Back to Journals » Drug Design, Development and Therapy » Volume 16
Remimazolam Tosylate Combined with Low-Dose Propofol Improves Sedation and Safety in Hysteroscopy
Authors Zhang F , Chang H, Qing W, Yu R, Liao Q , Tong J
Received 19 September 2022
Accepted for publication 5 November 2022
Published 29 November 2022 Volume 2022:16 Pages 4101—4108
DOI https://doi.org/10.2147/DDDT.S390403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Fan Zhang, Huan Chang, Wenxiang Qing, Rili Yu, Qin Liao, Jianbin Tong
Department of Anesthesiology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, 410013, People’s Republic of China
Correspondence: Jianbin Tong; Qin Liao, Department of Anesthesiology, The Third Xiangya Hospital, Central South University, No. 138 Tongzipo Road, Changsha, Hunan, 410013, People’s Republic of China, Email [email protected]; [email protected]
Background: Propofol is widely used for sedation of hysteroscopy. It can cause injection pain, respiratory depression, and hypotension. Remimazolam is a novel ultra-short-acting benzodiazepine. Clinical practice has found that the use of remimazolam alone often leads to body movement during hysteroscopy, which decreases the safety and comfort. Here this study is to investigate whether remimazolam combined with low-dose propofol can improve the sedation effect and safety of hysteroscopy.
Patients and Methods: In this prospective, randomized, parallel-controlled trial, women (18 to 60 years) undergoing hysteroscopy were randomly assigned to receive propofol (Group P), remimazolam tosylate (Group R), or remimazolam tosylate plus propofol (Group RP). Intraoperative sedation depth was kept at the bispectral index (BIS) value of 40– 60. 6 μg/kg alfentanil was used for analgesic before sedation. Intraoperative low pulse oxygen saturation (SpO2), body movement, injection pain, mean arterial pressure (MAP), heart rate (HR), and postoperative recovery time, dizziness, nausea and vomiting were recorded and compared.
Results: From February to July 2022, 193 patients were recruited and randomly assigned to group P (n=64), group R (n=64), or group RP (n=65). There was no significant inter-group difference of the intraoperative BIS values. The incidence of low SpO2, injection pain, hypotension, and postoperative dizziness in group RP were less than that in group P, and had no significant difference from group R. The incidence of body movement in group RP was less than that in group R, and had no significant difference from group P. Postoperative recovery time of group RP was shorter than that of the other two groups. No significant inter-group difference in bradycardia, nausea and vomiting was observed.
Conclusion: Remimazolam tosylate combined with low dose of propofol improved sedation and safety in hysteroscopy, and may be a more ideal sedative method for hysteroscopy.
Keywords: remimazolam tosylate, propofol, sedation, hysteroscopy
Introduction
Uterine diseases are widespread in gynecological patients.1,2 Hysteroscopy is one of the most common minimally invasive procedures for the diagnosis and treatment of uterine diseases.3,4 In order to relieve the anxiety and tension of patients and successfully complete hysteroscopy, propofol is widely used for hysteroscopic sedation.5,6 Propofol exerts its sedative effect mainly by activating gamma amino butyric acid (GABA) receptors.5 It can effectively sedate and improve the patients’ comfort,5,7 but it often causes injection pain, respiratory depression, hypotension and bradycardia.8,9 Remimazolam is an novel ultra-short-acting benzodiazepine.10 It has little inhibiting effect on respiration and hemodynamics,11 and the sedative effect of remimazolam can be rapidly reversed by flumazenil, which has a higher safety profile.12 However, in clinical practice, in the presence of opioid analgesics, sedation with remimazolam alone often causes body movement during hysteroscopy, resulting in difficulty for operative procedure and even an increased risk of uterine perforation. Therefore, it is necessary to find a more ideal hysteroscopic sedation method to improve the patients’ comfort and safety.
In this study, we combined propofol with remimazolam to confirm whether it could provide high quality of sedation and greatly reduce the adverse events during the hysteroscopy.
Materials and Methods
Study Design
We did a prospective, randomized, parallel-controlled trial in the Third Xiangya Hospital of Central South University, China. The trial protocol was approved by the Institutional Review Board of the Third Xiangya Hospital of Central South University (R22002) and registered at http://www.chictr.org.cn (date of registration: December 31st, 2021, ChiCTR2100055034, principal investigator: Dr. Qin Liao) before implementation. Written informed consent was obtained from all enrolled patients. This study complied with the 1964 Helsinki Declaration and its subsequent amendments.
Patients
Patients undergoing elective hysteroscopy were assessed for eligibility on admission. The inclusion criteria were women aged 18 to 60 years, planning to receive painless hysteroscopy, American Society of Anesthesiologists (ASA) class I or II. The exclusion criteria included severe hepatic or renal malfunction, cardiovascular diseases (eg, acute or decompensated heart failure, acute coronary syndrome, third degree atrioventricular block, or severe heart valve disease), craniocerebral diseases, psychiatric disorders, allergy to the drugs involved in this study (eg, benzodiazepines, propofol, opioids, flumazenil or naloxone), patients judged to have difficulty with airway management, or unwillingness to participate in the study for any reason.
Randomization and Masking
According to the above recruitment criteria, the enrolled subjects were randomly assigned to receive propofol (Group P), remimazolam tosylate (Group R), or remimazolam tosylate plus propofol (Group RP). A randomization scheme was generated by a biostatistician, who was independent of statistical analysis of data, using SPSS 25.0 software (SPSS Inc., Chicago, IL, USA). Randomized results were sealed in sequentially numbered envelopes until the end of the study.
To keep blindness, we have taken the following measures; 1) the drugs were prepared by a study nurse and covered with sterile towel, 2) the patients did not know the components of drugs, because the anesthesiologist in charge of anesthesia administered the drugs also covered with sterile towel, 3) the investigator who was responsible for postoperative follow-up and data processing, did not know the group assignment throughout the study period.
Procedures
Preoperative Preparation and Monitoring
All patients routinely fasted for 6 hours and were deprived of drinking for 2 hours before hysteroscopy. After entering the operating room, all patients inhaled oxygen (2 L/min) through a nasal catheter. Electrocardiogram (ECG), HR, non-invasive blood pressure (NIBP), MAP, SpO2, respiratory rate (RR), and BIS were monitored. Then, an intravenous access was established, and compound sodium chloride injection was infused according to the patient’s basic needs, vital signs and intraoperative circulation status.
Grouping and Intraoperative Intervention
After preparations were completed, all cases received intravenous injection of 6 μg/kg alfentanil (Yichang Humanwell Pharmaceutical Co., Ltd., China. LOT No., 13S09041, 13S11021) for analgesic preconditioning.
Patients in group P received 1.5 mg/kg propofol (Jiangsu Yingke Biopharmaceutical Co., Ltd., China. LOT No., 12201041) for anesthesia induction. When the patients had a BIS value of 40–60, hysteroscopy was started. In order to keep the intraoperative BIS value at 40–60, the continuous intravenous infusion rate of propofol was maintained at a dose of 4–10 mg/kg/h.
Patients in group R received 0.2 mg/kg remimazolam tosylate (Jiangsu Hengrui Medicine Co., Ltd., China. LOT No., 211029AK, 210209AU) for anesthesia induction, and a dosage of 0.4–1.0 mg/kg/h by continuous intravenous infusion for anesthesia maintenance to hold the intraoperative BIS value at 40–60. The induction and maintenance doses of remimazolam tosylate described above were based on previous studies, which reported that they were as effective as the induction (2 mg/kg) and maintenance doses (4–10 mg/kg/h) of propofol when used as general anesthesia.6,13,14 When the patients had a BIS value of 40–60, hysteroscopy was initiated.
Patients in group RP received 0.2 mg/kg remimazolam tosylate for anesthesia induction. The continuous intravenous infusion rate of remimazolam tosylate was maintained at a dose of 0.4 mg/kg/h, a relatively low maintenance dose of sedation based on previous reports.6,13,14 Therefore, 0.5 mg/kg propofol was intravenously injected before the start of hysteroscopy. Hysteroscopy was commenced when the patients had a BIS value of 40–60. If the depth of sedation was insufficient (BIS value > 60), the patients received intravenous injection of propofol 0.5mg/kg/time until the BIS value was maintained at 40–60.
If intraoperative analgesia was insufficient (painful face, MAP increase of more than 20% of baseline, HR > 100 beats/min (bpm) or sudden increase of 30 bpm over baseline, or RR > 20 times/min, and the above changes were related to surgical stimulation) in the above three groups, 2 μg/kg/time of alfentanil was administered intravenously. If excessive analgesia (hypotension, bradycardia or respiratory depression), no additional alfentanil was added during the procedure. Sedative and analgesic drugs were all stopped at the end of hysteroscopy.
Management of Perioperative Adverse Events
An appropriate depth of sedation and analgesia was maintained during the operation. If there was obvious respiratory depression, that was, SpO2 < 90%, or respiratory rate < 8 times/minute, lower jaw lifting, inhaled oxygen through a mask or mechanical ventilation would be performed. For the body movement affecting the operation, propofol of 0.5 mg/kg/time would be added in groups P and RP, and remimazolam tosylate of 0.05 mg/kg/time would be added in group R. If adverse hemodynamic events, including hypotension (less than 20% of basal blood pressure) or bradycardia (HR < 50 bpm) occured during the hysteroscopy, ephedrine or atropine would be used as necessary. To prevent postoperative nausea and vomiting, 8 mg of ondansetron hydrochloride was administered intravenously before the end of surgery. If the BIS values of the patients were not greater than 90, and the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score did not reach five at 30 minutes after the operation, the antagonist flumazenil would be used for antagonism in groups R and RP.
Criteria for Patients Leaving the Operating Room After Surgery
After hysteroscopy, all subjects were assessed for recovery of consciousness before leaving the operating room. When their BIS value was greater than 90 and the MOAA/S score reached five, they would leave the operating room and be sent back to the ward.
All of the above anesthesia procedures were carried out by the same project-trained anesthesiologist.
Outcomes
Outcome assessment and postoperative follow-up were performed by another anesthesiologist who had been trained before the study and was not involved in the patient’s anesthesia. The primary outcomes were the incidence of intraoperative low SpO2 and body movement. Low SpO2 was defined as intraoperative SpO2 < 90%. Body movement referred to visible body movement during the hysteroscopy.
The secondary outcomes included injection pain, hypotension, bradycardia, postoperative recovery time, and the incidence of postoperative dizziness, nausea and vomiting. Injection pain referred to the pain reported verbally by patients after the first injection. Hypotension was generally defined as intraoperative blood pressure lower than 20% of basal blood pressure. Bradycardia was defined as intraoperative HR < 50 bpm. Time from the end of the hysteroscopy to meet the criteria for leaving the operating room was defined as postoperative recovery time.
Statistical Analysis
In the preliminary research, the incidence of intraoperative low SpO2 in group P, group R and group RP was 38.5%, 4.7% and 4.2%, respectively. The incidence of intraoperative body movement was 6.7%, 30.6% and 5.2% in groups P, R and RP, respectively. The sample size was calculated using the following settings: two sided, the test level α = 0.05, the test power 1-β = 0.9, and three groups allocation were equal. 60 patients for each of the three groups were required to detect differences. Taking into account the 20% dropout rate, we ultimately needed to enroll 75 patients per group for a total of 225 patients.
Statistical analysis was performed using SPSS Statistics 25.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used to determine whether the continuous variables were in accordance with a normal distribution. Normally distributed continuous variables were expressed as mean ± standard deviation (SD), and continuous variables that did not conform to the normal distribution were expressed as the median (interquartile range). Bartlett's test was used to determine the homogeneity of variances for normally distributed continuous variables. One-way analysis of variance (ANOVA) was used to statistically evaluate the differences of the three groups in continuous variables with normal distribution and homogeneous variance. If the difference was significant, the Student-Newman-Keuls q test was further used for pairwise comparison of each group. Nonparametric test was used to statistically assess the differences of the three groups in non-normally distributed continuous variables. Enumeration data were presented as count (percentage) and compared with the χ2 test. A p value < 0.05 was considered statistically significant. For the significant results, multiple comparisons of the enumeration data (group P to group R, group P to group RP, group R to group RP) were performed, and the α lever was set at 0.017, following Bonferroni adjustment.
Results
From February to July 2022, 225 patients undergoing elective hysteroscopy were screened. 32 patients were excluded due to not meeting the recruitment criteria, refusing to participate, or changing the type of surgery. Finally, 193 patients were enrolled and randomly assigned to group P (n=64), group R (n=64) or group RP (n=65) (Figure 1).
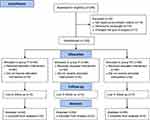 |
Figure 1 Consolidated standards of reporting trials (CONSORT) 2010 flow diagram of patients’ distribution. |
The baseline demographic characteristics, including age, height, weight, body mass index (BMI) and ASA classification, were of no significant difference among the three groups (P > 0.05, respectively) (Table 1). The duration of procedure, intraoperative mean BIS values (including after cervical dilation and before withdrawing the hysteroscope), and the total alfentanil dose were similar among the three groups (P > 0.05, respectively). The total dose of propofol in group RP (0.72 ± 0.29 mg/kg) was lower than that in group P (3.14 ± 0.79 mg/kg; p < 0.01), and the total dose of remimazolam in group RP (0.28 ± 0.05 mg/kg) was significantly lower than that in group R (0.36 ± 0.07 mg/kg; p < 0.01) (Table 1).
 |
Table 1 The Demographic Characteristics and Clinical Data of Patients |
Primary Outcomes
The incidence of intraoperative low SpO2 in group RP (2 [3.1%] of 65 patients) was less than that in group P (25 [39.1%] of 64 patients; p < 0.01), and had no significant difference from group R (2 [3.1%] of 64 patients; p > 0.05). During the hysteroscopy, the incidence of body movement in group RP (2 [3.1%] of 65 patients) was lower than that in group R (24 [37.5%] of 64 patients; p < 0.01), and had no significant difference from group P (3 [4.7%] of 64 patients; p > 0.05). However, body movement did not interfere with the operation or lead to dropout from the research (Table 2).
 |
Table 2 Primary Outcomes and Secondary Outcomes Among the Three Groups |
Secondary Outcomes
Secondary outcomes among the three groups were summarized in Table 2. The incidence of injection pain was as high as 60.9% in group P, but not occur in groups R and RP. The incidence of intraoperative hypotension in groups R (6 [9.4%] of 64 patients) and RP (10 [15.4%] of 65 patients) was significantly lower than that in group P (27 [42.2%] of 64 patients; p < 0.01, respectively), and there was no significant difference between group R and group RP (p > 0.05). No significant difference was found in the incidence of intraoperative bradycardia among the three groups (p > 0.05). The postoperative recovery time of group RP (169.60 ± 45.80 s) was significantly shorter than that of groups P (239.20 ± 50.22 s) and R (268.10 ± 39.88 s; p < 0.01, respectively). The incidence of postoperative dizziness in group P (21 [32.8%] of 64 patients) was higher than that in groups R (2 [3.1%] of 64 patients) and RP (1 [1.5%] of 65 patients; p < 0.01, respectively). The three groups did not experience obvious nausea and vomiting after hysteroscopy (Table 2).
All subjects successfully underwent hysteroscopy by the same experienced surgical team. Patients in groups R and RP were not given flumazenil, because their BIS values were all greater than 90, and the MOAA/S score reached five at 30 minutes after the operation. No serious adverse events requiring withdrawal from the trial were observed in the three groups of patients.
Discussion
This study aimed to investigate whether remimazolam combined with low-dose propofol could improve the sedation and safety in hysteroscopy. We found that, with intraoperative BIS values of 40–60, sedation with remimazolam and low-dose of propofol showed less incidence of low SpO2, body movement, injection pain, hypotension and postoperative dizziness, and faster postoperative recovery than with remimazolam or propofol alone. This information supports that the combination of remimazolam and low-dose propofol can provide high quality of sedation and safety for the hysteroscopy.
Previous studies have shown that propofol functions as a sedative via acting mainly on the β subunit of GABA receptors, directly opening the chloride (Cl−) channels, and then inducing postsynaptic inhibition.15,16 Remimazolam functions as a sedative via activating benzodiazepine receptors located on the α subunit of GABA receptors, increasing the frequency of Cl− channels opening, and then inducing postsynaptic inhibition.17,18 These support that the two drugs can play synergistic effect of sedation. In fact, in our study, the doses of propofol and remimazolam in group RP are less than that in group R and group P, when keeping BIS value at 40–60. In addition, previous studies have shown that low-dose propofol has less effects on activation of GABAergic neurons in kernel respiratory rhythmogenesis nucleus pre-Bötzinger complex (PrBo) and its respiratory depression,19 has less irritation on venous adventitia,20 has less inhibition on sympathetic nerve activity and myocardial contractility,21,22 and has less accumulation in adipose tissue.23 These provide explanation to the less incidence of respiratory and circulatory depression, injection pain, and postoperative dizziness in group RP. Moreover, propofol can decrease the release of the excitatory neurotransmitters glutamate and norepinephrine,24,25 it is helpful to keep the balance between excitability and inhibition in brain when remimazolam is used to sedate, and consequently decrease the body movement in group RP. The real reason remains elusive.
Our study confirmed the effectiveness and safety of remimazolam combined with low-dose of propofol for sedation in hysteroscopy. But several limitations still need to be considered in our study. First, for the sake of safety and to reduce individual differences within the groups, patients, with more than 60 years old or lower than 18 years old, with complex comorbidities, with ASA class III or higher were excluded. Second, since propofol and remimazolam could be distinguished by naked eyes, it is difficult to achieve complete blindness in this study. However, we have taken some measures to keep the patients and the recorder unaware of the group assignment. Third, it was a single-center investigation, and the findings of this study need to be further confirmed by multi-center studies.
Conclusion
Remimazolam tosylate combined with low-dose of propofol improved sedation and safety for hysteroscopy. It may be a more ideal sedative method for hysteroscopy.
Data Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request.
Acknowledgments
We thank our colleagues at the department of anesthesiology and gynecological surgery for their cooperation in facilitating this trial.
Funding
This study was supported by the New Xiangya Talent Projects of the Third Xiangya Hospital of Central South University (No. 20180303); the Scientific Research Project “Medical Empowerment and Talent Training Program” of the Chinese Red Cross Foundation Medical Empowerment Public Welfare Special Fund.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Boretto M, Maenhoudt N, Luo X, et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21(8):1041–1051. doi:10.1038/s41556-019-0360-z
2. Louie MY, Vegunta S. Abnormal uterine bleeding in perimenopausal women. J Womens Health. 2022;31(8):1084–1086. doi:10.1089/jwh.2022.0273
3. Zhou H, Lai KF, Xiang Q, et al. Oncological safety of diagnostic hysteroscopy for apparent early-stage Type II endometrial cancer: a multicenter retrospective cohort study. Front Oncol. 2022;12:918693. doi:10.3389/fonc.2022.918693
4. Qiu DE, Zhang WL, Liu J, et al. Comparison of the reproductive outcome between 2 and 4 mg daily doses of estradiol after hysteroscopic adhesiolysis: a propensity score matching analysis-retrospective cohort study. Front Endocrinol. 2022;13:775755. doi:10.3389/fendo.2022.775755
5. Bingol Tanriverdi T, Koceroglu I, Devrim S, et al. Comparison of sedation with dexmedetomidine vs propofol during hysteroscopic surgery: single-centre randomized controlled trial. J Clin Pharm Ther. 2019;44(2):312–317. doi:10.1111/jcpt.12793
6. Zhang X, Li S, Liu J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single-centre randomized controlled trial. BMC Anesthesiol. 2021;21(1):156. doi:10.1186/s12871-021-01373-y
7. Crespo J, Teran A. Endoscopy and sedation: an inseparable binomial for the gastroenterologist. Rev Esp Enferm Dig. 2018;110(4):250–252. doi:10.17235/reed.2018.5585/2018
8. Guo J, Qian Y, Zhang X, et al. Remimazolam tosilate compared with propofol for gastrointestinal endoscopy in elderly patients: a prospective, randomized and controlled study. BMC Anesthesiol. 2022;22(1):180. doi:10.1186/s12871-022-01713-6
9. Nolan PJ, Delgadillo JA, Youssef JM, et al. Dexmedetomidine provides fewer respiratory events compared with propofol and fentanyl during third molar surgery: a randomized clinical trial. J Oral Maxillofac Surg. 2020;78(10):1704–1716. doi:10.1016/j.joms.2020.05.015
10. Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021;12:690875. doi:10.3389/fphar.2021.690875
11. Sneyd JR, Rigby-Jones AE. Remimazolam for anaesthesia or sedation. Curr Opin Anaesthesiol. 2020;33(4):506–511. doi:10.1097/ACO.0000000000000877
12. Morimoto Y. Efficacy and safety profile of remimazolam for sedation in adults undergoing short surgical procedures. Ther Clin Risk Manag. 2022;18:95–100. doi:10.2147/TCRM.S304556
13. Zhang S, Wang J, Ran R, et al. Efficacy and safety of remimazolam tosylate in hysteroscopy: a randomized, single-blind, parallel controlled trial. J Clin Pharm Ther. 2022;47(1):55–60. doi:10.1111/jcpt.13525
14. Doi M, Morita K, Takeda J, et al. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543–553. doi:10.1007/s00540-020-02788-6
15. Sajeeda S, Kumar L, Verma R. An overview of analytical methods for the estimation of propofol in pharmaceutical formulations, biological matrices, and hair marker. Crit Rev Anal Chem. 2022;52(7):1694–1701.
16. Borghese CM, Wang HL, McHardy SF, et al. Modulation of alpha1beta3gamma2 GABAA receptors expressed in X. laevis oocytes using a propofol photoswitch tethered to the transmembrane helix. Proc Natl Acad Sci U S A. 2021;118(8):e2008178118. doi:10.1073/pnas.2008178118
17. Wallner M, Hanchar HJ, Olsen RW. Alcohol selectivity of beta3-containing GABAA receptors: evidence for a unique extracellular alcohol/imidazobenzodiazepine Ro15-4513 binding site at the alpha+beta- subunit interface in alphabeta3delta GABAA receptors. Neurochem Res. 2014;39(6):1118–1126. doi:10.1007/s11064-014-1243-0
18. McGoldrick MK, Galanopoulou AS. Developmental pharmacology of benzodiazepines under normal and pathological conditions. Epileptic Disord. 2014;16(1):S59–S68. doi:10.1684/epd.2014.0690
19. Jiang J, Jiao Y, Gao P, et al. Propofol differentially induces unconsciousness and respiratory depression through distinct interactions between GABAA receptor and GABAergic neuron in corresponding nuclei. Acta Biochim Biophys Sin. 2021;53(8):1076–1087. doi:10.1093/abbs/gmab084
20. Desousa KA. Pain on propofol injection: causes and remedies. Indian J Pharmacol. 2016;48(6):617–623. doi:10.4103/0253-7613.194845
21. Hoka S, Yamaura K, Takenaka T, et al. Propofol-induced increase in vascular capacitance is due to inhibition of sympathetic vasoconstrictive activity. Anesthesiology. 1998;89(6):1495–1500. doi:10.1097/00000542-199812000-00028
22. Massolo AC, Sgro S, Piersigilli F, et al. Propofol formulation affects myocardial function in newborn infants. Pediatr Cardiol. 2019;40(7):1536–1542. doi:10.1007/s00246-019-02182-4
23. Hayes E, Esteves A. Adherence to sedation targets with weight-based propofol and dexmedetomidine in patients with morbid obesity. Ann Pharmacother. 2022;10600280221108429. doi:10.1177/10600280221108429
24. Gelegen C, Miracca G, Ran MZ, et al. Excitatory pathways from the lateral habenula enable propofol-induced sedation. Curr Biol. 2018;28(4):580–587.e5. doi:10.1016/j.cub.2017.12.050
25. Han L, Fuqua S, Li Q, et al. Propofol-induced inhibition of catecholamine release is reversed by maintaining calcium influx. Anesthesiology. 2016;124(4):878–884. doi:10.1097/ALN.0000000000001015
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
