Back to Journals » Drug Design, Development and Therapy » Volume 8
Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure
Authors Kono Y, Fukuda S, Hanatani A, Nakanishi K, Otsuka K, Taguchi H, Shimada K
Received 1 June 2014
Accepted for publication 27 June 2014
Published 27 August 2014 Volume 2014:8 Pages 1175—1181
DOI https://doi.org/10.2147/DDDT.S68715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Yasushi Kono,1 Shota Fukuda,1 Akihisa Hanatani,2 Koki Nakanishi,2 Kenichiro Otsuka,2 Haruyuki Taguchi,1 Kenei Shimada2
1Department of Medicine, Osaka Ekisaikai Hospital, Osaka, Japan; 2Department of Internal Medicine and Cardiology, Osaka City University School of Medicine, Osaka, Japan
Background: Remote ischemic conditioning (RIC) is a treatment modality that suppresses inflammation and improves endothelial function, which are factors involved in the pathogenesis of heart failure (HF) with reduced left ventricular ejection fraction. Coronary flow reserve (CFR) is a physiological index of coronary microcirculation and is noninvasively measured by transthoracic Doppler echocardiography (TTDE). This study aimed to investigate the effects of RIC on CFR in healthy subjects and patients with HF, through the assessment by TTDE.
Methods: Ten patients with HF with left ventricular ejection fraction of less than 40%, and ten healthy volunteers were enrolled in this study. RIC treatment was performed twice a day for 1 week. Our custom-made RIC device was programmed to automatically conduct 4 cycles of 5 minutes inflation and 5 minutes deflation of a blood pressure cuff to create intermittent arm ischemia. CFR measurements and laboratory tests were examined before, and after 1 week of RIC treatment.
Results: One week of RIC treatment was well tolerated in both groups. RIC treatment increased CFR from 4.0±0.9 to 4.6±1.3 (mean ± standard deviation) in healthy subjects (P=0.02), and from 1.9±0.4 to 2.3±0.7 in patients with HF (P=0.03), respectively. Systolic blood pressure in healthy subjects, and heart rate in HF patients decreased after RIC treatment (both P<0.01).
Conclusion: This study demonstrated that a 1 week course of RIC treatment improved coronary microcirculation in healthy subjects and patients with HF associated with reduced left ventricular ejection fraction.
Keyword: echocardiography, coronary flow reserve, heart failure, preconditioning, rehabilitation
Introduction
Heart failure (HF), a syndrome resulting from a variety of cardiac injuries, leads to significant morbidity and mortality. It is now recognized that HF is a highly complex multifactor disorder in which a number of physiological systems participate. Patients with HF associated with reduced left ventricular (LV) ejection fraction (EF) have elevated inflammatory status and impaired endothelial function, and these factors appear to have prognostic implications.1–4 Despite advances in drug and/or device therapy for chronic HF with reduced LVEF, outcomes at the community level remain suboptimal.5,6 These unsatisfactory results warrant investigation of further therapeutic strategies to treat HF patients in their daily lives.
Remote ischemic conditioning (RIC) is a noninvasive, simple procedure whereby repeated brief episodes of ischemia at a site distant from the heart protect the heart from cellular injury. A number of clinical studies have confirmed positive cardioprotective effects of RIC in the setting of myocardial infarction,7 cardiac8–10 and non-cardiac surgery,11 and percutaneous coronary intervention.12 The mechanisms of RIC include suppressed inflammation8,13,14 and improved endothelial function, which are common pathogenic mechanisms in HF.15,16 We therefore hypothesized that RIC can provide beneficial effects on microvascular function in patients with HF. This study therefore aimed to investigate the effects of 1 week of RIC treatment on coronary flow reserve (CFR) as a physiological index of coronary microcirculation in both healthy subjects and patients with chronic HF with reduced LVEF, through the assessment by transthoracic Doppler echocardiography (TTDE).
Methods
Study population
This study consisted of ten healthy subjects (age, 31±4 years; ten men) and ten patients with chronic HF (age, 64±9 years; nine men, and one woman). Ten patients with HF (ischemic cardiomyopathy in six patients, and idiopathic dilated cardiomyopathy in four patients) whose morbidity was stable at the time of study enrollment were recruited. Patients were eligible for the study if they were hemodynamically stable, had decreased exercise tolerance, and had an LVEF of less than 40%. The exclusion criteria were as follows: more than moderate valvular heart disease; a history of peripheral arterial disease; chronic kidney disease (estimated glomerular filtration rate <60 mL/min/1.73 mm2) including maintenance hemodialysis; and the presence of other serious systemic diseases. Patients with unstable hemodynamics, or with obstructive narrowing (≥50%) in the left anterior descending (LAD) artery as confirmed by coronary angiography were also excluded because of ethical considerations regarding the safety of RIC.
Ten male physicians and technicians with normal echocardiographic findings, and no history of cardiovascular disease served as healthy subjects. To investigate the effect of repeated hyperemic stimulation with adenosine triphosphate (ATP) on CFR, TTDE examinations were conducted 1 week apart without RIC treatment, as a control study. Premenopausal women were not enrolled considering that the menstrual cycle might influence CFR.17 This study was approved by the Institutional Review Board of the Osaka Ekisaikai Hospital, and the subjects gave informed consent.
Study protocol
CFR and LV function were measured using TTDE before and after a 1 week course of RIC treatment. These TTDE examinations were performed at approximately the same time of day, considering circadian variation in CFR.18 Blood samples for the assessment of white blood cell counts, fasting lipids levels, and high-sensitive C-reactive protein levels, were performed immediately before each TTDE examination. Levels of high-sensitive tumor necrosis factor-α, high-sensitive interleukin-6, high-sensitive troponin T, and brain natriuretic peptide were assessed only in HF patients.
In each RIC treatment, three steps were performed by the subjects. First, the subject placed the cuff on their arm; second, they pushed the “run” button; and third, they removed the cuff after ending 4 cycles of inflation and deflation. RIC treatment was repeated each morning and evening for 1 week. In a supine position, cuffs placed on both right, and left upper arms were inflated to 200 mmHg pressure for 5 minutes (min). After 5 min inflation, each cuff was deflated at the same time. Four cycles of 5 min inflation and 5 min deflation of the blood pressure cuffs were automatically performed by our custom-made equipment (Figure 1). The system also counted four cycles of inflation and deflation and provided an alert when the four cycles ended. A physician (YK) confirmed that the subjects used the system correctly.
Echocardiography
TTDE examination was performed using Vivid 7 (GE Healthcare UK Ltd, Little Chalfont, UK) in the standard manner.19 LV end-diastolic and systolic volumes were obtained using the Simpson’s method and indexed by body surface area. LVEF was then calculated. Pulsed-wave Doppler examination of mitral inflow was performed to measure peak velocity (E). Early diastolic mitral annular velocity (e’) was also measured from tissue Doppler imaging in the septal wall. The ratio of E to e’ was then calculated (E/e’).
For the measurement of CFR, a modified foreshortened two-chamber view was applied to explore the flow in the distal portion of the LAD. If the angle between color flow and the Doppler beam was >20%, angle correction was performed. Coronary blood flow velocity was obtained at baseline and after intravenous infusion of ATP at a rate of 0.14 mg/kg per min for 2 min to produce hyperemia. Mean diastolic flow velocity (MDFV) was measured by tracing the contour of the spectral Doppler signal. CFR was calculated as the ratio of hyperemic to basal MDFV. For each variable in the CFR measurements, the values of three cycles were averaged. Expert sonographers who had >5 years experience in echocardiography performing approximately 100 CFR examinations, performed the TTDE examinations. They were blinded to the other information of each patient.
Statistical analysis
Values were expressed as the mean ± standard deviation. Comparisons of laboratory and echocardiographic data before and after RIC were made with a paired t-test. Heart rate, blood pressure, and MDFV after ATP infusion in pre- and post-RIC treatment were evaluated by two-way repeated measures analysis of variance, testing for RIC effects, ATP effects, and interactions. Differences were considered significant at P<0.05.
Results
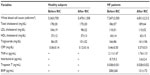
Clinical characteristics of healthy subjects and patients with HF are summarized in Table 1. RIC was completed and well tolerated in all healthy subjects and patients with HF. No significant hemodynamic or mechanical complications were observed at the end of the 1 week procedure.
Significant increases in CFR were observed in both healthy subjects (4.0±0.9 to 4.6±1.2, P=0.02) and patients with HF (1.9±0.4 to 2.3±0.7, P=0.03), as shown in Figure 2. Hemodynamics and MDFV values before and after RIC in each group are shown in Table 2. In two-way repeated measures analysis of variance, systolic blood pressure decreased after RIC treatment in healthy subjects (P=0.001). Heart rate decreased after RIC treatment in HF patients (P=0.01). MDFV increased during hyperemia in both groups (both P<0.001), whereas there were no significant interactions in terms of RIC.
  | Figure 2 Changes in CFR after 1 week of RIC treatment in healthy subjects and HF patients. |
Other echocardiographic parameters measured before and after RIC are shown in Table 3. There were no statistical differences in echocardiographic parameters before and after RIC in either group, except for LV end-systolic volume in healthy subjects (19±3 mL/m2 to 22±4 mL/m2, P=0.01). The laboratory data before and after RIC are summarized in Table 4. In patients with HF, tumor necrosis factor-α (P=0.2), interleukin-6 (P=0.1), troponin-T (P=0.2), and brain natriuretic peptide levels (P=0.07) tended to decrease after RIC treatment, but these reductions did not reach statistical significance.
In the control study (examinations without RIC treatment), there were no significant differences in any laboratory data, hemodynamics, or echocardiographic results before and after 1 week, except for MDFV before and after ATP infusion (P<0.001). These findings are summarized in Table 5.
Discussion
This study demonstrated that a 1 week course of RIC treatment improved the status of coronary microcirculation in healthy subjects and patients with HF associated with reduced LVEF without any adverse effects, supporting the widespread use of RIC in the daily lives of HF patients.
Developments in TTDE technology have enabled visualization of coronary blood flow and assessment of CFR in the LAD. This technique has been validated by the intracoronary Doppler flow wire technique advanced into the coronary vessels, leading to the accurate diagnosis of physiologically obstructive coronary artery disease.20,21 Furthermore, because of the noninvasive, relatively inexpensive, and physiological nature of TTDE, TTDE-derived CFR measurement is open to innovative applications, including serial measurement of coronary microvascular function,18,22,23 because CFR is considered to be an integrated parameter that reflects the severity of coronary microvascular dysfunction. We therefore used TTDE-derived CFR to evaluate the effect of RIC on coronary microcirculation in patients with HF.
Recently, measurement of TTDE-derived CFR has gained prognostic significance for stratifying patients at risk of developing cardiovascular diseases, beyond the assessment of individual traditional atherosclerotic risk factors. Several investigations have shown that CFR was reduced even in the absence of obstructive coronary narrowing, resulting in long-term cardiovascular events in patients with coronary risk factors, such as hypertension,24 diabetes,25 and chronic kidney disease.26 Importantly, reduced CFR was strongly associated with the development of HF, rather than coronary artery events.27 These observations indicate the potential of CFR as a therapeutic target for improving long-term outcomes in patients with impaired LV function as well as the close relationship between impaired coronary microcirculation and the future functional capability of the myocardium.
Ischemic preconditioning was first described in 1986 in a canine model of myocardial infarction.28 Because ischemic preconditioning has systemic effects that result in protection from ischemic-reperfusion injury for tissues remote from those undergoing preconditioning, RIC offers a clinically relevant stimulus.29 RIC can prevent adverse consequences of ischemia in potentially vulnerable target organs. Several mechanisms for RIC have been proposed in experimental settings, all of which are broadly related to the release of humoral mediators, including adenosine,30 bradykinin,31 and opioids,32 and the autonomic nervous system.33 Subsequent studies in humans have confirmed that RIC decreased inflammation8,13,18 and improved endothelial function.15,16 Moreover, enhanced inflammatory status and endothelial dysfunction are reported to be involved in the pathogenesis of HF.1–4 This study demonstrated that a 1 week course of RIC treatment resulted in favorable effects in HF patients with reduced LVEF, through the improvement of coronary microcirculation. In addition, our custom-made RIC device was programmed to administer four cycles of inflation and deflation of blood pressure cuffs; this device was to be used by the patients themselves. The findings of the present study indicated that a simple RIC device would enhance the use of RIC as a self-care HF treatment for inpatients as well as individuals outside the hospital setting, including home-care patients.
Study limitations
This study had several limitations. First, the sample size was small (n=20), which may reasonably explain that changes in some biomarkers did not reach statistical significance. In addition, this study focused on the investigation of the effect of a 1 week course of RIC treatment on LV function in healthy subjects and HF patients with reduced LVEF. The long-term effects of RIC treatment and their impact on clinical outcomes remain unknown, especially in patients with HF with preserved EF. Further study of larger populations with long-term follow-up should be conducted to confirm the results of the present study. Second, statistically significant decreases in systolic blood pressure and increases in LV end-systolic volume were observed following the RIC procedure in healthy subjects. Although the degree of change was too subtle to cause any systemic effects, their alterations should be carefully interpreted. Third, all subjects completed 1 week of RIC treatment without any complications based on self-reports. The RIC system should be improved to precisely record when it is activated. Finally, CFR was measured by TTDE method in the LAD alone, and the effect of RIC on circulation in other coronary vessels was not estimated. This was because the success rates in obtaining adequate coronary flow velocity in the LAD were higher than those in other coronary vessels.34 Concomitant measurement of CFR in an adjacent normal vessel may provide more mechanistic insights into the findings of this study.
Conclusion
This study demonstrated that a 1 week course of RIC treatment improved the status of coronary microcirculation in healthy subjects and patients with HF with no adverse effects, supporting the widespread use of RIC in the daily lives of HF patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Drexler H, Hayoz D, Munzel T, et al. Endothelial function in chronic congestive heart failure. Am J Cardiol. 1992;69(19):1596–1601. | |
Aukrust P, Ueland T, Lien E, et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83(3):376–382. | |
Tousoulis D, Charakida M, Stefanadis C. Inflammation and endothelial dysfunction as therapeutic targets in patients with heart failure. Int J Cardiol. 2005;100(3):347–353. | |
Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60(16):1455–1469. | |
Fonarow GC, Peterson ED. Heart failure performance measures and outcomes: real or illusory gains. JAMA. 2009;302(7):792–794. | |
Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147. | |
Bøtker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375(9716):727–734. | |
Cheung MM, Kharbanda RK, Konstantinov IE, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47(11):2277–2282. | |
Hausenloy DJ, Mwamure PK, Venugopal V, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370(9587):575–579. | |
Thielmann M, Kottenberg E, Kleinbongard P, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382(9892):597–604. | |
Ali ZA, Callaghan CJ, Lim E, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116(Suppl 11):I98–I105. | |
Hoole SP, Heck PM, Sharples L, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119(6):820–827. | |
Harkin DW, Barros D’Sa AA, McCallion K, Hoper M, Campbell FC. Ischemic preconditioning before lower limb ischemia – reperfusion protects against acute lung injury. J Vasc Surg. 2002;35(6):1264–1273. | |
Konstantinov IE, Arab S, Kharbanda RK, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19(1):143–150. | |
Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–H588. | |
Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46(3):450–456. | |
Hirata K, Shimada K, Watanabe H, et al. Modulation of coronary flow velocity reserve by gender, menstrual cycle and hormone replacement therapy. J Am Coll Cardiol. 2001;38(7):1879–1884. | |
Fukuda S, Shimada K, Maeda K, et al. Circadian variation in coronary flow velocity reserve and its relation to α1-sympathetic activity in humans. Int J Cardiol. 2012;157(2):216–220. | |
Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. | |
Hozumi T, Yoshida K, Akasaka T, et al. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998;32(5):1251–1259. | |
Caiati C, Montaldo C, Zedda N, Bina A, Iliceto S. New noninvasive method for coronary flow reserve assessment: contrast-enhanced transthoracic second harmonic echo Doppler. Circulation. 1999;99(6):771–778. | |
Fukuda S, Shimada K, Kawasaki T, et al. “Passive exercise” using whole body periodic acceleration: effects on coronary microcirculation. Am Heart J. 2010;159(4):620–626. | |
Kubo T, Fukuda S, Hirata K, et al. Comparison of coronary microcirculation in female nurses after day-time versus night-time shifts. Am J Cardiol. 2011;108(11):1665–1668. | |
Cortigiani L, Rigo F, Galderisi M, et al. Diagnostic and prognostic value of Doppler echocardiographic coronary flow reserve in the left anterior descending artery in hypertensive and normotensive patients [corrected]. Heart. 2012;97(21):1758–1765. | |
Cortigiani L, Rigo F, Gherardi S, et al. Additional prognostic value of coronary flow reserve in diabetic and nondiabetic patients with negative dipyridamole stress echocardiography by wall motion criteria. J Am Coll Cardiol. 2007;50(14):1354–1361. | |
Nakanishi K, Fukuda S, Shimada K, et al. Prognostic value of coronary flow reserve on long-term cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol. 2013;112(7):928–932. | |
Nakanishi K, Fukuda S, Shimada K, et al. Impaired coronary flow reserve as a marker of microvascular dysfunction to predict long-term cardiovascular outcomes, acute coronary syndrome and the development of heart failure. Circ J. 2012;76(8):1958–1964. | |
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. | |
Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87(3):893–899. | |
Pell TJ, Baxter GF, Yellon DM, Drew GM. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol. 1998;275(5 Pt 2):H1542–H1547. | |
Schoemaker RG, van Heijningen CL. Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol. 2000; 278(5):H1571–H1576. | |
Patel HH, Moore J, Hsu AK, Gross GJ. Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol. 2002;34(10):1317–1323. | |
Wolfrum S, Schneider K, Heidbreder M, Nienstedt J, Dominiak P, Dendorfer A. Remote preconditioning protects the heart by activating myocardial PKCepsilon-isoform. Cardiovasc Res. 2002;55(3):583–589. | |
Murata E, Hozumi T, Matsumura Y, et al. Coronary flow velocity reserve measurement in three major coronary arteries using transthoracic Doppler echocardiography. Echocardiography. 2006;23(4):279–286. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.