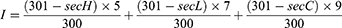Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Reparative Effects of Dandelion Fermentation Broth on UVB-Induced Skin Inflammation
Authors Zhang Y, Fu H, Zhang Y, Wang D, Zhao D , Zhang J , Li M , Wang C
Received 27 November 2021
Accepted for publication 9 March 2022
Published 15 March 2022 Volume 2022:15 Pages 471—482
DOI https://doi.org/10.2147/CCID.S351527
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yuzhi Zhang,1,2 Hao Fu,1,2 Yongtao Zhang,1,2 Dongdong Wang,1,2 Dan Zhao,1– 3 Jiachan Zhang,1– 3 Meng Li,1,2 Changtao Wang1– 3
1Beijing Key Lab of Plant Resource Research and Development, College of Chemistry and Materials Engineering, Beijing Technology and Business University, Beijing, People’s Republic of China; 2Institute of Cosmetic Regulatory Science, Beijing Technology and Business University, Beijing, People’s Republic of China; 3Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University, Beijing, People’s Republic of China
Correspondence: Meng Li; Changtao Wang, Beijing Technology and Business University, Beijing, 100048, People’s Republic of China, Tel +86 10-68984917, Fax +86 10-68984917, Email [email protected]; [email protected]
Objective: To evaluate the efficacy of the dandelion fermentation broth in repairing UVB-induced skin inflammation.
Methods: Detection of active ingredients in dandelion fermentation broth and water extract. The antioxidant capacity of dandelion fermentation broth was investigated by in vitro antioxidant experiments. The influence of the broth on the content of inflammatory factors interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-1β (IL-1β), and tumor necrosis factor (TNF-α), in human immortalized epidermal cells (HaCaT) is discussed on the basis of a UVB-induced HaCaT damage model. The effects of the broth on the contents of skin barrier-related proteins kallikrein-7 (KLK-7), filaggrin (FLG) and aquaporin (AQP3) in the UVB-induced damage and repair of the HaCaT mechanism are also comprehensively discussed. The effect of DF on the activation of MAPK pathway proteins was detected by PCR. A chicken embryo chorioallantoic membrane test is used to explore the safety of the dandelion fermentation broth.
Results: The results show that the dandelion fermentation broth is rich inTotal sugar, with good free radical scavenging ability and antioxidant effects; it can regulate the MAPK pathway, reduce the expression of inflammatory factors, adjust the skin barrier factors and good safety.
Conclusion: Dandelion fermentation broth exhibits repairing effect on UVB-induced skin inflammation.
Keywords: dandelion fermentation broth, ultraviolet radiation B, human immortalized epidermal cells, inflammation repair, medicine, daily chemical raw materials
Introduction
Dandelion (Taraxacum mongolicum Hand.-Mazz.), also known as Huanghua Diding, Granny Ding, etc., is a perennial herb of the Compositae family which is mainly distributed in North, Northeast, Northwest and Southwest China.1 Dandelion is rich in chemical components, among which polysaccharides, flavonoids, polyphenols, steroidal terpenoids, alcohols and other active components have been widely used in anti-tumor, anti-inflammation, anti-infection and other aspects.2–6 With advantages including good safety, dandelion is widely used in the fields of food, medicine and health care products under the above applications, and dandelion extraction methods are numerous, including traditional water extraction, ultrasonic extraction, alcohol extraction and so on.7
Microbial fermentation technology is a modern form of fermentation technology based on traditional processing methods, which has the functions of protecting the activity of traditional Chinese medicine from being destroyed, and modifying the structures of toxic ingredients. Research shows that compared with other extraction methods, using microorganism fermentation to extract active ingredients from natural raw materials such as plants results in active extracts with fewer side effects and greater effectiveness; moreover, the fermentation process can be used to produce new active substances, and the mild microorganism fermentation conditions can protect the active ingredients to a certain extent.8,9
An increasing number of studies have reported the application of fermentation to achieve reduced toxicity and increased efficiency. Lihua Zheng et al10 found that when the fermentation method is applied to gallnut, the use of Rhizopus can promote the production of L-lysine and improve the astringent effects of the gallnut. Pan et al11 studied the toxic effects of fermented croton through an acute toxicity test, inflammation and hemolysis, and found that fermentation treatment reduced the toxicity of croton.
The skin provides an effective barrier between an organism and the environment by helping to reduce the risk of physicochemical and microbiological damage. Ultraviolet radiation will damage the skin’s barrier function and cause dry skin, desquamate, excessive reactive oxygen species (ROS) production and so on, leading to skin inflammation.12–16 Because FLG, KLK7 and AQP3 each play an important role in maintaining the barrier function of the skin, TNF-α, IL-1β and IL-8 are the hallmark inflammatory factors of inflamed skin.As a downstream pathway from the cell to the cell and even the nucleus, MAPK plays a key role in the Development of inflammatory responses. Therefore, the regulation of FLG, KLK7, AQP3, TNF-α, IL-1β, IL-8 and MAPK pathway can indirectly reflect the degree of skin inflammation.
The anti-inflammatory effects of dandelion extract have been previously reported.17 However, the role of dandelion fermentation broth obtained by lactic acid bacteria in a UVB-induced cell inflammation model has not been reported. This study shows that the efficacy of dandelion fermentation broth is superior to that of dandelion water extract. Through the determination of its antioxidant capacity, skin barrier repair ability, ROS regulation ability, inflammatory factor regulation ability and other aspects, dandelion fermentation broth may become a safe and effective medicine and daily chemical raw material for the treatment of UVB-induced skin inflammation in the future.
Materials and Methods
Materials
Dandelion, Beijing Tongrentang Group; Lactplantibacillus plantarum, CICC-20261, China Center of Industrial Culture Collection; Human immortal keratinocyte (HaCaT), Cell Resource Centre, Beijing Union Medical College, China; Folin reagent, copper sulfate, anhydrous ethanol, rutin standard, sodium nitrate, Sinopharm Group; CCK8 kit, Biorigin; Shanghai Yiheng Technology Co., Ltd.; DMEM medium, pancreatin, PBS buffer, total antioxidant capacity test kit, Beyotime; TNF-α, IL-8, IL-1β, KLK-7, FLG and AQP3 ELISA kits, Nanjing Jiancheng Institute of Bioengineering; microplate reader, cell incubator, Shanghai Yiheng; 6-well plate, 96-well plate, Corning; UV ultraviolet light box, refrigerated high-speed centrifuge, Hunan Xiangyi Laboratory Instrument Development Co., Ltd.; Cell incubator, Shanghai Yiheng Technology Co., Ltd.
Preparation of Dandelion Fermentation Broth
Dandelion is pulverized with a high-speed multifunctional pulverizer and sieved. Purified water is added to the conical flask at a material-to-liquid ratio of 1:20. The solution is sealed and autoclaved, then cooled to room temperature. And treated with 5% Lactplantibacillus plantarum (The bacteria were cultured in MRS medium for 48 hours, shaken, and the absorbance at 600 nm is measured, and the absorbance is between 1–1.2.). It is then cultured in a shaker at 28°C and 180 r/min for 48 h, and centrifuged at 4800 r/min for 30 min to obtain the supernatant, which is the dandelion fermentation broth (DF).
Dandelion water extract (blank control) (DW): The method is as above, without lactic acid bacteria.
Determination of DF Content
2.3.1 Total sugar: According to the instructions of the total sugar kit, the standard is diluted with distilled water to 1.5, 1, 0.8, 0.6, 0.5, 0.4, 0.2 and 0.1 mg/mL, then 30 μL of Reagent III is added and it is mixed well and boiled in a water bath for 10 min (tightly covered to prevent water loss). It is then cooled to room temperature, supplemented with 180 μL of distilled water and mixed well. A sample of 200 μL is taken and the absorbance is measured at 540 nm. A standard curve is drawn with the concentration of the standard tube as the x-axis and the corresponding absorbance difference as the y-axis to obtain the standard equation y=kx+b. The absorbance difference measurement is added to the equation to calculate x (mg/mL). Sample measurement: A sample of 30 μL is taken, supplemented with 30 μL of Reagent III, mixed well and boiled in a water bath for 10 min (tightly covered to prevent water loss). It is then cooled to room temperature, supplemented with 180 μL of distilled water and mixed well. A sample of 200 μL is taken and the absorbance is measured at 540 nm.
2.3.2 Total phenols: The content of polyphenols is determined via the Folin method. A standard curve is established with gallic acid as a positive control. The regression equation is C=0.8591A+0.0793, R2=0.9954, where C is the total phenol content (mg/mL) in the sample and A is the absorbance of the sample.18
2.3.3 Total flavonoids: The NaNO2-AL(NO)3-NaOH colorimetric method is used to determine the content of flavonoids. Rutin is used as a positive control to establish a standard curve. The regression equation is C=0.8554A+0.0006, R2=0.992, where C is the total flavonoid content (mg/mL) in the sample and A is the absorbance of the sample.18
Determination of DF Antioxidant Activity
DPPH Free Radical Scavenging Experiment
DPPH solution (2×10−4 moL/L): 20 mg of DPPH is accurately weighed, dissolved in anhydrous ethanol solution and diluted to 250 mL.
The reaction solution is added to the test tube according to the order and added content in Table 1. Dandelion fermentation broth is referred to as the sample, diluted with water to obtain different concentrations. It is then mixed well, and the absorbance is measured at a wavelength of 517 nm in the microplate reader.19
 |
Table 1 Reagent Ratios |
Calculation of the inhibition rate of measuring solution to DPPH free radicals:
Inhibition rate = [1-(A0-A2)/A1]×100%
A1: Absorbance of DPPH free radicals when the measuring solution is not added.
A2: Absorbance of the measuring solution at the measuring wavelength.
A0: Absorbance of DPPH free radicals after adding the measuring solution.
Hydroxyl Radical Scavenging Experiment
A 0.5 mL sample of 6 mmoL/L ferrous sulfate solution is taken in a test tube, then 0.5 mL of sample and 0.5 mL of 6 mmoL/L H2O2 are added (the H2O2 needs to be added last to start the whole reaction). After mixing thoroughly, it is allowed to stand at room temperature for 10 min before 0.5 mL of 6 mmoL/L salicylic acid is added, and it is placed in a 37°C water bath for 30 min. The absorbance is measured at a wavelength of 510 nm in the microplate reader, and the obtained data is the absorbance value A1 of the sample tube. In the blank tube, 0.5 mL of distilled water is used to replace the 0.5 mL sample in the sample tube. The operation method is the same as that of the sample tube, and the absorbance value A2 of the blank tube is measured. The background value tube replaces 0.5 mL of salicylic acid in the sample tube with 0.5 mL of distilled water. The operation method is the same as that of the sample tube, and the absorbance value A3 of the sample tube is measured. This is conducted three times in parallel for each concentration.20
Hydroxyl radical scavenging rate/%= (A2+A3-A1) /A2×100
Determination of Total Antioxidant Capacity (ABTS Method)
Standard curve drawing: The standard is diluted with a solution prepared with distilled water. 10 mM of TroLox standard solution is diluted to 0.15, 0.3, 0.6, 0.9, 1.2 and 1.5 mM.
Sample measurement: 200 mL of ABTS working solution is added to each detection hole of a 96-well plate, and 10 mL of distilled water, PBS or other appropriate solution is added to the blank control well; 10 mL of Trolox standard of various concentrations is added to the standard curve detection wells, and 10 mL of various samples is added into the sample detection holes. After being gently mixed and incubated for 2–6 minutes, A734 is measured.21
Determination of Active Oxygen Content
HaCaT cells with good logarithmic growth were diluted with 2 mL DMEM complete culture medium to obtain cell suspension, and a blank control, model group and sample group are established. The number of cells per well are controlled to 300,000. After being cultured in a 37°C and 5% CO2 incubator for 12 hours, the medium is discarded and switched to serum-free DMEM. After being cultured in a 37°C and 5% CO2 incubator for 12 hours, the medium is discarded and a small amount of PBS (pH=7.4) is added. It is better just to cover the cells. The cells are then stimulated with UVB, while the blank group is not irradiated. Aspirate the PBS and add samples of different concentrations diluted in serum-free medium to the cells and incubate for 24 hours. 1:1000 is added to dilute 1 mL of DCFH-DA in a serum-free culture medium, then incubated in a 37°C cell incubator for 20 min, inverted every 3–5 minutes. The cells are washed three times with a serum-free cell culture medium to fully remove the DCFH-DA that has not entered the cells. The cell culture medium is then discarded, 1 mL of PBS is added, and pictures are taken.
Determination of DF Anti-Inflammatory Activity
Cell Viability
HaCaT cells with a good logarithmic growth phase are seeded in a 96-well culture plate and cultured overnight at 37°C and 5% CO2. The culture medium is aspirated and samples of different concentrations are added, with six parallels for each sample. The cell control group is cultured for 24 hours, then the culture medium is aspirated and 100 μL of serum-free DMEM and 10 μL of CCK8 are added to each well after washing with PBS. They are then cultured for 2–4 hours, and the absorbance value of each well is measured at a wavelength of 450 nm.
Cell viability = (measurement well OD value-blank control OD value)/(cell control OD value-blank control OD value) * 100%.
Inflammatory Factors
Enzyme-linked immunosorbent assay (ELISA) is used to detect the concentration of inflammatory HaCaT cytokines TNF-α, IL-8 and IL-1β. HaCaT cells with a good logarithmic growth phase are counted and treated in a 6-well culture plate. The number of cells in each well is about 5×105. They are then incubated overnight at 37 °C and 5% CO2, the medium is discarded and 1 mL of PBS is added. After a certain amount of UVB irradiation, the PBS is aspirated and samples are added to the cells for 24 hours (the blank control group is treated with a serum-free DMEM medium). They are aspirated again and the liquid is discarded, then washed with PBS, scraped with a cell scraper, collected in a centrifuge tube and centrifuged. The supernatant is then discarded to obtain cell pellets. 200 μL of lysis buffer is added to lyse the cells, and they are centrifuged at 4°C for 5 min to obtain the cell lysis supernatant for the determination of inflammatory factors. Refer to the manufacturer’s instructions for specific measurement methods.
Skin Barrier-Related Factors
As in 2.5.2, enzyme-linked immunosorbent assay (ELISA) is used to detect the concentration of inflammatory HaCaT cytokines KLK-7, FLG, and AQP3. Refer to the manufacturer’s instructions for specific measurement methods.
MAPK Pathway
Cell Culture and Extraction and Transcription of Total RNA
As in 2.5.2, cells from blank group, model group and sample group were collected.Follow the instructions of Trizol (Total RNA Extraction Reagent) to extract RNA, and store the extracted RNA in a refrigerator at −80 °C. The cDNA first-strand synthesis reaction was performed using EasyScript ® One-Step gDNA Removal and cDNA Synthesis SuperMix Reverse Transcription Kit. The reaction system is shown in Table 2.
 |
Table 2 Reverse Transcription System |
The reverse transcription system was mixed thoroughly, centrifuged briefly, and incubated at 42°C for 15 min, and heated at 85°C for 5 s to complete the reverse transcription. Fill up to 100 uL with double distilled water.
Design and Synthesis of Primers and Probes
Through the gene sequence obtained from NCBI, specific primers for 4 genes (including the housekeeping gene GADPH (internal reference gene)) were designed with Primer Express software. The primer sequences are shown in Table 3.
 |
Table 3 Primer Sequences for Real-Time PCR |
qPCR Detection
Perform qPCR operations according to the TransStart® Top Green qPCR SuperMix kit instructions. The total reaction system is shown in Table 4.
 |
Table 4 Real-Time PCR System |
Refer to the kit instructions to set the reaction conditions, a total of 40 cycles, 72 °C to collect fluorescence data. The reactants were mixed and then carried out on the QuantStudio3 fluorescent quantitative PCR instrument.
Safety Determination of DF
Chicken embryo chorioallantoic membrane test (HET-CAM): The allantoic membrane of 9-day-old chicken embryos is exposed, 0.3 mL of positive (0.1mol/L NaOH), negative control (0.9% NaCl), DF and DW are dropped directly on the surface of the CAM film to observe the CAM reaction, and the times of bleeding, coagulation and vascular dissolution are recorded within 5 minutes. The sample is tested with six chicken embryos and the reaction time method is used for the test. The calculation formula of the stimulus score (IS) is:
In this formula, the average time for the blood vessels to melt in the allantoic membrane after sample addition is the vascular melting time (SecL); the average time for bleeding in the allantoic membrane after sample addition is the bleeding time (SecH); and the average time for clotting to appear in the allantoic membrane after sample addition is the clotting time (SecC). The eye irritation of the tested substance is classified according to IS value in Table 5.
 |
Table 5 Evaluation Results of Stimulus Scoring Method |
Data Analysis
Excel is used for the experimental data statistics, GraphPad Prism 8 is used for graphing and the significance t-test is performed using IBM SPSS Statistics 22, with each experiment performed three times.
Results
Determination of DF Content
As shown in Table 6, the total sugars, phenols and flavonoids in DF and DW are determined. It can be seen from the table that the total phenols and flavonoids contents in DF and DW are not very different, but the total sugars in DF are significantly higher than those in DW (P<0.05), with the total sugar content as high as 17.57 mg/mL. The experimental results show that Lactplantibacillus plantarum fermentation can increase the content of total sugar, the primary active substance in the product, thereby improving the efficacy.
 |
Table 6 Contents of Active Ingredients in DF |
Determination of DF Antioxidant Activity
DPPH Free Radical Scavenging Experiment
The scavenging effect of DF on DPPH free radicals is shown in Figure 1. It can be seen that DF and DW have scavenging effects on DPPH free radicals and similar scavenging rates. Before the volume fraction of 5%, the scavenging rate increases exponentially with the increase of the sample volume fraction, then increases slowly with the increase of the volume fraction. When the volume fraction is 10%, the scavenging rate of DF on DPPH free radicals reaches above 90%. The IC50 of DF to scavenge DPPH free radicals is 1.05%, while that of DW is 1.09%, showing that DF has a better scavenging effect on DPPH free radicals.
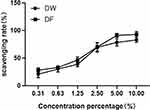 |
Figure 1 Scavenging effects of DW and DF on DPPH free radicals. |
Hydroxyl Radical Scavenging Experiment
The scavenging effect of DF on hydroxyl radicals is shown in Figure 2. It can be seen that DF and DW have scavenging effects on hydroxyl radicals. As the volume fraction of the sample increases, the clearance rate continues to increase. The IC50 of DF to scavenge hydroxyl free radicals is 36.3%, while that of DW is 37.42%, showing that DF has a better scavenging effect on hydroxyl free radicals.
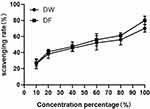 |
Figure 2 Scavenging effects of DW and DF on hydroxyl radicals. |
Determination of Total Antioxidant Capacity (ABTS Method)
Figure 3 shows the total antioxidant capacity of DF and DW. It can be seen from Figure 3 that the total antioxidant capacity of DF is greater than that of DW.
 |
Figure 3 Total antioxidant capacity of DW and DF. The student’s t-test was performed to determine statistical significance (*P < 0.05, versus the control group). |
Determination of Active Oxygen Content
The scavenging effect of DF on active oxygen is shown in Figure 4. It can be seen that the blank group has less fluorescence and a lower active oxygen content. After UVB irradiation, the active oxygen content increases significantly. After adding DW and DF, both have an obvious active oxygen scavenging effect, and that of DF is better.
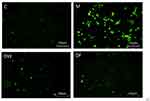 |
Figure 4 Scavenging effects of DW and DF on active oxygen (C: Control group; M: UVB irradiation model group). |
Determination of Anti-Inflammatory Activity of DF
Cell Viability
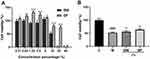
Figure 5A shows the effect of DF on the survival rate of HaCaT cells. It can be seen that the toxicity of DF on HaCaT cells is less than that of DW. The relative survival rate of HaCaT cells after culturing HaCaT cells in DF before the volume fraction of 5% is above 100%, showing that the fermentation of lactic acid bacteria can reduce the toxicity of the product on cells. Considering the cell growth status and cost issues, 2% DF and DW were selected for the next experiment.
Figure 5B shows that after UVB irradiation, the cells are damaged to a certain extent. After 24 hours of adding 2% DW and DF, the cell viability increases, indicating that DF has better reparative effects on cells.
Inflammatory Factors
Figure 6 shows the relative expression changes of TNF-α, IL-1β and IL-8 in HaCaT cells after treatment with DF. It can be seen that after UVB irradiation of HaCaT cells, the inflammatory factors are significantly increased, and DF can significantly reduce the expression of inflammatory factors to a higher reduction degree than that of DW. TNF-α, IL-1β and IL-8 are inflammatory factors whose expression increases sharply when skin inflammation occurs. The above results indicate that the inflammation reduction effect of DF is better than that of DW.
Skin Barrier Factor
Figure 7 shows the relative expression changes of skin barrier factors KLK-7, FLG and AQP3 in HaCaT cells after treatment with DF. It can be seen that after HaCaT cells are irradiated with UVB, KLK-7 is significantly increased, while FLG and AQP3 are significantly decreased. DF can significantly reduce the expression of KLK-7 with a better reduction effect than that of DW. DF also significantly increases the expression of FLG and AQP3 with a higher degree of improvement than that of DW. These results indicate that DF can repair skin inflammation more effectively than DW.
MAPK Pathway
As shown in Figure 8, compared with the blank group, UVB irradiation significantly reduced the expression of JNK, ERK, and P38 genes in cells. Compared with the model group, DF can significantly increase the expression of JNK, ERK, and P38 genes in cells. These results indicate that DF had better down-regulation effect on JNK, ERK, and P38 mRNA expression than DW.
Safety Determination of DF
Figure 9 shows irritation to blood vessels.The stimulation score of the positive control is 16.71 points, and obvious vascular hemolysis and strong irritation are shown. The stimulation score of the negative control (NaCl) is 0.04 points, and the blood vessels show no hemolysis. The stimulation scores of the two samples are 0.09 and 0.08 respectively, and the blood vessels show no hemolysis, indicating that neither DW nor DF irritates the eyes.
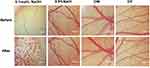 |
Figure 9 Safety determination of DW and DF. |
Discussion
This study has verified in several aspects that DF presents a very safe and effective treatment option for repairing inflammation caused by UVB. In the content determination and free radical experiments, It can be seen that microbial fermentation of dandelion has the effect of scavenging free radicals, and may increase the content of active substances with changes in structure or molecular weight. In the UVB-induced HaCaT inflammation model, radiation stimulates the production of ROS, the increase of inflammatory cytokine levels and the disorder of skin barrier factor levels. As expected, the dandelion extract obtained via the microbial fermentation method effectively reversed these trends.
The data shows that UVB irradiation reduced the expression of AQP3 and FLG-related proteins, and increased the level of KLK-7. Studies have shown that FLG participates in the formation of a stable keratinized protein shell, namely the cornified envelope (CE), which can promote the differentiation of the epidermis. In the stratum corneum, FLG is the main barrier against physical stimuli from the external environment. It is hydrolyzed into amino acids and their derivatives. The amino acids are also known as “hygroscopic free amino acids”. Their derivatives such as pyrrolidone carboxylic acid, uridine acid, etc., are responsible for hydration and water retention in the natural moisturizing factor (NMF).16,17 AQP3 water channel protease is a kind of protease that resides on the skin cell membrane, forming a nutrient channel dedicated to transporting water, controlling the entry and exit of moisture and nutrients in and out of the skin cells, and regulating moisture in the cells.22 Therefore, the low protein expression of FLG and AQP3 accelerates the loss of skin moisture and other forms of damage which affect the skin barrier. Desmoprotein plays a role in connecting and fixing keratinocytes, and strengthening the skin barrier. KLK7 is a member of the serine protease family, which can hydrolyze desmosomal proteins and cause the adhesion between stratum corneum cells to decrease. Therefore, the increase of KLK7 content will further hydrolyze desmosomes and cause skin desquamation.15,16,23 This study shows that DF effectively increases the expression of FLG and AQP3, and reduces the level of KLK-7, thereby playing a key role in repairing the skin barrier function and effectively reducing inflammation.
It has been reported in the literature that UVB-induced ROS production activates mitogen-activated protein kinase (MAPK) signaling and nuclear factor-κB (NF-κB),24 which further induce skin inflammation and may also upregulate reactive oxygen metabolites, participating in the inflammatory response to trigger or amplify inflammation.25,26 As such, maintaining an adequate antioxidant state may be an important way to reduce inflammatory diseases. In this study, by observing the state of cells in the UVB-induced HaCaT inflammation model, it can be seen that UVB irradiation of cells leads to an increase in the content of ROS and a decrease in the content of proteins related to the MAPK pathway. After DF treatment, the cells were repaired to a greater extent, the ROS content was significantly reduced and the protein content related to the MAPK pathway returned to normal.
In recent years, the microbial fermentation method has entered the fields of medicine, daily chemicals, food, etc., due to its advantages of reducing toxicity and increasing the content of active ingredients. R. M. Saleh26 found that the microbial fermentation of ginger increased its gingerol content and enhanced its antioxidant and antibacterial activity. Yang27 found that the fermentation of turmeric by lactic acid bacteria increased its curcumin content, reduced its cytotoxicity and increased its anti-inflammatory effect. In this study, using DW as the sample control (with the same preparation steps as those of DF, but without the use of lactic acid bacteria), it is found that DF can increase the content and effectiveness of the active ingredients. Thus, DF plays a role in repairing UVB-induced skin inflammation in many ways, including preventing oxidation, repairing the skin barrier and regulating inflammatory factors in cells.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Hu C. Taraxacum: phytoChemistry and health benefits. Chin Herb Med. 2018;10(4):353–361. doi:10.1016/j.chmed.2018.08.003
2. Bao T, Ke Y, Wang Y, et al. Taraxasterol suppresses the growth of human liver cancer by upregulating hint1 expression. J Mol Med. 2018;96(7):661–672. doi:10.1007/s00109-018-1652-7
3. Yang Y, Ying G, Wu S, Wu F, Chen Z. In vitro inhibition effects of hepatitis B virus by dandelion and taraxasterol. Infect Agent Cancer. 2020;15(1). doi:10.1186/S13027-020-00309-4
4. San Z, Fu Y, Li W, et al. Protective effect of taraxasterol on acute lung injury induced by lipopolysaccharide in mice. Int Immunopharmacol. 2014;2014(19):342–350. doi:10.1016/j.intimp.2014.01.031
5. Zheng F, Dong X, Meng X. Anti-inflammatory effects of taraxasterol on LPS-stimulated human umbilical vein endothelial cells. Inflammation. 2018;41(5):1755–1761. doi:10.1007/s10753-018-0818-3
6. Che L, Li Y, Song R, et al. Anti-inflammatory and anti-apoptosis activity of taraxasterol in ulcerative colitis in vitro and in vivo. Exp Ther Med. 2019;2019(18):1745–1751.
7. Saini RK, Keum YS. Carotenoid extraction methods: a review of recent developments. Food Chem. 2018;240:90–103. doi:10.1016/j.foodchem.2017.07.099
8. Yang GM, Tu X. Research progress on fermentation engineering to attenuate toxicity and synergize effect of toxic Chinese materia medica. J Food Sci Biotechnol. 2013;32(8):785–792.
9. Suga T, Hirata T. Biotransformation of exogenous substrates by plant cell cultures. Phytochemistry. 1990;29(8):2393–2406. doi:10.1016/0031-9422(90)85155-9
10. Lihua Zheng SJ. A brief introduction of Chinese nutgall fermentation processing. Chin J Chin Mater Medica. 1998;23(1):26–27.
11. Pan Y. Toxicological comparison between defatted croton seed powder and two fermentation products of fructus crotonis. J Food Sci Biotechnol. 2011;30(5):1673–1689.
12. Hwang E, Park SY, Lee HJ, et al. Vigna angularis water extracts protect against ultraviolet B-exposed skin aging in vitro and in vivo. J Med Food. 2014;17(12):1339–1349. doi:10.1089/jmf.2013.3017
13. Oh JE, Kim MS, Jeon KS, et al. A nuclear factor kappa B-derived inhibitor tripeptide inhibits UVB-induced photoaging process. J Dermatol Sci. 2014;76(3):196–204. doi:10.1016/j.jdermsci.2014.10.002
14. Haratake A, Uchida Y, Schmuth M, et al. UVB-induced alterations in permeability barrier function: roles for epidermal hyperproliferation and thymocyte-mediated response. J Invest Dermatol. 1997;108(5):769–775. doi:10.1111/1523-1747.ep12292163
15. Meguro S, Arai Y, Masukawa Y, Uie K, Tokimitsu I. Relationship between covalently bound ceramides and transepidermal water loss (TEWL). Arch Dermatol Res. 2000;292(2000):463–468. doi:10.1007/s004030000160
16. Yutaka T, Nakagawa H, Kondo H, Takema Y, Imokawa G. Decreased levels of covalently bound ceramide are associated with ultraviolet B-induced perturbation of the skin barrier. J Invest Dermatol. 2004;123(6):1102–1109. doi:10.1111/j.0022-202X.2004.23491.x
17. Rotblatt M. Herbal medicine: expanded commission E monographs. Ann Intern Med. 2000;133(6):487. doi:10.7326/0003-4819-133-6-200009190-00031
18. Sembiring E, Elya B, Sauriasari R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn J. 2018;10(1):123–127. doi:10.5530/pj.2018.1.22
19. Cheung L, Cheung P, Ooi VEC. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003;81(2):249–255. doi:10.1016/S0308-8146(02)00419-3
20. Kim JW, Minamikawa T. Hydroxyl radical-scavenging effects of spices and scavengers from brown mustard (Brassica nigra). Biosci Biotechnol Biochem. 1997;61(1):118–123. doi:10.1271/bbb.61.118
21. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi:10.1016/S0891-5849(98)00315-3
22. Benfeldt E, Serup J. Effect of barrier perturbation on cutaneous penetration of salicylic acid in hairless rats: in vivo pharmacokinetics using microdialysis and non-invasive quantification of barrier function. Arch Dermatol Res. 1999;291(9):517–526. doi:10.1007/s004030050447
23. Hao D, Wen X, Liu L, Wang L, Zhou X, Li Y. Sanshool improves UVB-induced skin photodamage by targeting JAK2/STAT3-dependent autophag. Cell Death Dis. 2019;19(2019):1–3.
24. Subedi L, Lee TH, Wahedi HM, Kim SY, Kim SY. Resveratrol-enriched rice attenuates UVB-ROS-induced skin aging via downregulation of inflammatory cascades. Oxid Med Cell Longev. 2017;2017:1–15. doi:10.1155/2017/8379539
25. Cooper S, Bowden G. Ultraviolet B regulation of transcription factor families: roles of nuclear factor-kappaB (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr Cancer Drug Targets. 2007;7(4):325–334. doi:10.2174/156800907780809714
26. Saleh RM, Kabli SA, Al-Garni SM, Al-Ghamdi MA, Abdel-Aty AM, Mohamed SA. Solid state fermentation by Trichoderma viride for enhancing phenolic content, antioxidant and antimicrobial activities in ginger. Lett Appl Microbiol. 2018;67(2):161–167. doi:10.1111/lam.13003
27. Yang E, Kwon H, Kang J, Kang B. Anti inflammatory and antiallergic activity of fermented turmeric by Lactobacillus johnsonii IDCC 9203. Microbiol Biotechnol Lett. 2008;36(2):121–127.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.