Back to Journals » Clinical Ophthalmology » Volume 16
Reproducibility of the Magnitude of Lens Rotation Following Implantation of a Toric Intraocular Lens with Modified Haptics
Authors Quesada GA, Quesada RA, Jones JJ, Straker BJK , Zhao W , Tsai L, Vilupuru S
Received 21 May 2022
Accepted for publication 5 September 2022
Published 29 September 2022 Volume 2022:16 Pages 3213—3224
DOI https://doi.org/10.2147/OPTH.S373976
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Gabriel A Quesada,1 Rodrigo A Quesada,1 Jason J Jones,2 Benjamin JK Straker,3 Wuchen Zhao,3 Linda Tsai,3 Srividhya Vilupuru3
1Centro de Oftalmología y Cirugía Plástica, San Salvador, El Salvador; 2Jones Eye Clinic, Sioux City, IA, USA; 3Johnson & Johnson Surgical Vision, Inc, Irvine, CA, USA
Correspondence: Srividhya Vilupuru, Johnson & Johnson Surgical Vision, 31 Technology Dr Suite 200, Irvine, CA, 92618, USA, Tel +1-949-581-5799, Email [email protected]
Purpose: To evaluate the reproducibility of magnitude of postoperative IOL rotation following implantation of a toric intraocular lens (IOL) with modified haptics, in comparison with a Proof-of-Concept (POC) study of prototype IOLs featuring the same haptic design.
Patients and Methods: A post-market, prospective, multicenter, single-arm, open-label clinical study was conducted. TECNIS Toric II IOL (Johnson & Johnson Vision, Irvine, CA, USA, Models ZCU150 to 600) were implanted in 125 subjects and evaluated at 1-day and 1-week postoperatively. An objective photographic method was used to determine postoperative IOL rotation. Uncorrected distance visual acuity (UCDVA), postoperative astigmatism, and surgeon satisfaction were also assessed. Rotation data were compared to the POC study in which two prototype non-toric monofocal IOLs, one with the same haptic design as Model ZCU, were studied.
Results: Mean absolute rotation was 0.82° ± 1.0° and 0.84° ± 0.92°at 1-day and 1-week visits, respectively. The percentage of eyes with ≤ 5° of absolute rotation was 98.9% and 99.5% at the 1-day and 1-week visits, respectively. The magnitude of rotation was similar to the POC study prototype IOLs. At 1-week, mean monocular UCDVA was 0.026 ± 0.135 (~20/21) logMAR and mean residual manifest refractive cylinder was 0.30 D ± 0.35 D. The mean signed axis difference (postoperative minus operative) of the TECNIS Toric II IOL was 0.23° ± 1.27° at 1-day and − 0.07° ± 1.25° at 1-week, indicating a clockwise drift. At 1-week, surgeons were very satisfied or satisfied with overall clinical outcomes and rotational stability in 98% of implanted eyes.
Conclusion: The TECNIS Toric II IOL, with frosted, squared haptics, demonstrated low magnitude of postoperative IOL rotation, excellent uncorrected distance vision, and minimal residual astigmatism. The POC study design was supported, demonstrating that prototype non-toric monofocal IOLs can predict clinical performance of toric IOLs with the same haptic design.
Keywords: toric IOL, astigmatism, IOL rotation, cataract, surgery, axis misalignment
Introduction
Approximately one-third of patients presenting for cataract surgery have at least one diopter (D) of corneal astigmatism and could therefore benefit from toric intraocular lenses (IOLs).1 Toric IOLs provide advantages to patients with corneal toricity compared to non-toric monofocal IOLs. These include reduced amounts of residual astigmatism following surgery, greater spectacle independence, and better uncorrected distance visual acuity (UCDVA).2 However, the importance of rotational stability of toric IOLs cannot be overstated, as postoperative alignment and stability of toric IOLs is critical for achieving optimal visual outcomes. Using a simple optical model, it has been shown that every 1 degree of toric IOL rotation results in a 3.3% reduction in astigmatic correction.3
Toric IOLs show the greatest amount of rotation within the first day following surgery, with little rotation observed from one day to one week and thereafter.4–6 Toric IOL rotation of ≥5 degrees (°) is considered to be clinically relevant, and rotation of ≥10° typically indicates the need for surgical intervention to reposition the toric IOL.7
Postoperative counterclockwise rotation of ≥5° has been reported in clinical studies evaluating the rotation of the TECNIS® Toric 1-Piece (Johnson & Johnson Vision, Irvine, CA, USA) monofocal IOL.4,8,9 In contrast, two additional studies reported that clockwise rotation was observed in the majority of subjects after surgery10,11 and another study showed a similar distribution of clockwise and counterclockwise rotation 3 months after surgery in subjects with long axial lengths.12 In a retrospective, multicenter case series in Japan, the incidence of repositioning surgery of the TECNIS® Toric 1-Piece was 1.8% (62/3451).13
To address this issue of postoperative rotation, two prototype non-toric monofocal IOLs with fiducial axis marks and frosted haptics were developed and evaluated for their postoperative IOL rotation compared to a control IOL without frosted haptics (TECNIS® 1-piece monofocal IOL with fiducial axis marks).14 In this proof-of-concept (POC) study, mean ± standard deviation (SD) absolute IOL rotation was found to be significantly lower at the 1 week visit with both Prototypes (Prototype 1: 0.88° ± 0.94° and Prototype 2: 0.71° ± 0.69°), compared to the control (2.24° ± 3.21°). In addition, both Prototypes showed ≤5° absolute rotation for all eyes at the 1 week visit, and no cases of ≥10° rotation at any time through 6 months postoperatively.14 Because the overall geometry of Prototype 2 was the same as the TECNIS® toric IOL (Model ZCT), that design was selected for commercialization under the brand name TECNIS Toric II 1-Piece IOL (Model ZCU).14
The TECNIS Toric II 1-Piece IOL (Model ZCU, Johnson & Johnson Vision, Irvine, CA, USA) is an ultraviolet light-absorbing, squared and frosted haptic (Figure 1) posterior chamber IOL that compensates for corneal spherical aberrations and corneal astigmatism.15 It was designed to deliver improved rotational outcomes within the first week following implantation. The purpose of this study was to evaluate the reproducibility of postoperative IOL rotation of the TECNIS Toric II (Model ZCU) IOL compared to the prototype non-toric monofocal IOL with fiducial axis marks (Prototype 2), reported previously by Vukich et al.14 In addition, visual performance, residual astigmatism, and surgeon satisfaction with the TECNIS Toric II (Model ZCU) IOL were assessed. Insights into the appropriateness of comparing rotational stability results between subjects of different races and ethnicities were also gained.
 |
Figure 1 High magnification photograph of the squared and frosted haptic, a design feature of both the TECNIS Toric II and Prototype 2 IOLs. Abbreviation: IOL, intraocular lens. |
Subjects and Methods
Study Design
A post-market, prospective, multicenter, single-arm, open-label clinical study (clinicaltrials.gov NCT04327518) was conducted at seven sites across the United States (US). The clinical study was reviewed and approved by Salus Institutional Review Board and Office of the Human Research Protection Program (OHRPP, University of California, Los Angeles). A total of 133 subjects were enrolled to achieve at least 200 evaluable eyes at the 1-week visit. Subjects were implanted with the commercially available TECNIS Toric II IOL (Model ZCU 1.50 diopter [D] to 6.00 D, see Table 1) in at least one eye and followed for 3 months. All subjects had a minimum of five scheduled study visits: preoperative examination of both eyes, then operative visit, 1-day, 1-week, and 3-month visits for each eye implanted. In subjects eligible for bilateral toric implantation, the second eye was implanted within one-month of the first eye surgery. Data from the 1-day and 1-week visits are presented in this manuscript. The long-term rotation data from the 3-month visits will be reported in a separate publication. The study was conducted in accordance with the Declaration of Helsinki and all subjects provided written informed consent. Local institutional review board approval was obtained.
 |
Table 1 TECNIS® Toric II Models ZCU, IOL Astigmatism Correction Range |
Rotation data from this study were compared with the POC data reported previously by Vukich et al, for a prototype non-toric monofocal IOL with fiducial axis marks (Prototype 2) with the same haptic design (Figure 1).14 In brief, that study was a prospective, randomized, multicenter paired-eye study using a similar study protocol and the same objective photographic method for measuring IOL rotation. A total of 99 eyes were implanted with the Prototype 2 IOL at one study site in Asia Pacific and two study sites in Latin America.14
Inclusion and Exclusion Criteria
Key inclusion criteria for participation in the study were age 22 years or older; scheduled to undergo unilateral or bilateral cataract extraction and posterior chamber IOL implantation; pre-existing corneal astigmatism of 1.00 D or greater; predicted toric IOL calculator residual refractive cylinder (considering surgically induced astigmatism and posterior corneal astigmatism) less than ≤0.50 D; potential candidate for achieving postoperative best-corrected distance visual acuity of 20/30 Snellen or better; clear intraocular media (other than cataract) in each eye; and available and willing to comply with the study procedures and provide signed informed consent and health insurance portability and accountability act (HIPAA) authorization.
Key exclusion criteria for the study were irregular corneal astigmatism; any corneal pathology/abnormality other than regular corneal astigmatism or corneal instability due to contact lens wear; previous corneal or intraocular surgery; any pupil abnormalities; dilated pupil size of less than 6.0 mm; recurrent severe anterior or posterior segment inflammation or uveitis; conditions associated with increased risk of zonular rupture; known ocular or systemic disease that may affect visual acuity or require surgical intervention during the study; use of systemic or ocular medications that may affect vision; pregnancy or plans to become pregnant; participation in any other clinical study within 30 days; and planned monovision correction (eye designated for near correction).
IOL Power and Targeted Refraction
In both studies, keratometry, axial length and anterior chamber depth (ACD) were measured to determine the appropriate lens power for implantation. Optical biometry methods (ie, IOLMaster® (Carl Zeiss Meditec AG, Germany) or LENSTAR® (Haag-Streit, USA)) were preferred; however, surgeons were allowed to use their preferred method for biometry. Surgeons used their personalized A-Constant or 119.3 for the TECNIS IOLs. The spherical equivalent lens power was calculated to achieve emmetropia (± 0.50 D) at distance for all study eyes. Intentional overcorrection or under-correction (ie, outside ± 0.50 D) was not allowed.
In the post-market study, in order to facilitate IOL selection, surgeons used the web-based TECNIS Toric calculator (www.TecnisToricCalc.com) or their preferred Toric Calculator for determination of lens axis placement. Preoperative keratometry, biometry data including axial length, incision location, spherical equivalent IOL power, and the surgeon’s estimated surgically induced corneal astigmatism were used as inputs for the Toric Calculator. These inputs were used to determine the axis of placement in the eye and the predicted residual refractive astigmatism.
Surgical Technique
Surgeons performed standardized, small-incision cataract surgery and implanted the study lenses in the capsular bag using a Johnson & Johnson Surgical Vision validated insertion system qualified for use with TECNIS Toric II lenses as specified in the Directions for Use.15
The TECNIS Toric II IOLs have two sets of four axis orientation marks 180° apart in the outer periphery of the anterior optic surface, to indicate the meridian of the lowest power (flat meridian). These axis orientation marks allow precise alignment of the flat meridian of the IOL with the steep meridian of the cornea. Surgeons were allowed to use their routine, preferred technique for IOL axis marking preoperatively for evaluation of the toric IOL alignment between the intended orientation and the actual IOL orientation during the surgery. However, surgeons were not allowed to perform additional refractive procedures during the operative procedure or throughout the postoperative study period (eg, LRI, PRK, LASIK or LASEK).
Study Endpoints
Lens Rotation
An objective photographic method, as detailed in two prior publications, was used to determine postoperative IOL rotation.14,16 Photographs were registered by matching scleral vessel and iris landmarks and lens rotation was evaluated by measuring IOL rotation, which was defined as the difference between IOL orientation at the end of the surgery and at the 1-day and 1-week postoperative visits. This was performed by two independent, masked analysts using the custom image analysis software described in an earlier publication.16 In cases of poor agreement between analysts (>3° difference in signed rotation value), both analysts repeated the analysis. Eyes were excluded (from photo analysis only) if agreement to within 3° could not be achieved following a second round of analysis.
Postoperative rotational stability was assessed by calculating the mean absolute rotation and percentage of implanted eyes with ≤5° absolute rotation for both the 1-day and 1-week time points. Mean signed lens rotation from operative was also calculated at 1-day and 1-week time points, with negative values indicating clockwise rotation. These methods for summarizing rotational data were identical to the previous POC study evaluating the non-toric prototype monofocal IOL.14
Visual Acuity and Refraction
Monocular UCDVA was measured at the 1-day and 1-week postoperative visits using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart at 4.0 meters (13 feet), under photopic (85–110 cd/m2) conditions. At the 1-week postoperative visit, manifest refractions were conducted at 4.0 meters, under the same photopic conditions, and monocular best corrected distance visual acuity (BCDVA) was also measured. Residual mean manifest cylinder was also calculated for all eyes at 1-week.
Surgeon Satisfaction Questionnaire
A 3-item questionnaire assessing subjective surgeon satisfaction with overall clinical outcomes, rotational stability, and UCDVA for each implanted eye was self-administered at 1-week. A 5-point scale was utilized for each question (1 = very dissatisfied, 2 = dissatisfied, 3 = undecided, 4 = satisfied and 5 = very satisfied).
Other Observations
Slit lamp biomicroscopy was performed at each postoperative visit to determine the presence or absence of any medical or lens findings, complications, or adverse events.
The current study was conducted in the US, whereas the POC study was conducted in Asia Pacific and Latin America. Therefore, lens rotation findings between subjects of different races and ethnicities, from the current and previous studies, were compared to determine if postoperative lens rotation results are generalizable across populations that vary demographically.
Statistical Analysis
Data from previous toric IOL studies were utilized to calculate the study sample size. With a sample size of 200 eyes, it was determined that the two-sided 95% confidence interval for the proportion of eyes within 5° of absolute rotation at 1-week would be within 3.0% of the observed proportion, assuming an expected proportion of 95% or higher.
IOL rotation of the TECNIS® Toric II IOL was analyzed by sub-model using general linear regression, with rotation as the outcome variable and lens model as the dependent variable. For monocular endpoints, including IOL rotation, all eyes implanted with the TECNIS® Toric II IOL were pooled for analyses, including both eyes from bilaterally implanted subjects and one eye from unilaterally implanted subjects. Only available data were used for analysis (ie, no data imputation was conducted for missing data).
For visual acuity, the values were converted to logMAR prior to analysis. Mean absolute rotation, axis misalignment, visual acuity and refractive data were analyzed for all visits using descriptive statistics, including the mean, SD, median, minimum and maximum, and 95% confidence interval. Linear regression was used to investigate potential correlation between axial length and absolute lens rotation. For visual acuity, the frequency and proportion of eyes achieving each acuity line was also calculated.
For questionnaire data, the frequency and proportion of each rating for each individual question were calculated. In addition, the frequency and proportion of eyes with medical findings, lens findings, complications and adverse events were calculated.
Results
This study was conducted from 11th June 2020 to 1st June 2021. A total of 133 subjects were enrolled and 125 subjects were treated/implanted. A total of 202 eyes were implanted with the TECNIS Toric II IOL in at least one eye: 80 subjects were treated bilaterally (77 subjects with study lens in both eyes, three subjects with non-study lens in the first eye and the study lens in second eye) and 45 subjects were treated unilaterally. For postoperative analysis, 6 eyes were excluded due to poor quality operative photos. For the 1-day visit, 5 eyes were excluded due to analyst disagreement. At the 1-week visit, 3 eyes were excluded due to analyst disagreement.
Subject demographics for both studies are provided in Table 2. The mean ± standard deviation age of the subjects implanted with the TECNIS Toric II IOL was 68.4 ± 8.3 years. In the US study, 42.4% were males and 57.6% were females, and most subjects were Caucasian and non-Hispanic/Latino in ethnicity. In comparison, the subject population from the POC study was similar in age (67.3 ± 8.7 years), but most subjects were either Asian (43.9%) or Hispanic/Latino (57.1%). There was also a greater proportion of female (69.4%) than male (30.6%) participants. Mean axial length was approximately 1.2 mm greater in this study compared to the Prototype 2 non-toric monofocal IOL study.
 |
Table 2 Participant Demographics at Baseline |
Mean Absolute IOL Rotation
Summary statistics for mean absolute IOL rotation at each postoperative time point are presented in Table 3. There were no significant differences in mean absolute IOL rotation of the TECNIS Toric II IOL by model at the 1-day (p = 0.491) and 1-week (p = 0.105) visits, therefore all data were pooled for further analysis. At the 1-day and 1-week visits, mean absolute IOL rotation of the TECNIS Toric II IOL was 0.82° ± 1.0° and 0.84° ± 0.92°, respectively. These data were comparable to the mean absolute IOL rotation of the Prototype version 2 non-toric monofocal IOL from the previous POC study, which was 0.73° ± 0.75° at 1-day and 0.71° ± 0.69° at 1-week.14 Boxplots of absolute lens rotation from operative to postoperative 1 day and 1 week visits are shown in Figure 2A and 2B, respectively. For both studies, there was no clinically significant change in mean absolute rotation between 1-day and 1-week postoperative. There was also no apparent difference in mean absolute rotation between IOL designs or between subjects of different race or ethnicity.
 |
Table 3 Mean Absolute Lens Rotation (°, Degrees) at the Postoperative 1-Day and 1-Week Visits |
 |
Figure 2 Boxplots of absolute IOL rotation in degrees (°) from operative to post-operative (A) 1-day and (B) 1-week visits. Abbreviation: IOL, intraocular lens. |
Frequency of IOL Rotation
For the TECNIS Toric II IOL, the percentage of eyes with ≤5° of rotation from the intended IOL axis of orientation at the 1-day and 1-week visits was 98.9% (187/189) and 99.5% (188/189), respectively (Table 3). There were zero eyes showing ≥10° of IOL rotation. For the Prototype 2 non-toric monofocal IOL from the earlier POC study, 100% of eyes showed ≤5° of IOL rotation at the 1-day and 1-week postoperative visits.14 The distribution of IOL rotation by degrees is shown in Figures 3A and 3B for the 1-day and 1-week postoperative visits, respectively.
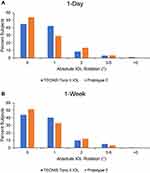 |
Figure 3 Absolute IOL rotation by degree (°) using photographic method at postoperative (A) 1-day and (B) 1-week visits. Abbreviation: IOL, intraocular lens. |
Direction of IOL Rotation
For both the TECNIS Toric II IOL and the Prototype 2 non-toric monofocal IOL with fiducial axis marks, analysis of the mean signed lens orientation showed a small negative change in orientation over time, indicating a clockwise drift. The mean signed rotation (postoperative minus operative) of the TECNIS Toric II IOL was 0.23° ± 1.27° at 1-day (P = 0.015) and −0.07° ± 1.25° at 1-week (P = 0.442). The mean signed rotation of the Prototype version 2 was not significantly different from zero at 1-day postoperative (0.07° ± 1.05°); however, was significantly lower than zero at 1-week postoperative (−0.23° ± 0.97°, 1-sample t-test, P = 0.03).14 As the magnitude of the mean signed rotation was small (less than 1°) for both lens types, these changes were not considered to be clinically meaningful.
IOL Rotation and Axial Length
A scatterplot of absolute IOL rotation with the TECNIS Toric II IOL versus axial length at postoperative 1-week is shown in Figure 4. Linear regression analysis showed no correlation between IOL rotation and axial length (R2 = 0.003, P = 0.42). Similar results were previously reported for the Prototype 2 non-toric monofocal IOL at 1-week (R2 = 0.002; P = 0.67).14
 |
Figure 4 Scatterplot of absolute IOL rotation by degree (°) at postoperative 1-week versus axial length (millimeters, mm) for the TECNIS Toric II IOL. Abbreviation: IOL, intraocular lens. |
Visual Acuity and Refraction
At the 1-day visit, mean monocular UCDVA with the TECNIS Toric II IOL was 0.09 ± 0.22 logMAR (~20/24). At the 1-week visit, mean monocular UCDVA and BCDVA were 0.03 ± 0.14 (~20/21) and −0.06 ± 0.10 (~20/17) logMAR for all study eyes, respectively. Frequencies of UCDVA and BCDVA at the 1-week visit are shown in Figure 5.
At the 1-week visit, the mean residual manifest refractive cylinder for all eyes was 0.30 D ± 0.35 D and the mean manifest refractive spherical equivalent for all eyes was 0.24 D ± 0.44 D. The mean difference between the manifest spherical equivalent at the 1-week postoperative visit and target spherical equivalent for all eyes was 0.04 D ± 0.43 D. The percentage of implanted eyes within 0.50 D and 1.00 D of intended cylinder were 84.0% and 98.5%, respectively.
Surgeon Satisfaction Questionnaire
At the 1-week visit, surgeons were very satisfied or satisfied with the overall clinical outcomes and rotational stability of the TECNIS Toric II IOL in 98.0% of all eyes. High rates of satisfaction were also recorded for UCDVA, with surgeons indicating they were very satisfied or satisfied with the UCDVA achieved following surgery in 97.5% of cases.
Serious and Device-Related Adverse Events
Two subjects experienced non-ocular serious adverse events in this study, for which hospitalization was required. Both were categorized as non-device related and resolved without sequelae. There were no instances of IOL repositioning and/or IOL exchange due to IOL misalignment.
Discussion
The TECNIS Toric II IOL (Model ZCU) demonstrated minimal postoperative rotation following implantation, which was reproducible between studies over the full dioptric power range. Mean absolute lens rotation was less than 1° at both the 1-day and 1-week visits, which was less than previously reported for the TECNIS Toric IOL (1° to 8.38°),4,9,10,12,17–19 confirming that the squared and frosted haptic design improved rotational stability. These data compared favorably with absolute rotation reported for other toric IOL models, including with the AcrySof Toric IOL (Alcon Laboratories, Inc., Fort Worth, TX, USA) (1.5° to 8.0°),5,9,18,20–24 the enVista Toric IOL (Bausch+Lomb, Inc. Rochester, NY, USA) (1.11° to 3.85°),25,26 and the HOYA XY-1 (HOYA, Tokyo, Japan) (5.43°).18
The squared and frosted haptic design of the TECNIS Toric II IOL reduces IOL rotation by increasing the static friction between the frosted haptic arms and the capsular bag.14,27 The HOYA 355 toric IOL (HOYA, Tokyo, Japan) was recently modified to the HOYA XY-1 toric IOL model, which included design changes such as introducing texture to the haptic surface and increasing the overall length.18 Osawa et al reported significantly reduced axis misalignment at 1-day with the HOYA XY-1 toric IOL (5.43° ± 4.67°) compared to the HOYA 355 (8.78° ± 6.54°). Presumably, the increased friction from the textured haptic contributed to this reduction.18
Only 1.1% of eyes at postoperative 1-day and 0.5% of eyes at postoperative 1-week showed >5° axis misalignment, with no surgical issues or complications observed in these eyes. No subjects showed >10° misalignment. These results were consistent with those for the Prototype 2 IOL with the same haptic design evaluated in the earlier POC study.14 Takaku et al reported that frequency of axis misalignment >10° was significantly reduced with the TECNIS Toric II IOL (1/61 eyes) compared to the TECNIS toric IOL (11/70 eyes) 3 months after surgery.27 The single eye with rotation >10° reported by Takaku et al may have been the result of using a test method with lower precision that the method used in this study. In comparison to the rotation data presented for the TECNIS Toric II IOL, Zhang et al reported that 31.7% and 5% of subjects implanted with the AcrySof Toric IOL showed >5° and >10° axis misalignment, respectively, at 1 week after surgery.22
For both the TECNIS Toric II IOL and the Prototype 2 non-toric monofocal IOL, analysis of the mean signed lens orientation showed a small negative change in orientation over time, indicating a clockwise drift (−0.07° with TECNIS Toric II IOL and −0.23° with Prototype 2 non-toric monofocal IOL at the 1-week visit). These findings were opposite to previous reports of counterclockwise rotation8,9,28 but consistent with two studies reporting clockwise rotation.10,11 However, given the small magnitude of mean clockwise rotation observed, these results were not considered to be clinically meaningful.
In the POC study of Prototype 2, Vukich et al reported no significant association between IOL rotation and axial length.14 This post-market study included subjects with longer axial lengths (range 20.67 to 29.52 mm, compared to a 21.75 to 25.93 with Prototype 2) and a similar outcome was observed. Shah et al previously reported significantly greater toric IOL rotation of the AcrySof Toric IOL with longer axial lengths, in subjects with a similar range of axial lengths (19.5 to 29.0 mm) to this study.20 Stability of the TECNIS Toric II IOL does not appear to be impacted by axial length.
Low residual refractive astigmatism (0.30 D ± 0.35 D at 1-week), excellent UCDVA (monocular 0.03 ± 0.14 [~20/21] and binocular −0.06 ± 0.10 [~20/17] logMAR) and very high rates of surgeon satisfaction were achieved with the TECNIS Toric II IOL, demonstrating the benefits of this toric lens design. Takaku et al also showed that the TECNIS Toric II IOL significantly reduced axis misalignment and residual manifest astigmatism, and improved UCDVA, compared to the TECNIS Toric 1-piece monofocal IOL.27
The POC study conducted with the prototype non-toric monofocal IOL14 was able to predict the clinical performance of the toric IOL in this study, as supported by both the rotation and visual acuity data. Both IOLs featured the same haptic design and overall geometry; the only difference was that the prototype design from the POC study had a non-toric monofocal optic (ie, provided defocus correction only). In terms of clinical development of toric IOLs, utilizing POC studies on IOLs with non-toric optics has the advantage of allowing evaluation of several prototype designs in a timely manner.
In the POC study, which was conducted in Asia Pacific and Latin America, the majority of subjects were Asian (43.9%) or Hispanic/Latino (57.1%). The current post-market study was conducted in the US, with almost all subjects being Caucasian (89.6%). To date, there have been no published studies evaluating the effect of race or ethnicity on rotational stability of toric IOLs. However, the consistency of the rotational stability results between the POC and post-market study suggest that race/ethnicity do not impact rotation for this model IOL and results from different races/ethnicities and regions around the world are generalizable across populations.
Conclusions
In the previous POC study, the Prototype 2 non-toric monofocal IOL with modified haptics demonstrated excellent rotational outcomes and was selected for commercialization as the TECNIS® Toric II IOL. In this post-market study, the TECNIS Toric II IOL (Model ZCU) demonstrated consistent and reproducible rotational outcomes over the full dioptric power range. Excellent visual acuity, minimal residual astigmatism and high surgeon satisfaction were achieved. The POC study design was supported, demonstrating that prototype non-toric monofocal IOLs can predict clinical performance of toric IOLs with the same haptic design, accelerating clinical development of toric IOLs.
Data Sharing Statement
The authors do not intend to share individual deidentified participant data. A summarized report with endpoints data tables based on statistical plan and analysis may be requested directly from the corresponding author for consideration. Access to anonymized data may be granted following review. Content with granted access will be available through email or other appropriate formats and for 3 months, upon review and consideration.
Acknowledgments
Manuscript preparation and editorial support provided were by Dr Carol Lakkis, iBiomedical Consulting (Neptune Beach, FL) and funded by Johnson & Johnson Vision Care, Inc. The following US-based surgeons participated in this clinical study: Jerry Hu, MD (Texas Eye & Laser Center, Hurst, TX), Daniel Chang, MD (Empire Eye & Laser Center, Bakersfield, CA), Jason Jones, MD (Jones Eye Clinic, Sioux City, IA), Michael Snyder, MD (Cincinnati Eye Institute, Cincinnati, OH), Jeffrey Whitman, MD (Key-Whitman Eye Center, Dallas TX), Kevin Miller, MD (The Regents of the University of California on behalf of its Los Angeles Campus UCLA Clinical Trials Contracts and Strategic Relations, Los Angeles, CA), Michael Greenwood, MD (Vance Thompson ND, Prof. LLC, West Fargo, ND).
Funding
This study was supported by Johnson & Johnson Surgical Vision, Inc., which participated in the design and conduct of the study.
Disclosure
The authors have made the following disclosures:
G.A.Q.: Consultant for Johnson & Johnson Surgical Vision, Zeiss, Alcon and Aurion Biotech
R.A.Q.: Consultant for Johnson & Johnson Surgical Vision, Zeiss, Alcon and Aurion Biotech
J.J.J.: Performs research supported by, speaks for and is consultant to Johnson & Johnson Surgical Vision
B.J.K.S.: Employee of Johnson & Johnson Vision Care
W.Z.: Employee of Johnson & Johnson Surgical Vision
L.T.: Employee of Johnson & Johnson Surgical Vision
S.V.: Employee of Johnson & Johnson Surgical Vision
The authors report no other conflicts of interest in this work.
References
1. Hoffmann PC, Hutz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J Cataract Refract Surg. 2010;36:1479–1485. doi:10.1016/j.jcrs.2010.02.025
2. Kessel L, Andresen J, Tendal B, et al. Toric intraocular lenses in the correction of astigmatism during cataract surgery: a systematic review and meta-analysis. Ophthalmology. 2016;123:275–286. doi:10.1016/j.ophtha.2015.10.002
3. Mencucci R, Favuzza E, Guerra F, et al. Clinical outcomes and rotational stability of a 4-haptic toric intraocular lens in myopic eyes. J Cataract Refract Surg. 2014;40:1479–1487. doi:10.1016/j.jcrs.2013.12.024
4. Inoue Y, Takehara H, Oshika T. Axis misalignment of toric intraocular lens: placement error and postoperative rotation. Ophthalmology. 2017;124:1424–1425. doi:10.1016/j.ophtha.2017.05.025
5. Miyake T, Kamiya K, Amano R, et al. Long-term clinical outcomes of toric intraocular lens implantation in cataract cases with preexisting astigmatism. J Cataract Refract Surg. 2014;40:1654–1660. doi:10.1016/j.jcrs.2014.01.044
6. Schartmuller D, Schriefl S, Schwarzenbacher L, et al. True rotational stability of a single-piece hydrophobic intraocular lens. Br J Ophthalmol. 2019;103:186–190. doi:10.1136/bjophthalmol-2017-311797
7. Visser N, Bauer NJ, Nuijts RM. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg. 2013;39:624–637. doi:10.1016/j.jcrs.2013.02.020
8. Potvin R, Kramer BA, Hardten DR, Berdahl JP. Toric intraocular lens orientation and residual refractive astigmatism: an analysis. Clin Ophthalmol. 2016;10:1829–1836. doi:10.2147/OPTH.S114118
9. Lee BS, Chang DF. Comparison of the rotational stability of two toric intraocular lenses in 1273 consecutive eyes. Ophthalmology. 2018;125:1325–1331. doi:10.1016/j.ophtha.2018.02.012
10. Li S, Li X, He S, et al. Early postoperative rotational stability and its related factors of a single-piece acrylic toric intraocular lens. Eye. 2020;34:474–479. doi:10.1038/s41433-019-0521-0
11. Yang JJ, Qin YZ, Qin L, Li JM. Comparison of the clinical efficacy of acrysof((r)) iq and tecnis((r)) toric intraocular lenses: a real-world study. Exp Ther Med. 2020;20:25. doi:10.3892/etm.2020.9153
12. He S, Chen X, Wu X, et al. Early-stage clinical outcomes and rotational stability of tecnis toric intraocular lens implantation in cataract cases with long axial length. BMC Ophthalmol. 2020;20:204. doi:10.1186/s12886-020-01465-2
13. Oshika T, Fujita Y, Hirota A, et al. Comparison of incidence of repositioning surgery to correct misalignment with three toric intraocular lenses. Eur J Ophthalmol. 2020;30:680–684. doi:10.1177/1120672119834469
14. Vukich JA, Ang RE, Straker BJK, et al. Evaluation of intraocular lens rotational stability in a multicenter clinical trial. Clin Ophthalmol. 2021;15:3001–3016. doi:10.2147/OPTH.S309214
15. Johnson & Johnson Surgical Vision, Inc. TECNIS® Toric II 1-Piece IOL directions for use. Available from: https://www.jnjvisionpro.com/sites/us/files/public/Products/Tecnis%20Toric%20II/z311395_02_final.pdf.
16. Kasthurirangan S, Feuchter L, Smith P, Nixon D. Software-based evaluation of toric IOL orientation in a multicenter clinical study. J Refract Surg. 2014;30:820–826. doi:10.3928/1081597X-20141117-01
17. Lubinski W, Kazmierczak B, Gronkowska-Serafin J, Podboraczynska-Jodko K. Clinical outcomes after uncomplicated cataract surgery with implantation of the tecnis toric intraocular lens. J Ophthalmol. 2016;2016:3257217. doi:10.1155/2016/3257217
18. Osawa R, Oshika T, Sano M, et al. Rotational stability of modified toric intraocular lens. PLoS One. 2021;16:e0247844. doi:10.1371/journal.pone.0247844
19. Brar S, Rathod DP, Nikhil RP, Ganesh S. Clinical outcomes, predictability and rotational stability following implantation of eyecryl toric versus tecnis toric intraocular lenses-A comparative study. Int Ophthalmol. 2021;41:3769–3780. doi:10.1007/s10792-021-01944-5
20. Shah GD, Praveen MR, Vasavada AR, et al. Rotational stability of a toric intraocular lens: influence of axial length and alignment in the capsular bag. J Cataract Refract Surg. 2012;38:54–59. doi:10.1016/j.jcrs.2011.08.028
21. Zhu X, Meng J, He W, et al. Comparison of the rotational stability between plate-haptic toric and c-loop haptic toric IOLs in myopic eyes. J Cataract Refract Surg. 2020;46:1353–1359. doi:10.1097/j.jcrs.0000000000000259
22. Zhang Z, Li H, Zhou J, et al. Clinical evaluation of toric intraocular lens implantation based on itrace wavefront keratometric astigmatism. BMC Ophthalmol. 2020;20:450. doi:10.1186/s12886-020-01726-0
23. Qiu X, Shi Y, Han X, et al. Toric intraocular lens implantation in the correction of moderate-to-high corneal astigmatism in cataract patients: clinical efficacy and safety. J Ophthalmol. 2021;2021:5960328. doi:10.1155/2021/5960328
24. Sasaki K, Eguchi S, Miyata A, et al. Anterior capsule coverage and rotational stability of an acrylic toric intraocular lens. J Cataract Refract Surg. 2021;47:618–621. doi:10.1097/j.jcrs.0000000000000489
25. Packer M, Williams JI, Feinerman G, Hope RS. Prospective multicenter clinical trial to evaluate the safety and effectiveness of a new glistening-free one-piece acrylic toric intraocular lens. Clin Ophthalmol. 2018;12:1031–1039. doi:10.2147/OPTH.S167726
26. Vokrojova M, Havlickova L, Brozkova M, Hlinomazova Z. Effect of capsular tension ring implantation on postoperative rotational stability of a toric intraocular lens. J Refract Surg. 2020;36:186–192. doi:10.3928/1081597X-20200120-01
27. Takaku R, Nakano S, Iida M, Oshika T. Influence of frosted haptics on rotational stability of toric intraocular lenses. Sci Rep. 2021;11:15099. doi:10.1038/s41598-021-94293-3
28. Kramer BA, Hardten DR, Berdahl JP. Rotation characteristics of three toric monofocal intraocular lenses. Clin Ophthalmol. 2020;14:4379–4384. doi:10.2147/OPTH.S285818
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

