Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Remnant Cholesterol is an Independent Predictor of New-Onset Diabetes: A Single-Center Cohort Study
Authors Xie G , Zhong Y, Yang S, Zou Y
Received 11 October 2021
Accepted for publication 23 November 2021
Published 3 December 2021 Volume 2021:14 Pages 4735—4745
DOI https://doi.org/10.2147/DMSO.S341285
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Antonio Brunetti
Guobo Xie,1 Yanjia Zhong,2 Shuo Yang,3 Yang Zou4
1Cardiology Department, Jiangxi Provincial People’s Hospital Affiliated to Nanchang University, Nanchang, Jiangxi, People’s Republic of China; 2Endocrinology Department, Jiangxi Provincial People’s Hospital Affiliated to Nanchang University, Nanchang, Jiangxi, People’s Republic of China; 3Cardiology Department, Dean County People’s Hospital, Jiujiang, Jiangxi, People’s Republic of China; 4From the Jiangxi Cardiovascular Research Institute, Jiangxi Provincial People’s Hospital Affiliated to Nanchang University, Nanchang, Jiangxi, People’s Republic of China
Correspondence: Yang Zou Email [email protected]
Purpose: Remnant cholesterol (RC) is the cholesterol of triglyceride-rich lipoproteins, which has a high degree of atherogenic effect. To date, epidemiological evidence supports that higher RC levels lead to a greater risk of adverse cardiovascular events in patients with diabetes, but the nature of the association between RC levels and diabetes risk remains unclear. This study was designed to assess the association of RC with the risk of new-onset diabetes and to investigate whether there is a causal relationship between the two.
Patients and Methods: The subjects included 15,464 individuals of the general population who participated in a health examination. Subjects were quartered according to the RC quartile, and the Cox proportional hazard model was used to assess the independent association between RC and new-onset diabetes.
Results: During an average observation period of 6.13 years, 2.41% of the subjects were diagnosed with new-onset diabetes. Kaplan–Meier analysis showed that the 13-year cumulative diabetes rates corresponding to the RC quartile were 8.62%, 2.49%, 12.78%, and 17.91%. Multivariate Cox regression analysis indicated that higher RC levels were independently associated with an increased risk of new-onset diabetes (HR: 2.44, 95% CI: 1.50– 3.89). Additionally, according to the results of receiver operating characteristic curve analysis, RC had the largest area under the curve (0.7314) compared to traditional lipid parameters in predicting new-onset diabetes.
Conclusion: These results indicated that RC is an important independent predictor of new-onset diabetes in the general population.
Keywords: diabetes, remnant cholesterol, independent predictor, cohort study
Introduction
Diabetes is an important public health problem worldwide and is common in the general population. Diabetes leads to multiple metabolic disorders and a variety of acute and chronic complications, affecting the whole body organ system.1–3 Due to the significant changes in lifestyle and the ageing of the population, the prevalence rate is increasing rapidly.2,4 As reported, the global prevalence rate of type 2 diabetes was 9.3% in 2019, and this figure is expected to increase to 10.9% by 2045 with nearly 700 million people worldwide living with diabetes.5 Additionally, according to the estimates of the International Diabetes Federation, only approximately 50% of people with diabetes have been diagnosed,6 and their potential disease burden will put great pressure on society and the health care system.1,5 Therefore, it is important to understand the changeable risk factors for the prevention of diabetes and the control of its complications.
Diabetes is an independent risk factor for cardiovascular disease and its complications. Active control of glucose can reduce the risk of cardiovascular disease to some extent. However, in recent years, an increasing number of studies have suggested that the main contributor to the increased risk of diabetes-related cardiovascular disease is not abnormal glucose but atherosclerotic lipid abnormalities, which show characteristic quantitative and qualitative changes.7,8 Among them, abnormalities in quantitative lipoproteins are characterized by increased levels of triglycerides (TG) and decreased levels of high-density lipoprotein cholesterol (HDL-C). Qualitative lipoprotein abnormalities include an increase in small dense low-density lipoprotein (LDL) and large, very low-density lipoprotein subfraction 1, as well as increased triacylglycerol content of LDL and HDL.7,8 Remnant cholesterol (RC) is cholesterol-rich in TG lipoprotein, which consists of chylomicron remnants, very low-density LDL and intermediate-density lipoprotein.9 In recent years, a number of epidemiological, biological, and genetic studies have shown that RC is the key component of atherosclerosis, and it has a high atherogenic effect.10–13 High levels of RC indicate a considerable risk of cardiovascular and cerebrovascular events,14–16 especially in patients with type 2 diabetes.17 Some recent data have shown that the concentration of serum RC is significantly increased in patients with type 2 diabetes17,18 and that higher levels of RC not only increase the risk of macrovascular disease in patients with diabetes but also lead to various complications of diabetes.17,19,20 However, the nature of the association between RC levels and diabetes risk remains unclear. The present study was designed to assess the association of RC with the risk of new-onset diabetes and to investigate whether there is a causal relationship between the two.
Materials and Methods
Study Population and Design
The present study used data from the NAGALA cohort study to investigate the association of RC with the risk of new-onset diabetes. The design details of the NAGALA cohort have been described elsewhere.21 In simple terms, this cohort was established in 1994 and continues to be included in the general population enrolled in the health checkup program at Murakami Memorial Hospital where approximately 60% of participants are checked once or twice a year. Considering that many participants were expected to undergo repeated examinations, the NAGALA cohort study conducted a follow-up survey of new-onset diabetes and fatty liver events. At present, the available data in the NAGALA cohort have been shared with the DRYAD database by Okamura et al, and these researchers have transferred the ownership of the data to the DRYAD database.22 According to the DRYAD data terms of service, researchers can make full use of the dataset for secondary analysis on the basis of the new research hypothesis. In previous studies, Okamura et al explored the relationship between ectopic fat obesity and diabetes.21 Based on a recent literature study, the present study established a new research hypothesis and extracted the dataset of the NAGALA cohort from 2004 to 2015, which included general data on 20,944 subjects who completed at least two health examinations. Subjects with the following characteristics were excluded from the study: (i) 1131 subjects diagnosed with diabetes or impaired fasting glucose at baseline; (ii) 2321 subjects who were taking oral medications at baseline; (iii) 416 subjects with viral/alcoholic hepatitis at baseline; (iv) 863 subjects with missing covariate data; (v) 739 subjects who drank excessively (alcohol consumption over 40 g/day for women and 60 g/day for men);23 and (vi) 10 subjects who did not participate in the study for unknown reasons. Since the design of the study had been authorized by the Murakami Memorial Hospital Ethics Committee in the previous study,21 the Ethics Committee of Jiangxi Provincial People’s Hospital exempted the repeated application for ethical approval for this study (ethical review No. 2021–066).
Data Collection and Measurement
Data collection and measurement of subjects in the NAGALA cohort have been described in previous studies.21 In short, trained health care workers used standardized self-administered questionnaires to collect information on the study population’s clinical disease history, medication history, lifestyle factors (history of smoking, drinking, and physical exercise), and general demographic data [waist circumference (WC), sex, weight, systolic/diastolic blood pressure (S/DBP), age, and height]. Blood samples for biochemical analysis were taken after fasting for 8 hours, and the levels of HDL-C, aspartate aminotransferase (AST), total cholesterol (TC), hemoglobin A1c (HbA1c), gamma-glutamyl transferase (GGT), fasting blood glucose (FPG), TG and alanine aminotransferase (ALT) were measured by an automatic analyzer.
Definition and Calculation
Alcohol consumption was classified by assessing the amount and type of alcohol consumed weekly over a period of nearly 1 month (heavy: more than 280 g/week; moderate: 140–280 g/week; light: 40–140 g/week; none or small: less than 40 g/week).23
Smoking status was assessed and classified by asking about smoking history at the baseline visit (none, past, and current).
Exercise habits were defined as participating in any type of exercise more than once a week.
Fatty liver was evaluated by ultrasound. First, colour Doppler ultrasound was performed by technicians through the AlokaSSD-650CL imaging system, and the diagnosis of fatty liver was then made by experienced gastroenterologists.24
The diagnosis of diabetes referred to the criteria of the American Diabetes Association,25 including FPG ≥ 7.0 mmol/L, HbA1c ≥ 6.5%, or starting diabetes treatment. Additionally, the self-reported diagnosis of diabetes from the subjects was also adopted.
Body mass index (BMI) was calculated as weight divided by height2;
Non-HDL-C was calculated as TC - HDL-C;26
LDL-C concentrations were calculated using the modified Friedewald equation: LDL-C (mg/dl) = Non-HDL-C x 90% - TG x 10%;26
RC was calculated as non-HDL-C - LDL-C.27
Statistical Analysis
RC was divided into four categories by quartile, and baseline data were statistically described according to RC categories. The differences between RC groups were compared using a nonparametric test, one-way ANOVA, or Chi-square test. The Kaplan–Meier method was used to calculate the cumulative incidence of diabetes in the general population. A Cox proportional hazard model was then established for univariate and multivariate analyses, and multiple linear regression was used to check the collinearity of covariates (Supplementary Table 1).28 In the present study, a total of three multivariate regression models were constructed according to the suggestion of STROBE’s statement,29 among which Model III was a completely adjusted model. In this model, in addition to adjusting the variables with a P value <0.05 in the univariate analysis, other variables (alcohol consumption and exercise habits) considered to be related to diabetes were also included in the model. Model II adjusted for covariables that changed RC-related diabetes matching risk by more than 10%.30 Model I was a fine-tuning model as it was adjusted only for BMI, sex, and age. Additionally, to observe whether RC and diabetes maintain a linear trend, the study included the RC quartile as a continuous variable in the multivariate regression model for trend analysis. If there was no obvious linear trend between RC and diabetes, the generalized additive model was further used to visualize the relationship between them through cubic spline smoothing. We also used the Cox regression model to perform an exploratory stratified analysis in different populations, and we determined whether there were significant differences among different stratifications by the likelihood ratio test. Finally, we also constructed receiver operating characteristic curves to estimate the ability of RC and other conventional lipids to predict new-onset diabetes.
Statistical software package R (version 3.4.3) and Empower Stats (R, version 2.0) were used to analyze all the data in this study. Bilateral P < 0.05 was considered as a significant standard.
Results
Baseline Characteristics of Subjects
The present study analyzed 15,464 subjects who met the inclusion and exclusion criteria. In this population, 8430 (54.51%) men and 7034 (45.49%) women had a mean age of 43.71 (8.90) years, and 2741 subjects (17.73%) had fatty liver disease at baseline. The mean follow-up was 6.13 years, ranging from 0.46 years to 13.14 years. During the follow-up period, 373 people were diagnosed with new-onset diabetes. Kaplan-Meier analysis showed that the 13-year cumulative incidence of diabetes corresponding to the quartile of RC was 8.62%, 2.49%, 12.78%, and 17.91% (log-rank P<0.0001).
The baseline characteristics of the study subjects by RC level are summarized in Table 1. With the increase in the level of RC, the proportion of men gradually increased, the proportion of women decreased, and the proportion of people who smoke and drink alcohol also gradually increased, suggesting that sex, smoking, and drinking may be associated with RC. In addition, in the group with higher RC levels, age, weight, TC, AST, DBP, non-HDL-C, ALT, WC, TG, HbA1c, LDL-C, SBP, BMI, GGT, and FPG were higher. In the lower RC group, more people maintained exercise habits and had higher HDL-C levels.
 |
Table 1 Baseline Characteristics of Four Groups |
Univariate Analysis
Table 2 summarizes the results of the univariate analysis of the association between covariates and new-onset diabetes. Without adjusting for other variables, all of the covariables, except exercise habits and alcohol consumption, were associated with new-onset diabetes, and only HDL-C was negatively associated with new-onset diabetes. Among all covariates with a positive correlation with diabetes, the variables reflecting blood glucose metabolism, such as FPG and HbA1c, had the strongest correlation with diabetes followed by RC and fatty liver.
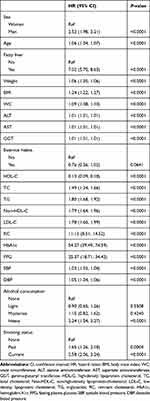 |
Table 2 Associations of Baseline Variables with Prevalence Diabetes by Univariate Regression Analyses |
Correlation Between RC and New-Onset Diabetes in the Multivariate Adjustment Model
Multivariate Cox proportional hazard models were established to evaluate the independent effect of RC on the risk of new-onset diabetes (Table 3). In the fine-tuning model (Model I), each additional 1 mmol/L RC increased the risk of new-onset diabetes by 355%, and the risk of diabetes increased gradually as RC increased (P-trend <0.001). Model II adjusted for fatty liver, HbA1c, BMI, FPG, SBP, and TC. The results of Model II showed that the positive correlation between RC and the risk of new-onset diabetes was slightly weaker than that of Model I [hazard ratios (HR) 2.74, 95% confidence interval (CI) 1.72–4.36], and the trend of linear positive correlation disappeared (P-trend=0.0722). After further adjustment of the covariates (Model III), the positive correlation between RC and diabetes decreased by 10.95% compared to Model II. With each 1 mmol/L increase in RC, the risk of new-onset diabetes increased by 144%, and the positive linear correlation between the RC and new-onset diabetes disappeared (HR: 2.44, 95% CI: 1.50–3.89; P-trend=0.2295), further suggesting that they are not linear correlations. On this basis, the generalized additive model was used to fit the correlation between the continuous variable, RC, and new-onset diabetes. The results indicated that the relationship between the two variables was nonlinear, and the inflection point of the curve correlation occurred when RC was approximately 0.6 mmol/L (Figure 1). In addition, to test the robustness of the main analysis results, we conducted a sensitivity analysis after excluding patients with a baseline diagnosis of fatty liver, and the results showed a stronger independent correlation (Supplementary Table 2). Overall, the multivariate analysis indicated that RC was an independent risk factor for new-onset diabetes, and the retrospective cohort design of this study suggested a causal association.
 |
Table 3 Multivariate Regression Analysis of the Relationship Between RC and New-Onset Diabetes |
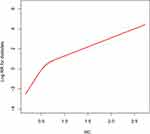 |
Figure 1 Nonlinear relationship between RC and the risk of new-onset diabetes. The area between the blue dotted lines shows 95% confidence interval. Abbreviation: RC, remnant cholesterol. |
Hierarchical Analysis and Interaction Test
Table 4 presents the results of a hierarchical analysis of RC and new-onset diabetes in different populations with the likelihood ratio test examining the differences among different subgroups. The results showed that although RC had a higher risk of new-onset diabetes among middle-aged, female, and non-obese people, there was no significant difference among groups according to the likelihood ratio test. RC-related diabetes risk had no significant interaction with age, sex, BMI, abdominal obesity, or exercise habits. Overall, the positive association of RC with new-onset diabetes was stable in the general population.
 |
Table 4 Stratified Associations Between RC and New-Onset Diabetes by Age, Sex, Abdominal Obesity, Habit of Exercise and BMI |
Predictive Value of RC for the Risk of New-Onset Diabetes
Table 5 shows the area under the receiver operating curve (AUROC) for RC and other conventional lipid parameters used to predict the risk of new-onset diabetes. Compared to TG, TC, non-HDL-C, HDL-C and LDL-C, RC had the largest AUROC (0.7314), a sensitivity of 71.85%, a specificity of 63.60%, and the best threshold was 0.5959 (Figure 2). Additionally, it is worth noting that the accuracy of RC in predicting the risk of new-onset diabetes increased by 12.66% compared to TC. We further incorporated the best threshold of RC as a cut-off point into the multivariate regression model, and the results showed that even in the fully adjusted model, higher RC was positively correlated with new-onset diabetes (Supplementary Table 3).
 |
Table 5 Areas Under the Receiver Operating Characteristic Curves for Each Lipid Parameters in Identifying Diabetes |
Discussion
This retrospective cohort study of 15,464 subjects examined the relationship of RC with new-onset diabetes and found that RC was an independent risk factor for new-onset diabetes, independent of other traditional diabetes risk factors. The results of the subgroup analysis further supported the stable association between RC and new-onset diabetes in the general population. To the best of our knowledge, the present study provides the first evidence of an independent association between RC and new-onset diabetes.
In recent years, RC has been widely studied in the cardiovascular field, and it is considered to be the main factor mediating the residual risk of major cardiovascular events.15,16 Animal experiments and in vitro studies have found that high levels of RC promote the process of atherosclerosis. Similar to LDL-C, RC can penetrate the arterial wall, be engulfed by macrophages and lead to foam cell formation. Moreover, RC also promotes foam cell formation by upregulating the expression of scavenger receptors.31–34 Additionally, high levels of RC have been shown to be associated with arterial wall inflammation in cases of intimal injury.35 These findings have also been confirmed in clinical observational studies, which have reported that RC is associated with the progression of atherosclerosis and is independent of other lipid factors. Genetic studies have further demonstrated that there is a causal link between higher levels of RC and coronary artery disease.15,36,37 In addition to these findings, a growing body of evidence links RC with diabetes and other metabolic diseases with higher levels of RC significantly increasing the risk of complications and major adverse cardiovascular events in patients with diabetes.17–20 According to Sascău et al, triglyceride-rich lipoproteins and their remnants are silenced promoters of atherosclerotic cardiovascular disease and other metabolic diseases.38 Hadi et al reported that RC is associated with prediabetes, and they suggested that RC might have a certain influence on glucose metabolism.39 However, the nature of the relationship of RC with new-onset diabetes is unclear, and in this context, the present study investigated the association between them. In the present study, the univariate analysis demonstrated that RC was an important marker second only to glucose metabolism parameters among many risk factors for diabetes. Further multivariate analysis demonstrated that the association between RC and diabetes was independent and not affected by other risk factors. The present study also assessed the association between RC and diabetes in different populations. Hierarchical Cox regression analysis showed that the risk of diabetes associated with RC was higher in middle-aged, female, and non-obese people. However, further interaction tests did not find significant differences among subgroups. These results indicated that the relationship of RC with new-onset diabetes is relatively stable in the general population. However, the number of new-onset diabetes cases in the present study may be too low, suggesting that further follow-up studies are needed.
Previous studies have shown that TG, HDL-C, and non-HDL-C in conventional lipid profiles are good lipid parameters for predicting diabetes risk.40,41 In the present study, the AUROC of non-HDL-C, TG, LDL-C, HDL-C, RC, and TC for predicting the risk of new-onset diabetes was calculated by ROC analysis. The results showed that the AUROC of RC was greater than that of conventional lipid parameters in predicting the risk of new-onset diabetes, suggesting that RC may be a better indicator of glucose metabolism disorders.42 Importantly, people with diabetes often have higher levels of RC,17,18 and measuring RC may help to distinguish between high-risk patients who are prone to diabetes.
The mechanisms that explain the association between RC and new-onset diabetes are uncertain, but insulin resistance (IR) may be involved in and mediate this association. In a previous study, Ohnishi et al analysed the relationship between blood lipid parameters and IR in 472 rural community residents, and they reported that fasting RC is closely related to IR.42 Subsequent studies by Funada et al found that postprandial RC is also an independent predictor of IR regardless of age, BMI, and other lipid profiles.43 These conclusions further suggest that RC has a stable association with IR; however, whether RC is a better alternative marker for IR needs further research. In addition, in recent clinical trials, some scholars have suggested that resistance exercise, Liraglutide and Empagliflozin can significantly reduce the level of RC in patients with type 2 diabetes, and Empagliflozin can significantly improve the status of IR in patients with type 2 diabetes.44–46 In addition to IR, RC may also directly cause β-cell dysfunction, which in turn leads to reduced insulin secretion.47 Additionally, Shirakawa et al reported that not only is the remnant lipoprotein particle size in patients with type 2 diabetes significantly larger than that in healthy people but that these remnant lipoproteins are also regulated by circulating lipoprotein lipase (LPL) and adiponectin.48 LPL and adiponectin are negatively correlated with remnant lipoproteins, and they may mediate the clearance of RC.49,50 Therefore, we speculated that when RC exceeds a certain threshold, the regulatory effect of LPL and adiponectin on RC may be weakened, thus further increasing the risk of diabetes.
Advantages and Limitations of Research
The present study confirmed the independent association between RC and new-onset diabetes for the first time in a large cohort, and it provided the AUROC and best threshold for RC in predicting new-onset diabetes.
The present study had several limitations. First, there was no oral glucose tolerance test to diagnose diabetes in this study. According to the results of a previous collaborative analysis of Asian and European diabetes epidemiological diagnostic criteria, approximately 68% of new cases of diabetes were detected in the European population only through FPG compared to 55% in Asia.51,52 Therefore, the actual incidence of diabetes may be higher. Second, IR measurement data were lacking in this study. Although the relationship of RC with IR has been demonstrated in previous studies, further research is needed to determine whether the relationship of RC with diabetes is mediated by IR. Third, the present study did not differentiate between types of diabetes. However, the findings of this study may be more applicable to assessing the risk of developing type 2 diabetes because the incidence of type 1 diabetes is extremely low in Japan.53 Fourth, the conclusion of this study is currently mainly applicable to Japanese people, and the association between RC and diabetes in other ethnic groups needs to be further studied. Finally, information, such as hip circumference, neck circumference, and dietary habits, may help to better understand the association of RC with diabetes, but these parameters were lacking in the dataset used in this study, suggesting that the present study may have had some unmeasured confounding.
Conclusion
In summary, the present study demonstrated that RC is an important independent predictor of new-onset diabetes in the general population, independent of traditional diabetes risk factors. These data suggested that RC has the potential to serve as a clinical biomarker for assessing the risk of new-onset diabetes in the general population. Considering the great potential harm of diabetes to the body, it is also necessary to measure RC while monitoring other blood lipid markers in the general population.
Abbreviations
RC, Remnant cholesterol; ROC, receiver operating characteristic curve; TG, triglycerides; HDL-C, high density lipoprotein cholesterol; LDL, low density lipoprotein; WC, waist circumference; S/DBP, systolic/diastolic blood pressure; GGT, gamma-glutamyl transferase; HbA1c, hemoglobin A1c; AST, aspartate aminotransferase; TC, total cholesterol; FPG, fasting blood glucose; ALT, alanine aminotransferase; HR, hazard ratios; CI, confidence interval; AUROC, area under the receiver operating curve; IR, insulin resistance.
Data Sharing Statement
The datasets that support the conclusions of this article can be found in the Dryad repository.
Ethics Statement
We confirm that the data accessed in this study complies with relevant data protection and privacy legislation.
Ethics Approval and Informed Consent
Informed consent for the use of study data had been authorized by the participants in the previous study, and research ethics has been approved by the Murakami Memorial Hospital Ethics Committee.
Acknowledgments
Thanks to Professor Wang for his guidance and comments on this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhou B, Lu Y, Hajifathalian K; NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. doi:10.1016/S0140-6736(16)00618-8
2. Pandey A, Chawla S, Guchhait P. Type-2 diabetes: current understanding and future perspectives. IUBMB Life. 2015;67(7):506–513. doi:10.1002/iub.1396
3. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278–316. doi:10.1210/er.2015-1137
4. Caspersen CJ, Thomas GD, Boseman LA, Beckles GL, Albright AL. Aging, diabetes, and the public health system in the United States. Am J Public Health. 2012;102(8):1482–1497. doi:10.2105/AJPH.2011.300616
5. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
6. International Diabetes Federation. IDF Diabetes Atlas.
7. Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014;63(12):1469–1479. doi:10.1016/j.metabol.2014.08.010
8. Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–899. doi:10.1007/s00125-015-3525-8
9. Twickler TB, Dallinga-Thie GM, Cohn JS, Chapman MJ. Elevated remnant-like particle cholesterol concentration: a characteristic feature of the atherogenic lipoprotein phenotype. Circulation. 2004;109(16):1918–1925. doi:10.1161/01.CIR.0000125278.58527.F3
10. Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. 2019;40(2):537–557. doi:10.1210/er.2018-00184
11. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118(4):547–563. doi:10.1161/CIRCRESAHA.115.306249
12. Salinas CAA, Chapman MJ. Remnant lipoproteins: are they equal to or more atherogenic than LDL? Curr Opin Lipidol. 2020;31(3):132–139. doi:10.1097/MOL.0000000000000682
13. Fujioka Y, Ishikawa Y. Remnant lipoproteins as strong key particles to atherogenesis. J Atheroscler Thromb. 2009;16(3):145–154. doi:10.5551/jat.E598
14. Kim JY, Park JH, Jeong SW, et al. High levels of remnant lipoprotein cholesterol is a risk factor for large artery atherosclerotic stroke. J Clin Neurol. 2011;7(4):203–209. doi:10.3988/jcn.2011.7.4.203
15. Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61(4):427–436. doi:10.1016/j.jacc.2012.08.1026
16. Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi:10.1016/j.atherosclerosis.2019.08.014
17. Yu D, Wang Z, Zhang X, et al. Remnant cholesterol and cardiovascular mortality in patients with type 2 diabetes and incident diabetic nephropathy. J Clin Endocrinol Metab;2021:dgab533. doi:10.1210/clinem/dgab533
18. Schaefer EJ, McNamara JR, Shah PK, et al. Elevated remnant-like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care. 2002;25(6):989–994. doi:10.2337/diacare.25.6.989
19. Jansson Sigfrids F, Dahlström EH, Forsblom C, et al. Remnant cholesterol predicts progression of diabetic nephropathy and retinopathy in type 1 diabetes. J Intern Med. 2021;290(3):632–645. doi:10.1111/joim.13298
20. Jørgensen PG, Jensen MT, Biering-Sørensen T, et al. Cholesterol remnants and triglycerides are associated with decreased myocardial function in patients with type 2 diabetes. Cardiovasc Diabetol. 2016;15(1):137. doi:10.1186/s12933-016-0454-x
21. Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes. 2019;43(1):139–148. doi:10.1038/s41366-018-0076-3
22. Okamura, Takuro et al. (2019), Data from: Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study, Dryad, Dataset. doi:10.5061/dryad.8q0p192
23. Hashimoto Y, Hamaguchi M, Kojima T, et al. Modest alcohol consumption reduces the incidence of fatty liver in men: a population-based large-scale cohort study. J Gastroenterol Hepatol. 2015;30(3):546–552. doi:10.1111/jgh.12786
24. Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102(12):2708–2715. doi:10.1111/j.1572-0241.2007.01526.x
25. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi:10.2337/dc11-S011
26. Chen Y, Zhang X, Pan B, et al. A modified formula for calculating low-density lipoprotein cholesterol values. Lipids Health Dis. 2010;9:52. doi:10.1186/1476-511X-9-52
27. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–635. doi:10.1016/S0140-6736(14)61177-6
28. Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72(6):558–569. doi:10.4097/kja.19087
29. Fitchett EJA, Seale AC, Vergnano S, et al. Strengthening the Reporting of Observational Studies in Epidemiology for Newborn Infection (STROBE-NI): an extension of the STROBE statement for neonatal infection research. Lancet Infect Dis. 2016;16(10):e202–e213. doi:10.1016/S1473-3099(16)30082-2
30. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi:10.1016/j.ijsu.2014.07.014
31. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10(4):328–335. doi:10.2174/138945009787846434
32. Chapman MJ, Ginsberg HN, Amarenco P, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345–1361. doi:10.1093/eurheartj/ehr112
33. Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. 1995;15(4):534–542. doi:10.1161/01.ATV.15.4.534
34. Mearns BM. Dyslipidaemia: role of remnant cholesterol in IHD. Nat Rev Cardiol. 2013;10(10):553. doi:10.1038/nrcardio.2013.132
35. Bernelot Moens SJ, Verweij SL, Schnitzler JG, et al. Remnant cholesterol elicits arterial wall inflammation and a multilevel cellular immune response in humans. Arterioscler Thromb Vasc Biol. 2017;37(5):969–975. doi:10.1161/ATVBAHA.116.308834
36. Hong LF, Yan XN, Lu ZH, et al. Predictive value of non-fasting remnant cholesterol for short-term outcome of diabetics with new-onset stable coronary artery disease. Lipids Health Dis. 2017;16(1):7. doi:10.1186/s12944-017-0410-0
37. Jørgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjærg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34(24):1826–1833. doi:10.1093/eurheartj/ehs431
38. Sascău R, Clement A, Radu R, Prisacariu C, Stătescu C. Triglyceride-rich lipoproteins and their remnants as silent promoters of atherosclerotic cardiovascular disease and other metabolic disorders: a review. Nutrients. 2021;13(6):1774. doi:10.3390/nu13061774
39. Hadi Alijanvand M, Aminorroaya A, Kazemi I, Amini M, Aminorroaya Yamini S, Mansourian M. Prevalence and predictors of prediabetes and its coexistence with high blood pressure in first-degree relatives of patients with type 2 diabetes: a 9-year cohort study. J Res Med Sci. 2020;25:31. doi:10.4103/jrms.JRMS_472_18
40. Athyros VG, Doumas M, Imprialos KP, et al. Diabetes and lipid metabolism. Hormones. 2018;17(1):61–67. doi:10.1007/s42000-018-0014-8
41. Ley SH, Harris SB, Connelly PW, et al. Utility of non-high-density lipoprotein cholesterol in assessing incident type 2 diabetes risk. Diabetes Obes Metab. 2012;14(9):821–825. doi:10.1111/j.1463-1326.2012.01607.x
42. Ohnishi H, Saitoh S, Takagi S, et al. Relationship between insulin-resistance and remnant-like particle cholesterol. Atherosclerosis. 2002;164(1):167–170. doi:10.1016/S0021-9150(02)00057-6
43. Funada J, Sekiya M, Otani T, Watanabe K, Sato M, Akutsu H. The close relationship between postprandial remnant metabolism and insulin resistance. Atherosclerosis. 2004;172(1):151–154. doi:10.1016/j.atherosclerosis.2003.09.016
44. Hattori S. Empagliflozin decreases remnant-like particle cholesterol in type 2 diabetes patients with insulin resistance. J Diabetes Investig. 2018;9(4):870–874. doi:10.1111/jdi.12781
45. Gavin C, Sigal RJ, Cousins M, et al. Resistance exercise but not aerobic exercise lowers remnant-like lipoprotein particle cholesterol in type 2 diabetes: a randomized controlled trial. Atherosclerosis. 2010;213(2):552–557. doi:10.1016/j.atherosclerosis.2010.08.071
46. Matikainen N, Söderlund S, Björnson E, et al. Liraglutide treatment improves postprandial lipid metabolism and cardiometabolic risk factors in humans with adequately controlled type 2 diabetes: a single-centre randomized controlled study. Diabetes Obes Metab. 2019;21(1):84–94. doi:10.1111/dom.13487
47. Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic beta-cell dysfunction. Diabetes. 2007;56(9):2328–2338. doi:10.2337/db07-0056
48. Shirakawa T, Nakajima K, Yatsuzuka S, et al. The role of circulating lipoprotein lipase and adiponectin on the particle size of remnant lipoproteins in patients with diabetes mellitus and metabolic syndrome. Clin Chim Acta. 2015;440:123–132. doi:10.1016/j.cca.2014.10.029
49. Dallinga-Thie GM, Franssen R, Mooij HL, et al. The metabolism of triglyceride-rich lipoproteins revisited: new players, new insight. Atherosclerosis. 2010;211(1):1–8. doi:10.1016/j.atherosclerosis.2009.12.027
50. Chan DC, Watts GF, Ng TW, et al. Adiponectin and other adipocytokines as predictors of markers of triglyceride-rich lipoprotein metabolism. Clin Chem. 2005;51(3):578–585. doi:10.1373/clinchem.2004.045120
51. DECODE Study Group, on behalf of the European Diabetes Epidemiology Study Group. Will new diagnostic criteria for diabetes mellitus change phenotype of patients with diabetes? Reanalysis of European epidemiological data. DECODE Study Group on behalf of the European Diabetes Epidemiology Study Group. BMJ. 1998;317(7155):371–375. doi:10.1136/bmj.317.7155.371
52. Qiao Q, Hu G, Tuomilehto J, et al. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care. 2003;26(6):1770–1780. doi:10.2337/diacare.26.6.1770
53. Neville SE, Boye KS, Montgomery WS, Iwamoto K, Okamura M, Hayes RP. Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev. 2009;25(8):705–716. doi:10.1002/dmrr.1012
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

