause were assessed only among postmenopausal women.
We used directed acyclic graphs (DAGs) to identify confounders.41 DAGs assume a causal relationship between the exposure and the outcome, and based on the current state of evidence and our expert knowledge on Norwegian women’s behavior, we assumed the most likely interrelations between covariates, exposure, and outcome. For instance, identified confounders for analysis of cumulative breastfeeding duration were age, parity, OC use, and education. We assumed that cumulative breastfeeding duration influenced the intensity and duration of smoking through adulthood (Supplementary Figure 1), and thus smoking intensity and duration was identified as a mediating factor. Likewise, cumulative breastfeeding duration likely affects BMI in middle adulthood through previous weight change, and thus we considered BMI a mediating variable and not a confounder, since our study sample mainly consisted mainly of women >45 years of age. In analysis of age at menarche, the model was adjusted for body type at age 7 years, parity for age at menarche, age at first birth, and education, OC use for age at menarche and education, age at first birth for OC use and education, age at menopause for BMI, OC use, smoking intensity and duration (pack-years), and parity, and MHT use for education and parity.
As our participants were recruited over a long period, we constructed a variable based on wave of enrollment and birth year, which was included as a stratification variable in the Cox regression models to account for calendar year and birth- cohort effects. This way, we allowed the baseline hazard function to vary between groups, but the regression coefficients were equal across groups. To test for linear trends across the categorical variables, we replaced the group identifier with the median value in each group (except for MHT and OC use) and included those variables in the multivariable models. For OC use and MHT, we modeled the categorical variables (never = 0, former = 1, current = 2) as a continuous metric. Finally, we conducted stratified analyses by current smoking status (ever-smoker = former and current smoker), as smoking is a strong risk factor for pancreatic cancer. As such, we assessed associations between cumulative breastfeeding duration, age at first birth, and pancreatic cancer incidence separately in never- and ever-smokers. Due to the limited number of pancreatic cases, we did not assess interactions.
All analyses were performed using Stata 15.1 (StataCorp, College Station, TX, USA).
Results
Study-Sample Characteristics
During a mean follow-up of 18.7 years, we identified 588 incident cases of pancreatic cancer. At baseline, women who had been diagnosed with pancreatic cancer during follow-up were generally older, less educated, less physically active, and more often current smokers and heavy smokers in terms of pack-years than pancreatic cancer–free women. They also had slightly higher prevalence of overweight and obesity and higher prevalence of diabetes. There was no major difference in height, weight, body type at age 7 years, or alcohol consumption between the groups. A slightly larger proportion of women with pancreatic cancer were >14 years of age at the time of menarche and had five children or more. They were also younger when they had their first child and reported shorter cumulative breastfeeding duration than pancreatic cancer–free women. Women who developed pancreatic cancer were more often current users of MHT and entered menopause at an earlier age than those without pancreatic cancer. There was also a larger proportion of never-users of OC among women who developed pancreatic cancer (Table 1).
 |
Table 1 Characteristics of the Study Sample at Enrollment |
Total Study Sample
Within the total study sample, breastfeeding >24 months was associated with substantially lower pancreatic cancer incidence than no breastfeeding (HR 0.37, 95% CI 0.21–0.65; P trend=0.001). For each month of additional breastfeeding, incidence of pancreatic cancer decreased by 2% (HR 0.98, 95% CI 0.96–0.99). The cubic spline regression confirmed an inverse linear relationship between cumulative breastfeeding duration and incidence of pancreatic cancer (Figure 2).
Current users of OCs had increased pancreatic cancer incidence in comparison to never-users (HR 2.20, 95% CI 1.08–4.49); however, the precision of the estimate was poor and analysis hampered by few current users of OCs among pancreatic cancer cases. Finally, age at menarche and parity displayed inverse associations with pancreatic cancer incidencee, but the 95% CIs were wide and included the value 1 (Table 2).
 |
Table 2 Associations Between Cumulative Breastfeeding Duration, Age at Menarche, Parity, OC use, and Pancreatic Cancer Incidence in the Study Sample |
Parous Women
Parous women who had breastfed >24 months had 64% lower pancreatic cancer incidence than those that had not breastfed (HR 0.36, 95% CI 0.20–0.64; P trend=0.001). The rate decreased by 3% per additional month of breastfeeding (HR 0.97, 95% CI 0.96–0.99). Age at first birth was inversely associated with pancreatic cancer incidence: each additional year of age was associated with a 3% decrease in incidence (HR 0.97, 95% CI 0.95–0.99). However the individual categories (≤19, 20–24, 25–30, ≥30 years, P trend=0.068) displayed poor precision of effect estimates (Table 3).
 |
Table 3 Associations Between Cumulative Breastfeeding Duration, Age at First Birth, and Pancreatic Cancer Incidence Among Parous Women in the Study Sample |
Postmenopausal Women
Among postmenopausal women, we observed an inverse association between increasing age at menopause and pancreatic cancer incidence: for each additional year, the rate decreased by 3% (HR 0.97, 95% CI 0.95–1.00). However, effect estimates for the individual categories (≤47, 48–49, 50–51, ≥52 years, P trend=0.07) had wide 95% CIs, which suggested poor precision. There was not enough evidence to confirm an association between MHT use and pancreatic cancer incidence (Table 4).
 |
Table 4 Associations Between Age at Menopause, MHT use, and Pancreatic Cancer Incidence Among Postmenopausal Women in the Study Sample |
Sensitivity Analyses
Breastfeeding >24 months was associated with 60% lower pancreatic cancer incidence in ever-smokers (HR 0.4, 95% CI 0.2–0.80, P trend=0.006). The corresponding figures for never-smokers were HR 0.41, 95% CI 0.15–1.11, and P trend=0.60. For each additional month of breastfeeding, pancreatic cancer incidence decreased by 3% in ever-smokers (HR 0.97, 95% CI 0.96–0.99) and 1% in never-smokers (HR 0.99, 95% CI 0.97–1.01; Supplementary Table 1). Spline regressions confirmed an inverse dose–response relationship between cumulative breastfeeding duration and pancreatic cancer incidence in ever-smokers, but not in never-smokers (Supplementary Figure 2 and 3).
Age at first birth was inversely associated with pancreatic cancer incidence in ever-smoking parous women (HR 0.97, 95% CI 0.94–1.00 per each additional year) and in never-smokers (HR 0.99, 95% CI 0.94–1.03); however, effect estimates for individual categories did not provide evidence of an association (P trend=0.16 for ever-smokers and P trend=0.68 for never-smokers, Supplementary Table 2).
Discussion
In this prospective cohort study of Norwegian women, we found a strong inverse association between cumulative breastfeeding duration and pancreatic cancer incidence. Specifically, incidence of pancreatic cancer was 63% lower among women who had breastfed >24 months than those who had never breastfed. Our results further provided evidence of an inverse linear dose–response trend between cumulative breastfeeding duration and pancreatic cancer incidence. Similar results were observed when restricting the analyses to parous women or ever-smokers. Therefore, the observed inverse association cannot be explained by longer breastfeeding duration in never-smokers, ie, residual confounding by smoking. A similar inverse association between breastfeeding >24 months and pancreatic cancer incidence was observed in never-smokers as well; however, the analysis was hampered by few pancreatic cancer cases, which rendered poor precision for the effect estimate.
Our finding of an inverse relationship between cumulative breastfeeding duration and pancreatic cancer incidence is supported by two other studies, although one was a case–control study of relatively few individuals that relied on recalled information collected after pancreatic cancer diagnosis.10,35 Six other cohort studies and one nested case–control study observed no association between breastfeeding and pancreatic cancer incidence.8,12,15,21,29,30 It is important to note, though, that — unlike the two aforementioned studies — some of these studies only assessed ever/never breastfeeding and did not take duration into account. Of those that observed no association between breastfeeding and pancreatic cancer incidence, only one specifically explored breastfeeding beyond 23 months and reported no significant association.29 In addition, only six cases of those women had breastfed >23 months, which limits the precision of those results. The Norwegian study by Heuch et al found similar results as ours, using a completely different sample of Norwegian women, which makes the probability of a random finding low.10 Heuch et al did not adjust their analysis for smoking; however, they argued that smoking was not very common in the birth cohort of women included in their study.10 We assumed that breastfeeding duration influenced lifetime smoking intensity and duration among our study participants; therefore, we identified smoking intensity and duration as a mediating factor and did not adjust our main analysis for that. However, we conducted stratified analysis based on current smoking status, and observed similar associations in ever- and never-smokers. Therefore, our study confirms that breastfeeding duration is inversely associated with pancreatic cancer incidence after accounting for smoking.
Still, the inverse association between cumulative breastfeeding duration and pancreatic cancer incidence is challenging to explain. It is well established that prolonged breastfeeding is associated with lower risk of breast cancer, which may be a result of the reduction in lifetime exposure to sex hormones, as lactation postpones resumption of the menstrual cycle after pregnancy and produces a period of infertility.42 Other mechanisms related to the exfoliation of damaged epithelial cells from breast tissue during lactation have also been proposed. Breastfeeding does however also alter circulating levels of other hormones, such as prolactin, which in addition to having an important role in lactation and reproduction, is also involved in many other biological activities.43 Previous epidemiological studies have reported positive associations between prolactin concentrations and cancer of the breast, endometrium, and ovaries, but no association with prostate cancer and an inverse association with pancreatic cancer.44–48 More specifically, a study of both men and women found that prospective cases had significantly lower concentrations of prolactin than healthy controls up to 35 months prior to pancreatic cancer diagnosis.46 On the other hand, recent animal experimental research has shown that elevated systemic prolactin levels in mice contributed to the progression of pancreatic intraepithelial neoplasia, a precursor to invasive ductal adenocarcinoma of the pancreas, and that prolactin released from tumor-associated macrophages may induce pancreatic tumorigenesis.49 As such, it remains unclear whether systemic or local concentrations of prolactin are relevant for pancreatic cancer development, if elevated or decreased concentrations play a role, and whether the effect is sex-dependent.
It is well known that during pregnancy, prolactin levels are highly superior to basal levels. However, research has shown that circulating concentrations of prolactin outside pregnancy and breastfeeding period are higher in nulliparous women compared to postlactation concentrations in parous women and inversely associated with the duration of breastfeeding of the first child.50,51 Therefore, women are exposed to high levels of prolactin during the relatively short periods of pregnancy and breastfeeding, but afterward they will have significantly lower exposure to prolactin than women who have never breastfed.50,51 As such, if we hypothesize that elevated prolactin levels are involved in pancreatic cancer development in women, long-term exposure to lower prolactin levels could potentially explain the inverse association between cumulative breastfeeding duration and pancreatic cancer incidence in women. Future studies should thus assess how breastfeeding duration for each subsequent child is associated with pancreatic cancer incidence.
Decreasing prolactin concentrations with increasing age at first birth has also been observed.5,19 As such, our finding of an inverse association between age at first birth and pancreatic cancer incidence could also be explained by lower prolactin concentrations by increasing age at first birth. However, our result regarding age at first birth contradicts several other studies, including a meta-analysis from 2016.8,10,12,20,29,30,35,52–55 It is also important to point out that although our results suggested a 3% decrease in incidence of pancreatic cancer per additional year of age, the effect estimates for the different categories were less precise. Therefore, it is also possible that our finding was a result of chance.
Finally, we cannot entirely rule out that cumulative breastfeeding duration is not a proxy for other beneficial behavior for pancreatic cancer incidence that our extensive questionnaire did not cover. For example, breastfeeding reduces the body burden of persistent organic pollutants, contaminants with endocrine-disrupting properties, some of which have been suggested to have carcinogenic effects.56 This was mentioned by Lo et al, who also found an inverse association between cumulative breastfeeding duration and pancreatic cancer.35 However, the association between persistent organic pollutants and pancreatic cancer has not been well studied and remains unclear.57 Nevertheless, the potential link between cumulative breastfeeding duration and pancreatic cancer incidence should be further explored in both experimental and epidemiological studies.
Current use of OCs was associated with a doubling of pancreatic cancer incidence in our cohort. However, this result, needs to be interpreted with caution, as it is based on only seven cases and the CI was very wide. A previous meta-analysis by Tang et al and the study by Butt et al based on all premenopausal women in Denmark found no effect of OC use on pancreatic cancer incidence, which was supported by a recent prospective cohort study.12,26,27 As our study sample consisted of women with a mean age of approximately 50 years at baseline, our study is not suitable for assessing the effects of current OC use in relation to pancreatic cancer incidence, as the prevalence of current use was very low.
We observed inverse associations between parity and pancreatic cancer incidence; however, our estimates had poor precision, which complicated further interpretation. Previous meta-analyses have also reported inverse associations, as well as more recent prospective studies, although they also reported imprecise effect estimates.8,23–25 It is however important to point out that our parity variable represented the number of live births or stillbirths that the women had experienced. We had no available information about previous abortions; therefore, nulliparous women could have experienced pregnancies that had been terminated through spontaneous or induced abortion. As such, they could have been exposed to sex hormones, although to a much lesser extent than women who experienced full-term pregnancies. This should be mentioned as a potential limitation of our study that could influence the results.
We did not identify an association for age at menarche or use of MHT and pancreatic cancer incidence, which is in line with the meta-analysis by Tang et al.26 Concerning age at menopause, we observed an inverse association: higher age at menopause was associated with decreased pancreatic cancer incidence, although the 95% CI was relatively wide, with an upper limit of 1. This finding mainly contradicts previous results, although the meta-analysis by Tang et al reported an inverse but insignificant association.26 It is important to emphasize that the individual estimates for each category of age at menopause had poor precision. Based on that, we believe that it is less likely that this finding reflects a true association. As such, our overall inconsistent results regarding use of exogenous hormones, reproductive factors, and pancreatic cancer incidence do not clearly support an important role for estrogens in pancreatic cancer etiology.
There are some strengths and limitations with our study that are worth mentioning. We used DAGs to identify and describe confounding factors and their interrelations. DAGs are based on assumptions about relationships between included variables, and in case our assumptions were wrong: the regression models could have been misspecified. However, our main finding of a strong inverse association between cumulative breastfeeding duration and pancreatic cancer incidence was consistent across both the age-adjusted and multivariable-adjusted models, as well as in models stratified by smoking status. In addition to the strong observed dose–response effect, this suggests a robust relationship. Further strengths of our study include the high number of women involved, the prospective design, and the use of updated information during follow-up, which decreased the risk of exposure misclassification. The fact that the NOWAC study contains detailed information on sociodemographic and lifestyle variables allowed us to adjust for known risk factors of pancreatic cancer.
However, as pancreatic cancer is a rare disease, we sometimes faced difficulties in terms of statistical power in subgroup analysis. In addition, we conducted many statistical analyses, and the chances of a random positive finding cannot be ruled out. In Norway, there were intensive campaigns to promote breastfeeding during the 1980s, and today 80% of mothers still breastfeed >6 months.58,59 Breastfeeding is common and encouraged to infant age of 1 year. Our results suggest that cumulative breastfeeding duration >2 years is associated with decreased incidence of pancreatic cancer; however, our findings may not be generalizable to a population with another breastfeeding pattern. Still, we hope that this study will trigger future research in the field of breastfeeding and pancreatic cancer incidence, as well as studies in younger women, which will be able to address associations with current OC use more properly. A future meta-analysis of pancreatic cancer incidence and cumulative breastfeeding duration is warranted.
Conclusion
This study suggests that cumulative breastfeeding duration and higher age at first birth are associated with reduced pancreatic cancer incidence. It does not support an important role for estrogen in pancreatic carcinogenesis, but may suggest an association between prolactin and pancreatic cancer, which requires further investigation.
Abbreviations
BMI, body-mass index; MHT, menopausal hormone therapy; NOWAC, Norwegian Women and Cancer; OCs, oral contraceptives.
Data-Sharing Statement
To access the data supporting the findings presented, kindly contact the person in charge of the NOWAC study (https://site.uit.no/nowac/contact-information).
Ethical Approval and Informed Consent
The NOWAC study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate (P REK NORD 141/2008), and the study was performed in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Acknowledgments
The authors thank the staff and participants of the NOWAC study for their valuable contributions.
Author Contributions
All authors contributed to data analysis or interpretation, drafting or revising the article, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cancer Registry of Norway. Cancer in Norway 2019 - Cancer Incidence, Mortality, Survival and Prevalence in Norway. Oslo: Cancer Registry of Norway; 2020.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
3. Zhao C, Gao F, Li Q, Liu Q, Lin X. The distributional characteristic and growing trend of pancreatic cancer in China. Pancreas. 2019;48(3):309–314. doi:10.1097/MPA.0000000000001222
4. World Cancer Research Fund [homepage on the Internet]. Diet, nutrition, physical activity and pancreatic cancer; 2018. Available from: https://www.wcrf-uk.org/uk/preventing-cancer/cancer-types/pancreatic-cancer.
5. Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol. 2015;44(1):186–198. doi:10.1093/ije/dyu240
6. Midha S, Chawla S, Garg PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016;381(1):269–277. doi:10.1016/j.canlet.2016.07.022
7. Collégiale des Universitaires en Hépato-Gastro-Entérologie [College of University Professors in Hepato-Gastro-Enterology]. Tumeurs du Pancréas [Pancreatic Tumors]. In: Hépato-gastro-entérologie-Chirurgie digestive [Hepato-Gastro-Enterology – Digestive Surgery]. Elsevier Health Sciences; 2018:379–387.
8. Andersson G, Borgquist S, Jirstrom K. Hormonal factors and pancreatic cancer risk in women: the malmo diet and cancer study. Int J Cancer. 2018;143(1):52–62. doi:10.1002/ijc.31302
9. Fernandez E, La Vecchia C, D’Avanzo B, Negri E. Menstrual and reproductive factors and pancreatic cancer risk in women. Int J Cancer. 1995;62(1):11–14. doi:10.1002/ijc.2910620104
10. Heuch I, Jacobsen BK, Albrektsen G, Kvale G. Reproductive factors and pancreatic cancer risk: a Norwegian cohort study. Br J Cancer. 2008;98(1):189–193. doi:10.1038/sj.bjc.6604095
11. Ji BT, Hatch MC, Chow WH, et al. Anthropometric and reproductive factors and the risk of pancreatic cancer: a case-control study in Shanghai, China. Int J Cancer. 1996;66(4):432–437. doi:10.1002/(SICI)1097-0215(19960516)66:4<432::AID-IJC4>3.0.CO;2-X
12. Kabat GC, Kamensky V, Rohan TE. Reproductive factors, exogenous hormone use, and risk of pancreatic cancer in postmenopausal women. Cancer Epidemiol. 2017;49:1–7. doi:10.1016/j.canep.2017.05.002
13. Karlson BM, Wuu J, Hsieh CC, Lambe M, Ekbom A. Parity and the risk of pancreatic cancer: a nested case-control study. Int J Cancer. 1998;77(2):224–227.227. doi:10.1002/(sici)1097-0215(19980717)77:2<224::aid-ijc10>3.0.co;2-b
14. Kvale G, Heuch I, Nilssen S. Parity in relation to mortality and cancer incidence: a prospective study of Norwegian women. Int J Epidemiol. 1994;23(4):691–699. doi:10.1093/ije/23.4.691
15. Lee E, Horn-Ross PL, Rull RP, et al. Reproductive factors, exogenous hormones, and pancreatic cancer risk in the CTS. Am J Epidemiol. 2013;178(9):1403–1413. doi:10.1093/aje/kwt154
16. Lucenteforte E, Zucchetto A, Bosetti C, et al. Reproductive and hormonal factors and pancreatic cancer risk in women. Pancreas. 2011;40(3):460–463. doi:10.1097/MPA.0b013e31820bf986
17. Masoudi S, Momayez Sanat Z, Mahmud Saleh A, Nozari N, Ghamarzad N, Pourshams A. Menstrual and reproductive factors and risk of pancreatic cancer in women. Middle East J Dig Dis. 2017;9(3):146–149. doi:10.15171/mejdd.2017.65
18. Prizment AE, Anderson KE, Hong CP, Folsom AR. Pancreatic cancer incidence in relation to female reproductive factors: iowa women’s health study. JOP. 2007;8(1):16–27.
19. Sadr-Azodi O, Konings P, Brusselaers N. Menopausal hormone therapy and pancreatic cancer risk in women: a population-based matched cohort study. United European Gastroenterol J. 2017;5(8):1123–1128. doi:10.1177/2050640617702060
20. Teras LR, Patel AV, Rodriguez C, Thun MJ, Calle EE. Parity, other reproductive factors, and risk of pancreatic cancer mortality in a large cohort of U.S. women (United States). Cancer Causes Control. 2005;16(9):1035–1040. doi:10.1007/s10552-005-0332-4
21. Duell EJ, Travier N, Lujan-Barroso L, et al. Menstrual and reproductive factors in women, genetic variation in CYP17A1, and pancreatic cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort. Int J Cancer. 2013;132(9):2164–2175. doi:10.1002/ijc.27875
22. Lujan-Barroso L, Xhang W, Olson SH, et al. Menstrual and reproductive factors, hormone use and risk of pancreatic cancer: analysis from the international pancreatic cancer case- control consortium (PanC4). Pancreas. 2016;45(10):1401–1410. doi:10.1097/MPA.0000000000000635
23. Guan H-B, Wu L, Wu Q-J, Zhu J, Gong T. Parity and pancreatic cancer risk: a dose-response meta-analysis of epidemiologic studies. PLoS One. 2014;9:3. doi:10.1371/journal.pone.0092738
24. Zhu B, Zou L, Han J, et al. Parity and pancreatic cancer risk: evidence from a meta-analysis of twenty epidemiologic studies. Sci Rep. 2014;4:5313. doi:10.1038/srep05313
25. Teng Y, Saito E, Abe SK, et al. Female reproductive factors, exogenous hormone use, and pancreatic cancer risk: the Japan Public Health Center-based prospective study. Eur J Cancer Prev. 2017;26(5):378–384. doi:10.1097/CEJ.0000000000000358
26. Tang B, Lv J, Li Y, Yuan S, Wang Z, He S. Relationship between female hormonal and menstrual factors and pancreatic cancer: a meta-analysis of observational studies. Medicine. 2015;94:7. doi:10.1097/MD.0000000000000177
27. Butt SA, Lidegaardi Ø, Skovlund C, et al. Hormonal contraceptive use and risk of pancreatic cancer—A cohort study among premenopausal women. PLoS One. 2018;13:10. doi:10.1371/journal.pone.0206358
28. Luo A-J, Feng R-H, Wang X-W, Wang F-Z. Older age at first birth is a risk factor for pancreatic cancer: a meta-analysis. Hepatobiliary Pancreatic Dis Int. 2016;15(2):125–130. doi:10.1016/S1499-3872(16)60063-2
29. Skinner HG, Michaud DS, Colditz GA, et al. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol Biomarkers Prev. 2003;12(5):433–438.
30. Stevens RJ, Roddam AW, Green J, et al. Reproductive history and pancreatic cancer incidence and mortality in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1457–1460. doi:10.1158/1055-9965.EPI-08-1134
31. Christopher K. Breastfeeding perceptions and attitudes: the effect of race/ethnicity and cultural background. Soc Today. 2012;10:2.
32. Dereuddre R, Van de Putte B, Bracke P. Ready, willing, and able: contraceptive use patterns across Europe. Eur J Popul. 2016;32(4):543–573. doi:10.1007/s10680-016-9378-0
33. Jung EM, Kim HS, Park H, Ye S, Lee D, Ha EH. Does exposure to PM10 decrease age at menarche? Environ Int. 2018;117:16–21. doi:10.1016/j.envint.2018.04.020
34. Kingsley M. The influence of income and work hours on first birth for Australian women. J Popul Res. 2018;35(2):107–129. doi:10.1007/s12546-018-9200-4
35. Lo AC, Soliman AS, El-Ghawalby N, et al. Lifestyle, occupational, and reproductive factors in relation to pancreatic cancer risk. Pancreas. 2007;35(2):120–129. doi:10.1097/mpa.0b013e318053e7d3
36. Lund E, Dumeaux V, Braaten T, et al. Cohort profile: the Norwegian Women and Cancer Study–NOWAC–Kvinner og kreft. Int J Epidemiol. 2008;37(1):36–41. doi:10.1093/ije/dym137
37. Lund E, Kumle M, Braaten T, et al. External validity in a population-based national prospective study-the Norwegian Women and Cancer Study (NOWAC). Cancer Causes Control. 2003;14(10):1001–1008. doi:10.1023/B:CACO.0000007982.18311.2e
38. Borch KB, Ekelund U, Brage S, Lund E. Criterion validity of a 10-category scale for ranking physical activity in Norwegian women. Int J Behav Nutr Phys Act. 2012;9:2. doi:10.1186/1479-5868-9-2
39. Rylander C, Sandanger TM, Engeset D, Lund E. Consumption of lean fish reduces the risk of type 2 diabetes mellitus: a prospective population based cohort study of Norwegian women. PLoS One. 2014;9(2):e89845. doi:10.1371/journal.pone.0089845
40. Skeie G, Mode N, Henningsen M, Borch KB. Validity of self-reported body mass index among middle-aged participants in the Norwegian Women and Cancer study. Clin Epidemiol. 2015;7:313–323. doi:10.2147/CLEP.S83839
41. Hernán MA, Robins JM. Graphical representation of causal effects. In: Causal Inference: What If. Boca Raton: Chapman & Hall/CRC; 2020:69–82.
42. World Cancer Research Fund [homepage on the Internet]. Lactation and the risk of cancer; 2018. Available from: https://www.wcrf.org/dietandcancer/exposures/lactation-breastfeeding.
43. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523–1631.
44. Hankinson SE, Willett WC, Michaud DS, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91(7):629–634. doi:10.1093/jnci/91.7.629
45. Levina VV, Nolen B, Su Y, et al. Biological significance of prolactin in gynecologic cancers. Cancer Res. 2009;69(12):5226–5233. doi:10.1158/0008-5472.CAN-08-4652
46. Nolen BM, Brand RE, Prosser D, et al. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS One. 2014;9(4):e94928. doi:10.1371/journal.pone.0094928
47. Stattin P, Rinaldi S, Stenman UH, et al. Plasma prolactin and prostate cancer risk: A prospective study. Int J Cancer. 2001;92(3):463. doi:10.1002/ijc.1191
48. Tworoger SS, Hankinson SE. Prolactin and breast cancer risk. Cancer Lett. 2006;243(2):160–169. doi:10.1016/j.canlet.2006.01.032
49. Tandon M, Coudriet GM, Criscimanna A, et al. Prolactin promotes fibrosis and pancreatic cancer progression. Cancer Res. 2019;79(20):5316–5327. doi:10.1158/0008-5472.CAN-18-3064
50. Hietala M, Olsson H, Jernstrom H. Prolactin levels, breast-feeding and milk production in a cohort of young healthy women from high-risk breast cancer families: implications for breast cancer risk. Fam Cancer. 2008;7(3):221–228. doi:10.1007/s10689-007-9178-0
51. Nagata C, Wada K, Nakamura K, Hayashi M, Takeda N, Yasuda K. Associations of body size and reproductive factors with circulating levels of sex hormones and prolactin in premenopausal Japanese women. Cancer Causes Control. 2011;22(4):581–588. doi:10.1007/s10552-011-9731-x
52. Kreiger N, Lacroix J, Sloan M. Hormonal factors and pancreatic cancer in women. Ann Epidemiol. 2001;11(8):563–567. doi:10.1016/S1047-2797(01)00219-8
53. Lin Y, Kikuchi S, Tamakoshi A, et al. Association of menstrual and reproductive factors with pancreatic cancer risk in women: findings of the Japan collaborative cohort study for evaluation of cancer risk. J Gastroenterol. 2006;41(9):878–883. doi:10.1007/s00535-006-1869-z
54. Navarro Silvera SA, Miller AB, Rohan TE. Hormonal and reproductive factors and pancreatic cancer risk: a prospective cohort study. Pancreas. 2005;30(4):369–374. doi:10.1097/01.mpa.0000160301.59319.ba
55. Zhang Y, Coogan PF, Palmer JR, Strom BL, Rosenberg L. A case-control study of reproductive factors, female hormone use, and risk of pancreatic cancer. Cancer Causes Control. 2010;21(3):473–478. doi:10.1007/s10552-009-9478-9
56. Hardell E, Carlberg M, Nordstrom M, van Bavel B. Time trends of persistent organic pollutants in Sweden during 1993–2007 and relation to age, gender, body mass index, breast-feeding and parity. Sci Total Environ. 2010;408(20):4412–4419. doi:10.1016/j.scitotenv.2010.06.029
57. Gasull M, Pumarega J, Kiviranta H, et al. Methodological issues in a prospective study on plasma concentrations of persistent organic pollutants and pancreatic cancer risk within the EPIC cohort. Environ Res. 2019;169:417–433. doi:10.1016/j.envres.2018.11.027
58. Statistics Norway [homepage on the Internet]. Norwegian women breastfeed as recommended; 2003. Avialable from: https://www.ssb.no/en/helse/artikler-og-publikasjoner/norwegian-women-breastfeed-as-recommended.
59. Thorvaldsen G. Was there a european breastfeeding pattern? Hist Fam. 2008;13(3):283–295. doi:10.1016/j.hisfam.2008.08.001
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.