Back to Journals » Drug Design, Development and Therapy » Volume 16
Remimazolam for the Prevention of Emergence Delirium in Children Following Tonsillectomy and Adenoidectomy Under Sevoflurane Anesthesia: A Randomized Controlled Study
Authors Yang X, Lin C, Chen S , Huang Y , Cheng Q, Yao Y
Received 11 July 2022
Accepted for publication 20 September 2022
Published 30 September 2022 Volume 2022:16 Pages 3413—3420
DOI https://doi.org/10.2147/DDDT.S381611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Xue Yang,1,* Chuantao Lin,2,* Sisi Chen,3 Yuezhou Huang,3 Qiong Cheng,1 Yusheng Yao3
1Department of Neurology, Shengli Clinical Medical College of Fujian Medical University, Fuzhou, People’s Republic of China; 2Department of Anesthesiology, Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, People’s Republic of China; 3Department of Anesthesiology, Shengli Clinical Medical College of Fujian Medical University, Fuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiong Cheng, Email [email protected]
Purpose: To identify the effectiveness of remimazolam at the end of tonsillectomy and adenoidectomy for preventing emergence delirium in children under sevoflurane anesthesia.
Patients and Methods: One hundred and four patients aged 3– 7 years scheduled for tonsillectomy and adenoidectomy under sevoflurane anesthesia were recruited. Patients were randomly assigned to receive either remimazolam 0.2 mg kg– 1 (intervention, n=52) or 0.9% normal saline (control, n=52) at the end of the procedure. The primary outcome was the incidence of emergence delirium, defined as a Pediatric Anesthesia Emergence Delirium (PAED) score ≥ 10. Secondary outcomes were peak PAED score, emergence time, postoperative pain intensity, length of postanesthesia care unit (PACU) stay, parental satisfaction, and postoperative behavior changes three days postoperatively.
Results: Emergence delirium occurred in 6 of 51 (12%) patients receiving remimazolam versus 22 of 50 (44%) patients receiving saline (risk difference 32% [95% confidence interval, 16% to 49%], relative risk 0.27 [95% confidence interval, 0.12 to 0.60]; P< 0.001). The peak PAED scores (median [interquartile range]) were lower in the remimazolam group than in the saline group (7 [6– 8] versus 9 [8– 11], P< 0.001). Likewise, parental satisfaction was improved in the remimazolam group compared with the saline group (9 [8– 10] versus 8 [7– 8], P< 0.001). There was no difference between groups concerning postoperative pain scores, length of PACU stay, or postoperative behavior changes.
Conclusion: In children undergoing tonsillectomy and adenoidectomy, administration of remimazolam 0.2 mg kg– 1 at the end of the surgery, compared with 0.9% saline, resulted in a significantly lower likelihood of emergence delirium after sevoflurane anesthesia.
Keywords: remimazolam, general anesthesia, pediatric anesthesia, emergence delirium
Introduction
Sevoflurane, as an inhalation anesthetic for the induction and maintenance of general anesthesia, is the most commonly used in pediatric patients. However, emergence delirium is a well-known postoperative behavior disorder after sevoflurane anesthesia in the pediatric population,1 with a reported incidence of up to 67%.2 This phenomenon is characterized by screaming, thrashing, kicking, and nonpurposeful restlessness,3 which may cause dissatisfaction among parents and self-injury and may increase the workload of postanesthesia care unit (PACU) nurses.
Rapid awakening from sedation was considered to be an independent risk factor.4 Several pharmacological interventions have been studied to prevent emergence delirium from sevoflurane anesthesia, allowing a smooth emergence, such as nonopioid analgesics, opioids,5 propofol,6 α2 agonists,7 and midazolam.8 However, drugs administered for treatment may result in delayed extubation and prolonged PACU stays,9 even following short surgical operations.10
Remimazolam is a novel ultrashort-acting benzodiazepine that has been used for procedural sedation.11 Clinical trials have shown that remimazolam can provide rapid onset sedation with prompt recovery while minimizing respiratory depression and blood pressure perturbation. Given that it is rapidly hydrolyzed to an inactive metabolite, it is not affected by hepatic or renal impairment.12 Thus, remimazolam has a predictable duration and controlled sedation. However, its effect on preventing emergence delirium after sevoflurane anesthesia and long-term negative behavioral changes after discharge has not been defined.
Therefore, we conducted this prospective, double-blind, randomized, placebo-controlled clinical trial in children following tonsillectomy and adenoidectomy surgery. Our primary hypothesis was that a single bolus injection of postoperative remimazolam would reduce the incidence of emergence delirium after sevoflurane anesthesia.
Materials and Methods
Study Design
This investigator-initiated, prospective, randomized, double-blind, placebo-controlled superiority study was approved by the Ethics Committee of Fujian Provincial Hospital (K2021-12-022, Supplementary File 1), Fuzhou, China. We registered the study protocol at the Chinese Clinical Trials Registry (http://www.chictr.org.cn, ChiCTR2100054959, 29/12/2021). Before study enrollment, each subject’s legal representatives ensured their written consent form (Supplementary File 2). We conducted this clinical trial at Fujian Provincial Hospital, following the provisions of the Declaration of Helsinki (as revised in 2013) and the Good Clinical Practice principles.13 Our manuscript was prepared according to the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement.14
Eligible subjects aged 3–7 years old with an American Society of Anesthesiologists (ASA) physical status of I or II were scheduled to undergo elective bilateral tonsillectomy and adenoidectomy under general anesthesia. Exclusion criteria included neuropsychiatric diseases, cardiopulmonary disease, acute upper respiratory tract infection/recovery period, developmental delay, and intake of any sedative drug before surgery. An independent researcher not involved in the study prepared a computer-generated nonblocked randomization sequence with a 1:1 ratio and sealed it in sequentially numbered, opaque envelopes. On the morning of surgery, an independent research nurse, who was not involved in the patients’ care or data collection, opened the envelope and prepared the study medications in identical syringes. All subject’s parents, attending anesthesiologists, caring nurses, and investigators involved in the study were blinded to the group assignments.
Procedures
All patients received no premedication and were accompanied by a parent to the operating room, where an anesthetist, blinded to the group allocation, induced general anesthesia. Standard monitoring, including peripheral oxygen saturation, electrocardiography, and noninvasive blood pressure, was conducted on all subjects. General anesthesia was induced with 5% sevoflurane in 100% oxygen at 6 L min–1 via a facemask. Intravenous access was secured after achieving an adequate depth of anesthesia. Propofol 2 mg kg–1, mivacurium 0.2 mg kg–1, and sufentanil 2 μg kg–1 were administered to facilitate endotracheal intubation. Anesthesia was maintained by inhalation of sevoflurane in a 50% nitrous oxide and 50% oxygen mixture. Volume-controlled mechanical ventilation was administered to maintain an end-tidal carbon dioxide partial pressure of 35–45 mmHg. Before the surgical procedure, patients received 0.15% ropivacaine infiltration around the peritonsillar fossa and intravenous flurbiprofen axetil (a nonsteroidal anti-inflammatory drug) 1 mg kg–1 for postoperative analgesia. After completion of the operation, sevoflurane and nitrous oxide were discontinued. At the same time, children were randomized to receive either intravenous remimazolam 0.2 mg kg–1 diluted in 10 mL saline (intervention group, n=52) or an equivalent volume of 0.9% saline (control group, n=52). After tracheal extubation, the children were accompanied by one of the child’s parents after arrival at the PACU. If delirium occurred, propofol 1 mg kg–1 was administered as a rescue medication and repeated if the delirium did not subside.
Outcome Assessments
The primary outcome was the incidence of emergence delirium. We used the Pediatric Anesthesia Emergence Delirium (PAED) scale15 to evaluate emergence delirium every 5 min for the first 30 min in the PACU. The PAED scale consists of five items: eye contact, the purposefulness of actions, awareness of surroundings, restlessness, and inconsolable. Items 1, 2, and 3 are scored as follows: 4=not at all, 3=just a little, 2=quite a bit, 1=very much, 0=extremely. Items 4 and 5 are scored reversely. Emergence delirium was defined as a global PAED score ≥10. Secondary outcomes included the peak PAED score, emergence time, postoperative pain intensity, length of PACU stay, parental satisfaction, and postoperative behavior changes 3 days postoperatively. We defined emergence time as the interval from discontinuation of inhalation anesthetic to eye-opening on verbal command. Postoperative pain intensity was assessed using the Face, Legs, Activity, Cry, and Consolability (FLACC) scale16 every 10 min during the first 30 min in the PACU. The FLACC pain scale is a composite of five behaviors (face, legs, activity, cry and consolability), and each item is graded on zero to two by an observer. When the FLACC score was ≥4, morphine 25 μg kg−1 intravenously was administered as rescue analgesia. Preoperative anxiety was evaluated using the modified Yale Preoperative Anxiety Scale (m-YPAS)17 before the anesthesiologist’s visit in the preoperative holding area. The length of PACU stay was defined as the time between arrival in the PACU and readiness for discharge from the PACU (defined as a modified Aldrete score of ≥9). Parental satisfaction was self-reported using the numeric rating scale (NRS, range 0–10) 24 hours after surgery, where 0 equals unsatisfied and 10 equals completely satisfied. Negative postoperative behavioral changes were determined on postoperative day three via telephone interview using the 27-item Post Hospitalization Behavior Questionnaire (PHBQ).18 The PHBQ consists of the following subscales: general anxiety and regression, separation anxiety, anxiety about sleep, eating disturbance, aggression toward authority, and apathy withdrawal. For each item, the parent was invited to compare the child’s current behavioral problems with the period before hospitalization (1=much less, 2=less, 3=unchanged, 4=more, and 5=much more). Intraoperative adverse events, such as postoperative nausea or vomiting (PONV), bradycardia (defined as heart rate <50 beats min–1), oxygen desaturation (defined as peripheral capillary oxygen saturation <90%), and laryngospasm, were also recorded. A single investigator who performed the above assessments was blinded to the group allocation.
Statistical Analysis
Our sample size was calculated based on the incidence of emergence delirium using the online Power and Sample Size Calculators (http://powerandsamplesize.com/Calculators/). Our previous study reported that the incidence of emergence delirium was 49% in children under sevoflurane anesthesia.19 We considered an absolute 30% reduction in the incidence of emergence delirium between groups to be clinically relevant. Therefore, a sample size of 48 patients per group was estimated to provide a power of 90% to reject the null hypothesis of equal proportion using a 2-sample, 2-sided equality test at the 0.05 significance level. After accounting for potential dropouts (due to withdrawals and other unexpected events), we concluded to enroll 104 patients for this study.
IBM SPSS for Windows version 25.0 software was used for statistical analyses. Continuous variables are presented as the mean ± standard deviation (SD) or median (interquartile range, IQR). Continuous variables were analyzed using an independent t-test or the Mann–Whitney U-test. All group comparisons are reported as absolute differences with 95% confidence intervals (CIs) and P values. Categorical variables are expressed as numbers (percentages, %) and were compared by Pearson’s χ2 test or Fisher’s exact test as appropriate. The criterion for rejection of the null hypothesis was P<0.05.
Results
A CONSORT flow diagram of subject eligibility, randomization, and follow-up is presented in Figure 1. A total of 114 subjects were enrolled in this study between January 2022 and May 2022. Ten subjects were excluded for the following reasons: four failed to meet the inclusion criteria, and six declined to participate. One subject in the remimazolam group and two in the saline group violated the study protocol. Consequently, 51 subjects in the remimazolam group and 50 subjects in the saline group were included in the final analysis. Demographic characteristics (age, height, weight, sex, and ASA status) and perioperative clinical data (m-YPAS score, duration of surgery, and anesthesia time) were similar in both groups (Table 1).
 |
Table 1 Demographic and Peri-Operative Characteristics |
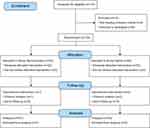 |
Figure 1 Consolidated Standards of Reporting Trials (CONSORT) flow diagram. Adapted from Schulz K, Altman D, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340:c332.14 |
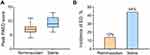
The peak PAED scores (median [IQR]) were lower in the remimazolam group than in the saline group (7 [6–8] vs 9 [8–11], P<0.001; Figure 2), with a median difference of −2 (95% CI −1 to −3). Emergence delirium occurred in 6 of 51 (12%) patients receiving remimazolam versus 22 of 50 (44%) patients receiving saline (risk difference 32% [95% CI, 16% to 49%], relative risk=0.27 [95% CI, 0.12 to 0.60]; P<0.001).
As detailed in Table 2, the median emergence time in the remimazolam group (21 min, IQR 19–23 min) was slightly longer than that in the saline group (14 min, IQR 13–15 min), with a median difference of 7 min (95% CI 6 to 8 min), P<0.001. However, comparisons between the remimazolam and saline groups were similar for the length of PACU stay (median difference −1 min, 95% CI −2 to 0 min; P=0.165). In addition, parental satisfaction scores were significantly higher in the remimazolam group than in the saline group (P<0.001). There were no significant differences in the postoperative pain scores (P=0.221). The peak FLACC pain score was 2.0 (IQR, 1.0–2.0) in the remimazolam group and 2.0 (IQR, 2.0–2.0) in the control group. Considering the incidence of negative behavioral changes on postoperative day three, no differences were detected between groups (remimazolam 14 of 51 [27%] vs saline 18 of 50 [36%], P=0.356). Postoperative nausea or vomiting occurred in 2 of 50 (4%) subjects in the saline group and 1 of 51 (2%) subjects in the remimazolam group. No episodes of bradycardia, hypotension, laryngospasm, or hypoxemia were identified during the study period.
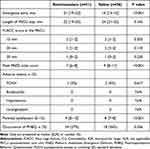 |
Table 2 Postoperative Outcomes and Complications |
Discussion
This study demonstrated that intravenous remimazolam 0.2 mg kg–1 administered at the end of tonsillectomy significantly decreased the incidence of delirium after sevoflurane anesthesia in pediatric patients. Furthermore, intravenous remimazolam effectively improves parental satisfaction without delaying the length of PACU stay or increasing clinically relevant adverse events.
The mechanism of emergence delirium is still unclear, but it may be correlated with rapid awakening, postoperative pain, preoperative anxiety, type of surgery, and preschool age.20,21 In this randomized clinical trial, approximately half of the subjects experienced emergence delirium (defined as PAED scores ≥10) under general anesthesia in the control group, consistent with previous studies.22 Administering remimazolam 0.2 mg kg–1 at the end of surgery dramatically decreased the incidence of emergence delirium (from 44% to 12%), indicating that remimazolam is an alternative adjunct for preventing emergence delirium.
Rapid awakening has been described as a contributing factor to emergence delirium.23 The emergence delayed by an average of 7 min would suggest that the mechanism of action of the remimazolam transition may facilitate sevoflurane washout before the point of emergence. Interestingly, the results of the current study showed that the length of PACU stay was similar between the two groups. One explanation may be that remimazolam has a short elimination half-life and lacks cumulative properties.24 Our study showed that patients who suffered from inconsolable emergence delirium received timely treatment with propofol without delaying the discharge time from the PACU, which is in line with a previous study.6
A clinical study indicated that postoperative pain was an independent risk factor for emergence delirium,25 which must be considered when assessing emergence delirium. In the current study, we used 0.15% ropivacaine infiltration and intravenous 1 mg kg−1 flurbiprofen axetil injection to manage postoperative pain. Thus, the possibility of pain acting as a confounding factor was minimized. It has been suggested that “no eye contact”, “no purposeful action”, and “no awareness of surroundings” significantly correlate with emergence delirium; acute pain behavior tends to exhibit abnormal facial expressions, crying, and inconsolability.26 In addition, the FLACC scale was used to distinguish delirium from irritability caused by pain to the greatest extent, which showed no significant difference in both groups. A previous study also demonstrated that emergence delirium correlates with an increased anxiety.27 However, our results demonstrated that children in the remimazolam and control groups had similar preoperative anxiety scores. In this study, children were accompanied by one of their parents in the preoperative holding area and PACU, which may help reduce their fear of unfamiliar surroundings.
There are several limitations to the study. First, we did not assess the dose–response relationship between remimazolam and emergence delirium. A single 0.2 mg kg–1 intravenous dose of remimazolam was selected based on our previous study28 and the pilot data. Second, our study was conducted as a single-center design, and we only included pediatric patients undergoing tonsillectomy and adenoidectomy, which is a risk factor for emergence delirium. Thus, the external validity of our findings needs to be further assessed. Third, the duration of follow-up was limited to 3 days, so the long-term effectiveness of remimazolam on emergence delirium is unclear.
Conclusion
The findings of this trial support that remimazolam 0.2 mg kg–1 administered at the end of surgery decreased the incidence of emergence delirium without delaying PACU discharge in children following tonsillectomy and adenoidectomy surgery under sevoflurane anesthesia.
Data Sharing Statement
After publication, the individual deidentified participant data underlying published results, the study protocol, and the statistical analysis plan can be accessed upon reasonable request from the corresponding author (Dr Qiong Cheng, [email protected]).
Acknowledgments
This study was supported by the Comfort Medical Research Project of Fujian Strait Medical and Health Exchange Association (No. 2020-HYH-03), the Medical Innovation Project of Fujian Province (No.2019-CXB-6), and the Natural Science Foundation of Fujian Province (No. 2021J01378).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Veyckemans F. Excitation and delirium during sevoflurane anesthesia in pediatric patients. Minerva Anestesiol. 2002;68(5):402–405.
2. Komazaki M, Mihara T, Nakamura N, et al. Preventive effect of ramelteon on emergence agitation after general anesthesia in pediatric patients undergoing tonsillectomy: a randomized, placebo-controlled clinical trial. Sci Rep. 2020;10(1):21996. doi:10.1038/s41598-020-79078-4
3. Malarbi S, Stargatt R, Howard K, et al. Characterizing the behavior of children emerging with delirium from general anesthesia. Paediatr Anaesth. 2011;21(9):942–950. doi:10.1111/j.1460-9592.2011.03646.x
4. Moore AD, Anghelescu DL. Emergence Delirium in Pediatric Anesthesia. Paediatr Drugs. 2017;19(1):11–20. doi:10.1007/s40272-016-0201-5
5. Kim MS, Moon BE, Kim H, et al. Comparison of propofol and fentanyl administered at the end of anesthesia for prevention of emergence agitation after sevoflurane anesthesia in children. Br J Anaesth. 2013;110(2):274–280. doi:10.1093/bja/aes382
6. Aouad MT, Yazbeck-Karam VG, Nasr VG, et al. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology. 2007;107(5):733–738. doi:10.1097/01.anes.0000287009.46896.a7
7. Shi M, Miao S, Gu T, et al. Dexmedetomidine for the prevention of emergence delirium and postoperative behavioral changes in pediatric patients with sevoflurane anesthesia: a double-blind, randomized trial. Drug Des Devel Ther. 2019;13:897–905. doi:10.2147/DDDT.S196075
8. Kawai M, Kurata S, Sanuki T, et al. The effect of midazolam administration for the prevention of emergence agitation in pediatric patients with extreme fear and noncooperation undergoing dental treatment under sevoflurane anesthesia, a double-blind, randomized study. Drug Des Devel Ther. 2019;13:1729–1737. doi:10.2147/DDDT.S198123
9. Ramlan AAW, Pardede DKB, Marsaban AHMS, et al. Efficacy of 0.5 mg/kg of propofol at the end of anesthesia to reduce the incidence of emergence agitation in children undergoing general anesthesia with sevoflurane. J Anesthesiol Clin Pharmacol. 2020;36(2):177–181. doi:10.4103/joacp.JOACP_257_19
10. Cho EJ, Yoon SZ, Cho JE, et al. Comparison of the effects of 0.03 and 0.05 mg/kg midazolam with placebo on prevention of emergence agitation in children having strabismus surgery. Anesthesiology. 2014;120(6):1354–1361. doi:10.1097/ALN.0000000000000181
11. Lee A, Shirley M. Remimazolam: a review in procedural sedation. Drugs. 2021;81(10):1193–1201. doi:10.1007/s40265-021-01544-8
12. Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127(3):415–423. doi:10.1016/j.bja.2021.05.027
13. Mentz RJ, Hernandez AF, Berdan LG, et al. Good clinical practice guidance and pragmatic clinical trials: balancing the best of both worlds. Circulation. 2016;133(9):872–880. doi:10.1161/CIRCULATIONAHA.115.019902
14. Schulz K, Altman D, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340:c332. doi:10.1136/bmj.c332
15. Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100(5):1138–1145. doi:10.1097/00000542-200405000-00015
16. Crellin DJ, Harrison D, Santamaria N, et al. Systematic review of the face, legs, activity, cry and consolability scale for assessing pain in infants and children: is it reliable, valid, and feasible for use? Pain. 2015;156(11):2132–2151. doi:10.1097/j.pain.0000000000000305
17. Kain Z, Mayes L, Cicchetti D, et al. The Yale Preoperative Anxiety Scale: how does it compare with a ”gold standard”? Anesth Analg. 1997;85(4):783–788. doi:10.1213/00000539-199710000-00012
18. Vernon DT, Schulman JL, Foley JM. Changes in children’s behavior after hospitalization. Some dimensions of response and their correlates. Am J Dis Child. 1966;111(6):581–593. doi:10.1001/archpedi.1966.02090090053003
19. Yao Y, Sun Y, Lin J, et al. Intranasal dexmedetomidine versus oral midazolam premedication to prevent emergence delirium in children undergoing strabismus surgery: a randomized controlled trial. Eur J Anesthesiol. 2020;37(12):1143–1149. doi:10.1097/EJA.0000000000001270
20. Mason KP. Pediatric emergence delirium: a comprehensive review and interpretation of the literature. Br J Anaesth. 2017;118(3):335–343. doi:10.1093/bja/aew477
21. Urits I, Peck J, Giacomazzi S, et al. Emergence delirium in perioperative pediatric care: a review of current evidence and new directions. Adv Ther. 2020;37(5):1897–1909.
22. Larsen LG, Wegger M, Lé Greves S, et al. Emergence agitation in pediatric day case surgery: a randomized, single-blinded study comparing narcotrend and heart rate variability with standard monitoring. Eur J Anesthesiol. 2022;39(3):261–268. doi:10.1097/EJA.0000000000001649
23. Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. 2003;96(6):1625–1630. doi:10.1213/01.ANE.0000062522.21048.61
24. Lohmer LL, Schippers F, Petersen KU, et al. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020;60(4):505–514. doi:10.1002/jcph.1552
25. Seo IS, Seong CR, Jung G, et al. The effect of sub-Tenon lidocaine injection on emergence agitation after general anesthesia in pediatric strabismus surgery. Eur J Anesthesiol. 2011;28(5):334–339. doi:10.1097/EJA.0b013e3283426ed6
26. Somaini M, Engelhardt T, Fumagalli R, et al. Emergence delirium or pain after anesthesia--how to distinguish between the two in young children: a retrospective analysis of observational studies. Br J Anaesth. 2016;116(3):377–383. doi:10.1093/bja/aev552
27. Kain ZN, Mayes LC, Caldwell-Andrews AA, et al. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics. 2006;118(2):651–658. doi:10.1542/peds.2005-2920
28. Yao Y, Guan J, Liu L, et al. Discharge readiness after remimazolam versus propofol for colonoscopy: a randomized, double-blind trial. Eur J Anesthesiol. 2022. doi:10.1097/EJA.0000000000001715
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.