Back to Journals » OncoTargets and Therapy » Volume 14
Remodels the Immunosuppressive Tumor Microenvironment by Combination of Bacillus Calmette–Guérin and Anti-PD-L1 in an Orthotopic Triple-Negative Breast Cancer Mouse Model
Authors Lu Y , Huang X, Liu X, He Y, Hu Z, Xu JW, Cao G, He W
Received 26 November 2020
Accepted for publication 3 March 2021
Published 30 March 2021 Volume 2021:14 Pages 2247—2258
DOI https://doi.org/10.2147/OTT.S294129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Yuan Lu, 1, 2 Xin Huang, 1, 2 Xiaoke Liu, 1, 2 Yu He, 1, 2 Zhe Hu, 1 Weize Xu, 1, 2 Gang Cao, 1– 3 Wenbo He 1, 2
1State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, 430070, People’s Republic of China; 2College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, 430070, People’s Republic of China; 3College of Biomedicine and Health, Huazhong Agricultural University, Wuhan, 430070, People’s Republic of China
Correspondence: Wenbo He
College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, 430070, People’s Republic of China
Tel +86 15337203990
Email [email protected]
Background: Targeting immunosuppressive tumor microenvironment (TME) is one of the important therapeutic strategies for triple-negative breast cancer (TNBC). The application of Bacillus Calmette–Guérin (BCG) in the clinical treatment of bladder cancer has shown that BCG is a strong inducer of immune activation and can remodel the immunosuppressive state of the TME. Meanwhile, previous studies have demonstrated that the 4T1 TNBC mouse model does not respond to anti-PD-L1 treatment alone. Therefore, it is necessary to explore the effect of BCG on TNBC, as well as the potential efficacy of BCG combined with anti-PD-L1.
Materials and Methods: In this study, we studied the effects of BCG treatment on the lymphocytes and transcriptome in the TME of an orthotopic TNBC mouse model, and evaluated the efficacy of combination therapy with BCG and anti-PD-L1 on the tumor.
Results: We found that three-dose BCG treatment could significantly inhibit tumor growth, while the single-dose BCG treatment was able to up-regulate the expression of chemokine-related genes and anti-tumor effect genes, down-regulate the expression of immunosuppressive-related genes, and increase tumor-infiltrating lymphocytes. The combination therapy of BCG and anti-PD-L1 has produced a marked oncolytic effect.
Conclusion: These findings emphasize that BCG treatment can relieve the immunosuppressive state of the TME, and indicate that the combination therapy of BCG and anti-PD-L1may be an efficacious treatment measure for TNBC.
Keywords: TME, BCG, PD-L1, TNBC, immunotherapy, combination therapy
Introduction
Targeting the tumor microenvironment (TME) is considered a promising strategy for tumor treatment.1 The TME is the cellular environment in which the tumor exists. Apart from the tumor cells, the TME includes vascular endothelial cells (ECs), cancer-associated fibroblasts (CAFs), various resident or migratory immune cell subsets and extracellular matrix (ECM). The TEM “immunologically cold” state induced by the synergistic interaction of tumor cells and other types of cells allows the tumor to be immunosuppressive and evade the elimination of the body’s immune system.2,3 Therefore, the TME should be treated by “lighting a fire” and reverted from “cold state” into “hot state”, therefore become sensitive to the immunological surveillance.4
Oncolytic therapy with microorganisms, such as oncolytic virus, can cause immunogenic death of tumor cells and reduce TME immunosuppression.4–7 However, Oncolytic virus is easy to be eliminated and sometimes cannot induce a strong enough immune response to diminish TME immunosuppression. The BCG vaccine originally used to prevent tuberculosis has been proved to have strong immunostimulatory properties,8,9 and BCG treatment can create “immunologically hot” state by recruiting lymphocytes into TME and therefore remodeling TME.
Although BCG has been successfully employed on immunotherapy for non-muscle invasive bladder cancer (NMIBC) to reduce the recurrence rate of NMIBC, its anti-tumor efficacy on other tumor types, such as breast cancer, is worth further investigating.10,11 Triple-negative breast cancer (TNBC) is a particularly aggressive subtype of breast cancer.12,13 Due to the lack of overexpression of estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 gene, TNBC cannot benefit from endocrine therapy or targeted therapy (such as Herceptin) and it is very desperate to develop a new strategy for TNBC treatment.13–15 The application of BCG on diminishing TME immunosuppression and activating anti-tumor immunity may be an efficacious therapeutic measure for TNBC. However, BCG treatment can induce immune resistance or immune evasion by up-regulating the expression of PD-L1. In turn, these suggested that the combination of immune screening site blockade (anti-PD-L1) and BCG should have a better oncolytic efficacy.16–18
The current study aimed firstly to investigate the efficacy of BCG on the TNBC, along with the variations of lymphocytes and gene expressions in the TME. We found that BCG treatment could reduce the immunosuppression in TME of TNBC mice, accompanied by up-regulation of the PD-L1 expression. Consequently, we further evaluated whether the combination of BCG immunotherapy and PD-L1 blocker can enhance and optimize the therapeutic effect on TNBC.
Materials and Methods
Plasmid, Antibody and Drug
pHKO-luc, pSPAX and pMD2.G were provided by Professor Fuqiang Xu (Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences).
Antibodies for flow cytometry analysis, CD49b-APC (clone DX5), CD3-PE (clone 17A2), CD4-FITC (clone RM4.5) and CD8a-PacBlue (clone 53–6.7) were purchased from BioLegend (United States of America). Anti-PD-L1 blocker BMS-202 (cat. no. HY-19745) was obtained from MedChemExpress (United States of America).
BCG Strain, Cell Line
BCG strain (ATCC® 35737™) was obtained from the American Type Culture Collection (ATCC). BCG were grown in DifcoTM Middlebrook 7H9 broth (Becton Dickinson) containing 0.5% glycerol, 0.05% Tween-80, and 10% oleic acid albumin dextrose catalase (OADC, BD, USA). BCG was amplified in a biochemical incubator at 37°C.
4T1 cells were purchased from the Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. In order to construct the 4T1-luc cell line, the plasmid pHKO-luc, pSPAX and pMD2.G were co-transfected into HEK 293T at a ratio of 1:1:1 for 60 hours. The supernatant was collected and concentrated with lentivirus concentrate to obtain lentivirus-luc. Lentivirus-luc was used to infect 4T1 cells, and single positive cells were picked and proliferated to obtain 4T1-luc cell lines expressing luciferase. 4T1, 4T1-luc cells were grown in RIPM1640 medium containing 10% FBS, 1mM Sodium Pyruvate (Gibco), 1× GlutaMAXTM-1 (Gibco) and 1×MEM NEAA (Gibco).
Establishment of Orthotopic TNBC Mouse Model
All animal studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the Research Ethics Committee of Huazhong Agricultural University. The use of mice has been approved by the Research Ethics Committee of Huazhong Agricultural University, Hubei, China (HZAURA-2020-0002). Balb/c female mice (6–8 weeks old) were purchased from the Experimental Animal Center of Huazhong Agricultural University. In order to construct a mouse model of TNBC, 4T1 (1×106) cells or 4T1-luc (1×106) cells in 10 μL of PBS were implanted into the fat pad of the fourth pair of left breasts of mice.
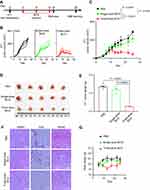
BCG Treatment
In order to verify the oncolytic effect of BCG, animals were randomly divided into three groups on the 5th d after tumor inoculation. In single-dose BCG treatment group, mice were injected with 10 μL of BCG in PBS with OD600 = 2 on the 5th d after tumor inoculation. In three-dose BCG treatment group, mice were injected with 10 μL of BCG in PBS with OD600 = 2 on the 5th, 9th and 13th days after tumor inoculation. In PBS group, mice were injected with 10 μL PBS on the 5th, 9th and 13th days after tumor inoculation (Figure 1A). The vernier calipers were used to measure the long diameter (L) and short diameter (W) of the tumor mass every 2–3 days. Calculated the tumor volume according to the ellipsoid volume calculation formula (that is, 1/2×L×W2). The weight data were recorded every 2–3 days, the mice were killed by anesthesia 25 d after BCG injection. The tumors were separated from the surrounding fascia, weighted and photographed. The spleens, lungs and kidneys were analyzed by histopathology to identify the side effects of BCG treatment.
Flow Cytometric Analysis
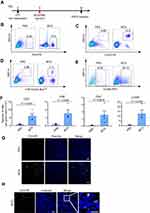
In order to analyze the effect of BCG treatment on tumor-infiltrating lymphocytes, mice were anesthetized and sacrificed to separate tumor masses 9 d after BCG inoculation. The tumors were separated from the surrounding fascia, minced into pieces by sterile scissors and ground. Cell clumps were removed through a 70-μm cell strainer to obtain a single-cell suspension. Lymphocytes were separated with The Mouse Tumor Infiltrating Tissue Lymphocyte Separation Kit (Solarbio). After washing twice with PBS containing 1% BSA (FACS solution) and blocking with anti-mouse CD16/CD32 for 30 min, the tumor-infiltrating lymphocyte suspension was incubated with antibodies CD49b-APC, CD3-PE, CD4-FITC and CD8a-PacBlue. After incubating for 1 h, these samples were washed once with PBS containing 0.2% BSA and analyzed by a flow cytometer Cytoflex LX (Beckman Coulter). At the same time, the untreated tumors were sectioned and stained with anti-mouse CD3-PE antibody for 1 h and Hoechst for 1 min. Confocal images were collected by an Olympus FV1000 microscope on a 40-fold objective.
Transcriptome Analysis
In order to analyze the effect of the single-dose BCG treatment on gene expression in the tumor of the TNBC mouse model, the mice were anesthetized and sacrificed to separate the tumor mass 9 d after BCG inoculation. After extracting the total RNA from the tumor block, we used the Whole RNA-seq Lib Prep Kit for Illumina (ABclonal) to build the RNA-seq library, and then send them to the sequencing company (Genewiz) for high-throughput sequencing. The up-stream data analysis using the nf-core rnaseq pipeline.19 The parameters “–aligner hisat2 –skipBiotypeQC –genome mm10” were used. DEseq2 was used for differential gene expression analysis and export the normalized count matrix.20 By Cytoscape/ClueGo software, the differentially expressed genes were enriched with GO by the GO-ImmunesystemProcess-EBI-UniProt-GOA-ACAP-ARAP-08.05.2020 library. The filtering conditions were as follows: p ≤ 0.05, Correction Method Used = Bonferroni step down, Min GO Level = 3, Max GO Level = 8, Kappa Score Threshold = 0.4. Adjust the Preferred Layout in software Cytoscape/ClueGo to Prefuse Force Directed Layout to plot the GO enrichment results.
To confirm the representative genes expression in tumor with real-time PCR, we utilized the isolated total RNA from the tumor block as a template to synthesize cDNA following the protocol of the ReverTra Ace qPCR RT Master Mix (TOYOBO, Osaka, Japan). Next, PCR was performed with a PowerUp™ SYBR™ Green Master Mix (Applied Biosystems, Foster City, CA, USA) kit on the ABI QuantStudio 3 Real-Time PCR System (Applied Biosystems) to determine the mRNA expression of Cxcl10, Ifng, Pdcd1, Cd274. The primers of the genes used in PCR were as follows: Cxcl10-F: 5′-CCAAGTGCTGCCGTCATTTTC-3′, Cxcl10-R: 5′-GGCTCGCAGGGATGATTTCAA-3′; Ifng-F: 5′-GCCACGGCACAGTCATTGA-3′, Ifng-R: 5′-TGCTGATGGCCTGATTGTCTT-3′; Pdcd1-F: 5′-GCTCCAAAGGACTTGTACGTG-3′, Pdcd1-R: 5′-TGATCTGAAGGGCAGCATTTC-3′; Cd274-F: 5′-GCTCCAAAGGACTTGTACGTG-3′, Cd274-R: 5′-TGATCTGAAGGGCAGCATTTC-3′. The expression of β-actin was utilized for normalization. The primers of β-actin used in PCR were as follows: β-actin-F: 5′-CGTTGACATCCGTAAAGACC-3′, β-actin-R: 5′-AACAGTCCGCCTAGAAGCAC-3′. The relative expression values of Cxcl10, Ifng, Pdcd1, Cd274 were all analyzed by the 2−ΔΔCt method.
Single-Dose BCG and Anti-PD-L1 Combination Therapy
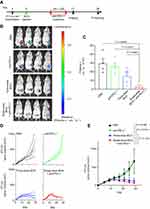
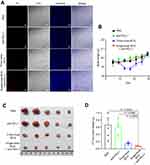
In order to verify the oncolytic effect of single-dose BCG and anti-PD-L1 combination therapy on TNBC mouse model, the mice were randomly divided into four groups on the 5th d after tumor inoculation (Figure 2A). In single-dose BCG combined with anti-PD-L1 treatment group, the mice were injected with 10 μL of BCG in PBS with OD600=2 on the 5th d after tumor inoculation. 9 d after BCG injection, the mice were intraperitoneally injected with BMS202 at a dose of 20 mg/kg for 7 consecutive days. In the three-dose BCG treatment group and PBS group, the mice were treated according to the previous method. In anti-PD-L1 treatment group, the mice were injected with 10 μL PBS on the 5th d after tumor inoculation. 9 d after PBS injection, the mice were intraperitoneally injected with BMS202 at a dose of 20 mg/kg for 7 consecutive days. Record the length (L) and short (W) diameter of each mouse’s tumor with a vernier caliper every 3–4 days. Calculate the total tumor volume of each animal according to the ellipsoid volume calculation formula (that is, 1/2×L×W2). 21 d after tumor inoculation, D-luciferin (Promega) at a dose of 150 mg/kg was intraperitoneally injected into 4 mice in each group. The mice were imaged using the small animal in vivo imager (PerkinElmer) 15 min after injection. Use imaging software (PerkinElmer) to quantitatively analyze the expression of luciferase in the mouse model. 30 d after tumor implantation, the mice were killed by anesthesia and the tumors were photographed and weighed. In addition, according to previous studies, Propidium Iodide was used to detect the cell death in tumor.21
Statistical Analysis
Data were analyzed using Prism 8.0 software (GraphPad Software, San Diego, CA, USA). The experiment of tumor-infiltrating lymphocyte analysis is repeated two times. The experiments of BCG treatment or single-dose BCG treatment combined with anti-PD-L1 blocker that inhibits the growth of TNBC were repeated three times. Quantification of the tumor-infiltrating lymphocytes from the TNBC mouse model, real-time PCR and average tumor weight of mice are represented as the mean ± standard error and the P-values were assessed by two-tailed unpaired Welch’s t-test. The average tumor volume of mice is represented as the mean ± standard deviation and the P-values were assessed by a two-tailed unpaired Student’s t-test. Corresponding quantification of luciferase expression is represented as the mean ± standard error and the P-values were assessed by two-tailed unpaired Welch’s t-test.
Results
BCG Treatment Recruited Infiltrating Lymphocytes in the TME of TNBC Mouse Model
To evaluate the immunostimulatory efficacy of BCG for TNBC in vivo, we established an orthotopic TNBC mouse model for flow cytometric analysis of infiltrating lymphocytes (Figure 3A, Supplementary Material Figure 1). As shown in Figure 3B–F, compared with the PBS treatment group, the BCG treatment group contained more CD3+, CD4+, CD8+ T cells and NK cells, indicating that BCG treatment can increase the number of lymphocytes in the TME of the TNBC mouse model. The results of CD3 staining on frozen sections of tumors also showed that there were more CD3+ T cells in the tumors of the BCG treatment group (Figure 3G and H). These data indicate that BCG treatment can change the immune suppression state of the TME by recruiting more lymphocytes.
BCG Treatment Inhibited the Growth of TNBC
In order to evaluate the efficacy of BCG on TNBC mouse model, we recorded tumor volume data to observe the effect of BCG treatment on TNBC mouse model (Figure 1A). As shown in Figure 1B and C, while tumor volume in single-dose BCG group had a slower increase than that of PBS group, those of three-dose BCG group had slowest increase among three groups. The volume and weight of tumor in three-dose BCG group were also the smallest among the three groups 25 d after inoculation (Figure 1D and E). These data suggested single-dose BCG treatment could moderately slow TNBC progression and three-dose BCG showed more powerful inhibition on TNBC.
To evaluate the safety of BCG dose, we carried out weight monitoring and Hematoxylin-Eosin staining (H&E-staining) of main organs (lung, kidney and spleen) in three groups of mice. There were no pathological changes observed in lungs, kidneys and spleens among the three groups (Figure 1F), which indicated that neither single-dose BCG nor three-dose BCG could cause organic damage in mouse. However, compared with the single-dose BCG group and PBS group, the body weight of mice in the three-dose BCG group decreased obviously during the treatment (Figure 1G), which may be related to the stronger immunostimulation induced by multiple BCG treatment. In order to optimize the BCG immunotherapy, it is necessary to further study the effect of BCG treatment on gene expression in the TME of TNBC.
BCG Treatment Changed the Expression of Immune Screening Site Genes in the TME of TNBC
To explore the effect of BCG on gene expression in the TME, we performed RNA-Seq analysis on 4T1 tumors treated with PBS and single-dose BCG. From the RNA-Seq data, we identified 6863 differentially expressed genes (DEGs) between two groups (Figure 4A and B). To determine the functions of the DEGs induced by BCG treatment, we performed Gene Ontology (GO) analysis on these DEGs (Figure 4C). The results showed that the functions of these DEGs were mainly belonging to the T cell activation signaling pathways, such as T cell proliferation (GO:0042098), T cell differentiation (GO:0042098). We found that the coding chemokines genes (Ccl5, Cxcl9, Cxcl10) in the single-dose BCG treatment group were significantly up-regulated (Figure 4B and D). CCL5 and CXCL9 are more prone to lymphocyte infiltration when they are both present in tumor tissue.22 This suggests that BCG treatment may increase lymphocyte infiltration in 4T1 tumors, which is in line with the results of the previous tumor-infiltrating lymphocyte flow analysis and the representative expression of the genes (Figure 4E).
Meanwhile, the results of our RNA-seq also revealed that BCG treatment could up-regulate genes that are essential for lymphocyte activation (Cd69 and Klrk1) and anti-tumor effects (Ifngr1 and Gzma), and down-regulate immunosuppressive genes (Tigit and Vegfa) (Figure 4B and D). The expression changes of these genes confirmed BCG treatment can change the immune suppression state of the TME. This implies that the combination of BCG and immune screening sites blockers, such as anti-PD-L1 inhibitors, may have a better effect on TNBC.
Single-Dose BCG Treatment Combined with Anti-PD-L1 Blocker Efficiently Inhibited TNBC
Based on RNA-seq analysis, we performed anti-PD-L1 treatment on the 9 d after BCG injection (Figure 2A). The live imaging of mice showed that although three-dose BCG treatment effectively alleviated the progression of TNBC compared with the PBS group and the anti-PD-L1 group, all mice of single-dose BCG combined with anti-PD-L1 treatment showed very little or no detectable luciferase signal indicated tumor growth (Figure 2B and C). The data of tumor volume proved that single-dose BCG combined with anti-PD-L1 treatment can significantly inhibit the growth of the breast cancer (Figure 2D and E).
Compared with PBS treatment, anti-PD-L1 treatment and three-dose BCG treatment, the tumors of single-dose BCG combined with anti-PD-L1 treatment contained a higher number of Propidium Iodide-positive cells (Figure 5A), indicating that the combined treatment of BCG and anti-PD-L1 has a better oncolytic effect. The mouse body weight monitoring data showed that single-dose BCG combined with anti-PD-L1 treatment can effectively alleviate the weight loss of mice caused by three-dose BCG treatments (Figure 5B), which may indicate that single-dose BCG combined with anti-PD-L1 treatment has more safeties. The results of tumor size and weight in each group showed that the combined treatment of BCG and anti-PD-L1 has a marked therapeutic effect on the TNBC mouse model, which can eliminate most of the tumor mass (Figure 5C and D).
Discussion
In the present study, we investigated the efficacy of BCG treatment on the orthotopic TNBC mouse model and confirmed that single-dose and three-dose BCG treatments can produce anti-TNBC efficacy in varying degrees. The three-dose intratumoral injections of BCG can significantly inhibit the growth of tumors in the TNBC mouse model, along with an obvious weight loss. While the single-dose intratumoral injection of BCG is not completely to inhibit TNBC progress, it can induce numerous infiltrating CD4+, CD8+ T cells and NK cells in the TME of TNBC mouse model. These results demonstrate that the single-dose BCG treatment is enough to recruit a large number of lymphocytes and remodel the TME of TNBC. The reason for its weak oncolytic effect may be related to immune evasion.
The up-regulation of programmed death ligand 1 (PD-L1) is one of the main reasons why immune evasion appears during BCG treatment. Previous studies have confirmed that BCG vaccination induces PD-L1 expression on antigen-presenting cells.23 BCG immunotherapy can up-regulate the expression of PD-L1 in dendritic cells in mouse models of airway inflammation, CD4+ T cells in patients with urothelial carcinoma (UCa) and tumor cells in rat bladder cancer model.16,24,25 However, there are few reports about the effect of BCG treatment on TNBC immune screening sites. In this study, by using RNA-seq analysis, we confirmed that the expression of PD1/PD-L1 was up-regulated during BCG treatment on TNBC, which suggests that although BCG treatment increase lymphocytes infiltration in the TME, it unavoidably induces immune resistance. This result also indicates that BCG immunotherapy and immune screening site-blocking should be combined for the TNBC treatment.
Immune screening site blockade represented by anti-PD1/PD-L1 has made surprising progress in clinical tumor treatment, but there is also an obstacle of low response efficiency.26,27 The previous study suggests that PD-L1 is highly expressed in 4T1 cells, but has no effect on anti-PD-L1 therapy,21 mainly due to the distinct immunosuppressive TME. In the current study, we confirmed that the single-dose BCG and anti-PD-L1 combined treatment has a significant oncolytic efficacy, which indicates that the BCG treatment can relieve the immunosuppressive state in the TME of the 4T1 mouse model, thereby enhancing the response efficiency of anti-PD-L1 treatment. These data imply that BCG treatment can be employed to pretreat those specific tumors which are not sensitive to anti-PD1/PD-L1 treatment, and thereby produce beneficial effects with the combined treatment.
Our research provides a preliminary theoretical basis and support for the widespread application of BCG in clinical tumor treatments and it also provides data on increasing the response efficiency of anti-PD1/PD-L1 treatment. Meanwhile, this research has some limitations. This study is limited to an orthotopic triple-negative breast cancer mouse model. Moreover, the research of BCG to relieve the immunosuppressive state of the tumor microenvironment is mainly focused on the immune activated T cells, such as CD4+, CD8+, NK. However, TME comprises various cell types and extracellular components that are surrounding tumor cells. Besides the immune-activated T cells, which had been investigated in this experiment, some other immune cell subgroups, such as Treg and MDSC, may have specified effects on relieving the immunosuppressive state of TME. Therefore, further studies on more tumor models and TME components are needed to uncover the mechanism of the efficacy of BCG treatment on remodeling TME. Meanwhile, the introduction of immune-enhancing genes into BCG has made some progress,28 which highlights the further research direction of BCG and anti-PD-L1 combined therapy.
In conclusion, this study proved that BCG treatment can up-regulate the expression of chemokine-related genes and anti-tumor effect-related genes, down-regulate the expression of immunosuppressive-related genes and increase lymphocyte infiltration in the TME of TNBC. But it is accompanied by the up-regulation of PD1 and PD-L1 expression. The combined treatment of BCG and anti-PD-L1 efficiently relieves the immunosuppressive state of the TME and has a marked efficacy on TNBC. Our results indicate that the combination therapy of BCG and anti-PD-L1 may be a promising option for TNBC targeted therapy.
Data Sharing Statement
RNA-Seq data that support the findings of this study have been deposited at the National Genomics Data Center (NGDC) under accession number PRJCA003587 (https://bigd.big.ac.cn/bioproject/browse/PRJCA003587). All other data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Professor Fuqiang Xu for reagents; Nan Zhang, Weijia Zhang for technical assistance. This research was supported by the Fundamental Research Funds for the Central Universities (2662018PY025, 2662019YJ004) and the National Key Research and Development Program of China (2017YFD0500300).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Gang Cao reports no conflicts of interest in this work. The other authors report patent Remodeling the immunosuppressive tumor microenvironment by a combination of Bacillus Calmette–Guérin and anti-PD-L1 in triple-negative breast cancer is pending.
References
1. Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019;20(4):840. doi:10.3390/ijms20040840
2. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
3. Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. doi:10.1126/science.aaa6204
4. Achard C, Surendran A, Wedge ME, Ungerechts G, Bell J, Ilkow CS. Lighting a fire in the tumor microenvironment using oncolytic immunotherapy. EBioMedicine. 2018;31:17–24. doi:10.1016/j.ebiom.2018.04.020
5. Koske I, Rossler A, Pipperger L, et al. Oncolytic virotherapy enhances the efficacy of a cancer vaccine by modulating the tumor microenvironment. Int J Cancer. 2019;145(7):1958–1969. doi:10.1002/ijc.32325
6. Xu B, Ma R, Russell L, et al. An oncolytic herpesvirus expressing E-cadherin improves survival in mouse models of glioblastoma. Nat Biotechnol. 2018. doi:10.1038/nbt.4302
7. Shi G, Yang Q, Zhang Y, et al. Modulating the tumor microenvironment via oncolytic viruses and CSF-1R inhibition synergistically enhances anti-PD-1 immunotherapy. Mol Ther. 2019;27(1):244–260. doi:10.1016/j.ymthe.2018.11.010
8. Bast RC
9. Yamazaki-Nakashimada MA, Unzueta A, Berenise Gamez-Gonzalez L, Gonzalez-Saldana N, Sorensen RU. BCG: a vaccine with multiple faces. Hum Vaccin Immunother. 2020;16(8):1841–1850. doi:10.1080/21645515.2019.1706930
10. Kamat AM, Colombel M, Sundi D, et al. BCG-unresponsive non-muscle-invasive bladder cancer: recommendations from the IBCG. Nat Rev Urol. 2017;14(4):244–255. doi:10.1038/nrurol.2017.16
11. Tse J, Singla N, Ghandour R, Lotan Y, Margulis V. Current advances in BCG-unresponsive non-muscle invasive bladder cancer. Expert Opin Investig Drugs. 2019;28(9):757–770. doi:10.1080/13543784.2019.1655730
12. Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293(2):247–269. doi:10.1007/s00404-015-3859-y
13. Lyons TG. Targeted therapies for triple-negative breast cancer. Curr Treat Options Oncol. 2019;20(11):82. doi:10.1007/s11864-019-0682-x
14. Nedeljkovic M, Damjanovic A. Mechanisms of chemotherapy resistance in triple-negative breast cancer-how we can rise to the challenge. Cells. 2019;8(9):957. doi:10.3390/cells8090957
15. Lyons TG, Traina TA. Emerging novel therapeutics in triple-negative breast cancer. Adv Exp Med Biol. 2019;1152:377–399.
16. Wang Y, Liu J, Yang X, et al. Bacillus Calmette-Guerin and anti-PD-L1 combination therapy boosts immune response against bladder cancer. Onco Targets Ther. 2018;11:2891–2899. doi:10.2147/OTT.S165840
17. Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev. 2017;54:58–67. doi:10.1016/j.ctrv.2017.01.007
18. Wu Y, Enting D, Rudman S, Chowdhury S. Immunotherapy for urothelial cancer: from BCG to checkpoint inhibitors and beyond. Expert Rev Anticancer Ther. 2015;15(5):509–523. doi:10.1586/14737140.2015.1015419
19. Ewels PA, Peltzer A, Fillinger S, et al. The nf-core framework for community-curated bioinformatics pipelines. Nat Biotechnol. 2020;38(3):276–278. doi:10.1038/s41587-020-0439-x
20. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12). doi:10.1186/s13059-014-0550-8
21. Wang Q, Wang Y, Ding J, et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579(7799):421–426. doi:10.1038/s41586-020-2079-1
22. Dangaj D, Bruand M, Grimm AJ, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell. 2019;35(6):885–900e810. doi:10.1016/j.ccell.2019.05.004
23. Copland A, Sparrow A, Hart P, et al. Bacillus Calmette-Guerin induces PD-L1 expression on antigen-presenting cells via autocrine and paracrine interleukin-STAT3 circuits. Sci Rep. 2019;9(1):3655. doi:10.1038/s41598-019-40145-0
24. Gouveia ACC, Braga FG, Mota M, et al. Enhanced expression of PD-L1 and IFN-gamma on dendritic cells is associated with BCG-induced Th2 inhibition. Cytokine. 2017;99:163–172. doi:10.1016/j.cyto.2017.09.005
25. Eich ML, Chaux A, Guner G, et al. Tumor immune microenvironment in non-muscle-invasive urothelial carcinoma of the bladder. Hum Pathol. 2019;89:24–32. doi:10.1016/j.humpath.2019.04.003
26. Salmaninejad A, Valilou SF, Shabgah AG, et al. PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234(10):16824–16837. doi:10.1002/jcp.28358
27. Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv324.
28. Yamada H, Matsumoto S, Matsumoto T, Yamada T, Yamashita U. Murine IL-2 secreting recombinant Bacillus Calmette-Guerin augments macrophage-mediated cytotoxicity against murine bladder cancer MBT-2. J Urol. 2000;164(2):526–531. doi:10.1016/S0022-5347(05)67417-4
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.