Back to Journals » Veterinary Medicine: Research and Reports » Volume 7
Reliability of clinical monitoring for the diagnosis of babesiosis in dogs in Nigeria
Authors Adebayo O, Ajadi R, Omobowale T, Omotainse S, Dipeolu M, Nottidge H, Otesile E
Received 12 January 2016
Accepted for publication 18 April 2016
Published 1 July 2016 Volume 2016:7 Pages 85—90
DOI https://doi.org/10.2147/VMRR.S104072
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Video abstract presented by Olufunke Omowunmi Adebayo
Views: 599
Olufunke Omowunmi Adebayo,1 Rasheed Adetola Ajadi,2 Temidayo Olutayo Omobowale,3 Samuel Olatunbosun Omotainse,4 Morenike Atinuke Dipeolu,5 Helen Oyebukola Nottidge,3 Ebenezer Babatunde Otesile2
1Veterinary Teaching Hospital, 2Department of Veterinary Medicine and Surgery, College of Veterinary Medicine, Federal University of Agriculture, Abeokuta, 3Faculty of Veterinary Medicine, University of Ibadan, Ibadan, 4Department of Veterinary Pathology, 5Department of Veterinary Public Health and Reproduction, College of Veterinary Medicine, Federal University of Agriculture, Abeokuta, Nigeria
Abstract: Babesiosis accounts for a high percentage of hospital cases in canines in Africa, with about 40% mortality in the cases presented. In Nigeria, records show an estimated 30% annual morbidity when diagnosis is largely based on clinical and laboratory findings. This study monitored clinical indices associated with canine babesiosis. One hundred and three babesiosis-suspected dogs were selected on the basis of clinical signs of anorexia, fever, presence of ticks, and enlarged lymph nodes or spleen when clinical parameters were recorded at the time of presentation. Parasite detection was done using thin blood smears; that is, the presence of Babesia merozoites was compared between capillary and cephalic blood. Blood was also assayed for hematology and blood chemistry using automated blood analyzers. The babesiosis-infected dogs’ outcome was monitored. Data obtained were analyzed using chi-square test, analysis of variance, and Pearson’s correlation. Results based on thin blood smears showed that 61.1% of the dogs were positive for Babesia species. Breed disposition, sex, and age did not significantly influence the incidence of Babesia canis, while mean rectal temperatures did not differ significantly between the cases (P>0.05). Heart rate and pulse rates of Babesia-positive dogs were significantly (P<0.05) higher than those that were negative. The packed cell volume between the cases was not significantly different, with the values in the positive and negative case obtained being 26.4% ±11.26% and 31.6%±11.9%, respectively, with a range of 6% to 50% and 10% to 47% observed, respectively. Normal leukogram was also observed in 62% of the Babesia-positive cases while 22.2% and 15.8% had leukocytosis and leukopenia, respectively. Most of the positive cases whose results were based on thin blood smear were treated with 5% oxytetracycline for 5 days and fully recovered. Pearson’s correlation was used to give relationship in the observed data. This study concluded that clinical indices are not reliable markers in the diagnosis of canine babesiosis.
Keywords: canine babesiosis, Babesia species, dogs, clinical parameters, markers
Introduction
Canine babesiosis, an important tick-borne protozoan disease caused mainly by both large and small Babesia species, is very endemic to different parts of the world, and presents varying clinical, hematological, and pathological manifestation depending on the species and subspecies involved.1,2
Diagnosis of canine babesiosis is done mainly using clinical signs, history, and identification of the parasite in thin blood smears.3 Recently, the use of molecular means of identification through DNA extraction, amplification, and sequencing of the Babesia spp. has become a common practice in different parts of the world.4,5 However, in Nigeria, where Babesia canis rossi accounts for the majority of the Babesia spp. involved in canine babesiosis,6 diagnosis of the disease is routinely done using nonspecific clinical signs and identification of the parasites on thin blood smears, which is mainly subjective as identification depends on the expertise of the laboratory personnel.7 With the prevalence of canine babesiosis reportedly recorded between 2% and 43.6% in all parts of Nigeria and the high cost of molecular means of identification of the Babesia spp., there is a dire need for prompt, easy, and cost-effective means of detecting this disease.
This study evaluated the sensitivity and specificity of clinical parameters associated with the diagnosis of naturally occurring babesiosis in Nigeria.
Materials and methods
One hundred and three dogs, of which 48 were males and 55 were females, of different ages and breeds, and having clinical signs suggestive of canine babesiosis, were included in this study following informed owners’ consent.
The dogs were located in the southwestern part of Nigeria in three different locations (Veterinary Teaching Hospital of the Federal University of Agriculture, Abeokuta; Ministry of Agriculture, Veterinary Hospital Complex, Ita-eko; Mokola Veterinary Clinic, Ibadan, Nigeria). The inclusion criteria consisted of presence or history of tick infestation, anorexia, and any two of the following clinical signs: persistent fever, splenomegaly, anemia, hemoglobinuria, and jaundice. Excluded were dogs with a history of treatment in the preceding 3 weeks, presence of concurrent disease, or detection of Ehrlichia canis morula on capillary blood smears.
Pertinent signalment such as breed, age, and sex was noted; also recorded were other clinical parameters such as rectal temperature, heart rate, pulse rate, capillary refill time, and respiratory rates.
Blood was collected in a 5 mL sodium ethylenediaminetetraacetic acid bottle for the use of parasite detection from a thin blood smear and to determine the packed cell volume (PCV). Giemsa staining technique was employed for the thin blood smear while an automated blood analyzer was used for the PCV.
Statistical analysis
Data were analyzed using Student’s t-test and were presented as mean ± standard deviation for clinical parameters and levels of plasma bilirubin, plasma proteins, and serum enzymes. Level of significance was set at 0.05. One-way analysis of variance was used for data from the Babesia positive and negative cases. Pearson’s correlation coefficient was used to determine correlation between clinical parameters in the Babesia-positive case. Age and sex distribution between cases was analyzed using the chi-square test.
Results
On the basis of the thin blood smears, the overall prevalence of canine babesiosis in this study population was 61.1%. Parasite detection was observed more on cephalic (57.4%) than on capillary (34.4%) thin blood smears, while 8.2% were observed on both sites. Most observed clinical findings associated with the disease were tick infestation (86.6%), fever (57.2%), and anorexia (49%) in positive cases. In the negative cases, however, tick infestation, fever, and anorexia had percentages of 90.1%, 46.4%, and 36%, respectively. Table 1 shows the distribution of the study population using clinical parameters.
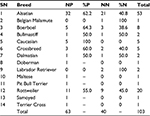
The breed distribution (Table 2) revealed a higher number of positive cases in three breeds, namely, Alsatian (62.2%), Rottweiler (55.0%), and Boerboel (64.3%), out of the 14 breeds present.
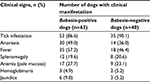  | Table 1 Distribution of study population according to clinical signs and parameters used for their selection |
Among sexes, 66.5% prevalence was observed in males (31/48) and in females prevalence was observed in 56.2% (30/55). Adult dogs (<9 months old) showed a prevalence of 59.1% (39/68) while puppies (<9 months old) had 64.7% (22/35), with equally no significant difference between the cases and the outcome (P=0.67; odds ratio 0.79).
Mean rectal temperatures were not significantly different between cases (P>0.05) with positive cases having a mean ± standard deviation of 39.8°C±0.95°C and negative cases having 39.6°C±0.89°C. Higher temperatures (>39.4°C) were seen in positive cases (40/63) compared to the negative case (23/40). A total of 61% were pyrexic while 35% and 4% had normal and subnormal temperatures, respectively. Forty-seven-point-five percent (19/40) of pyrexic cases were males, while 52.5% (21/40) were females. Regarding the age case, 67.5% (27/40) of pyrexic cases were adults while 32.5% (13/40) were puppies. An odds ratio of 0.90 was arrived at for the relationship of pyrexia observed between adults and puppies within cases.
The study revealed significantly higher values of mean heart rate in positive case (P<0.05; 128±19.7 bpm) compared to the negative case (118±17.7 bpm) while rates >130 bpm of both heart and pulse rates were seen more often (33/63) in cases that were positive with canine babesiosis. Normal rates (90–120 bpm) were observed in 28/63 and 23/40 of Babesia-positive and Babesia-negative cases, respectively.
Conversely, the mean respiratory rates were not significantly different between those that were positive and those negative for Babesia spp. However, increased respiratory rates (>34 cycles per minute) were observed in 21/63 of positive and 14/40 of the negative cases, while decreased respiratory rates (<18 cycles per minute) were seen in 3% of the total number of cases. Approximately 62% of the total number of canines had normal respiratory rates (18–34 cycles per minute).
The PCV and capillary refill time did not differ significantly between dogs who were positive or negative for Babesia spp. The severity of anemia varied between the canines that were positive for Babesia spp.; based on the result of the variations in packed cell volume it was observed that out of the 63 dogs, 21 (34.3%), 14 (22%), eight (12.7%), and 20 (31.7%) had no anemia (>30%), mild anemia (25% to 30%), moderate anemia (18% to 24%), and severe anemia (<18%), respectively. Table 3 compares the severity of anemia observed in the positive and negative cases.
The mean PCV in the positive and negative cases were 26.4% (26.4±11.26) and 28.8% (28.8±10.5), respectively, with ranges of 6% to 50% and 10% to 47%, respectively. In the study, three out of the 63 positive cases had hypoxia and respiratory distress, while two within this case had severe anemia. Only three of the positive case had congestion of the mucous membranes. Table 4 shows variation in PCV values within the breed of dogs.
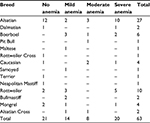  | Table 4 Severity of anemia within breeds positive with Babesia spp. |
In this report, the white blood cell (WBC) count, the mean neutrophil count, and the mean lymphocyte count did not significantly differ between dogs that were positive and negative to Babesia spp. In addition, normal leukogram was also observed in 62% of the Babesia-positive cases. Approximately 22% and 15.8% had leukocytosis and leukopenia, respectively, within the positive case, as shown in Table 5. In this report, variable leukogram was observed as Babesia-positive cases showed as normal leukogram (62%), leukocytosis (22%) and leukopenia (15.8%) in cases observed, thus, supporting the report by Di Cicco and Birkenheuer.1 Table 6 shows breed-wise leukocytic changes in dogs.
  | Table 5 Leukocytic changes in Babesia-positive dogs |
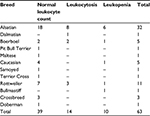  | Table 6 Leukocytic changes within breeds positive to Babesia spp. |
Most of the positive cases were treated with 5% oxytetracycline (5 mg/kg) for 5 days and a total of 50/63 of the positive cases recovered, while eleven dogs died. Of the positive cases, three were treated with a combination of berenil (3.5 mg/kg) and oxytetracycline (5 mg/kg) or doxycycline (5 mg/kg); 44/63 were treated with only oxytetracycline of 5% or 5.5% concentration. Out of the 44/63 cases treated with oxytertacycline (5% or 5.5%) alone, a mortality of seven was recorded.
One-way analysis of variance showed a value of 0.34, revealing no significant findings between the PCV values in both cases. However, the PCV in the positive case on the basis of the Pearson’s correlation table (Table 7) showed a significant positive correlation (0.005) with temperatures of affected dogs and a negative nonsignificance (–0.462) with heart rate.
Table 7 shows that the rectal temperatures of the dogs infected with Babesia spp. were positively correlated to the PCV (P=0.005, r=0.358) and negatively correlated to the WBC counts (P=0.003, r=–0.390). However, the PCV was negatively correlated to WBC counts (P=0.009, r=–0.342). Total plasma protein level was significantly and positively correlated to plasma albumin (P=0.000, r=0.763) and globulin (P=0.000, r=0.775) levels but negatively correlated to total bilirubin level (P=0.000, r=–0.550). Also, the plasma globulin level was significantly and negatively correlated to total bilirubin level (P=0.000, r=–0.600).
Discussion
From this study, clinical findings such as tick infestation, fever, and anorexia were most commonly observed in both Babesia-positive and negative dogs (tick infestation: 90.1% [35/40]; fever: 46.4% [18/40]; and anorexia 36% [14/40]). This result revealed that the aforementioned clinical findings of canine babesiosis are nonspecific, as previously observed among Egyptian dogs.11 However, an observable difference was seen with splenomegaly and jaundice between the cases (splenomegaly: positive case 19.6% [12/63], negative case 20.6% [8/40]; jaundice: positive case 9.8% [6/63], negative case 5.2% [2/40]). Among the 14 breeds of dogs present, the most affected were the foreign and smaller breeds, while moderate susceptibility was observed among the Alsatian breeds. Ogo et al reported that although breed susceptibility has not been established for canine Babesia infection,5 a 50% to 55% seropositivity of Babesia canis vogeli infection in Greyhounds was observed,12–14 while American Staffordshire Terrier and American Pit Bull Terrier breeds had 15% to 93% seropositivity.14–16 Furthermore, a study conducted in 2009 showed that prevalence was highest in crossbreeds and lowest in Doberman.17 In this study, Alsatian and Rottweiler breeds were overpresented as 58.8% and 55%, respectively, while only one indigenous breed was presented and positive for Babesia infection.
For the sexes, outcome observed for positive males and females was 65% (31/48) and 55% (30/55), respectively, with an 1.20 odds ratio of male dogs having the disease more than females. Bashir et al stated that the odds of canine babesiosis were 2.63 times higher in male than female dogs,17 which is similar to this study. Furthermore, it has been suggested that male dogs were more prone to the disease than the females due to bite wounds and blood transmission during fighting among male dogs.11,17 Inasmuch as the fact that blood transmission of canine babesiosis has not been established in Nigeria, there is a probability that this could occur. In addition, Babesia-positive cases were observed in 65% males and 55% females.
For susceptibility among ages, 57.3% (39/68) and 62.8% (22/35) were positive among adults and puppies, respectively, while the odds ratio was 0.79; P>0.05. This result agrees with an earlier report which stated that age does not have any influence on the animals’ susceptibility to the disease.8 In addition, nonspecific or innate factors (genetics or age) possessed by the host act as natural protective elements;9,10 that is, the immunity of an individual could be primed to withstand or be susceptible to a disease challenge at a particular point in time.
Other clinical parameters, such as pulse and heart rates, in the positive case gave significantly higher values compared to the negative case, and this could be due to the cardiac pathology reported in cases of canine babesiosis; also the heart rate of dogs that were positive to Babesia parasites was significantly higher than (P<0.05) those that were negative for the parasite. Increased heart rate was reported as a common finding in uncomplicated canine babesiosis.18–22
Based on the study, in the positive cases, a higher number of dogs were observed to have severe anemia (31.7%) and normal packed cell volume (33.3%) when compared to those with moderate or mild anemia. While the presence of anemia in this study supports the reports by Lobetti2 and Zygner et al,4 variable hematology was observed in both positive and negative cases and the presenting state of the dog could account for this result. Also, factors such as nutrition or the presence of unidentified causes during investigation using thin blood smears could account for this result.
The Pearson’s correlation shown in Table 7 reveals that the temperature of dogs positive for Babesia spp. was significantly correlated with the PCV (P<0.05). This agrees with reports stating significant correlation between body temperature and heart rate, red blood cell count, PCV, and platelet count.22 In addition to this study, the body temperature of dogs positive to the Babesia parasite showed negative correlation with WBC count from positive dogs (P<0.05).
Conclusion
Canine babesiosis with a prevalence of 61% appears to be a common cause of illness among the dog population in Nigeria. Commonly determined physiological parameters such as temperature and respiratory rates did not change significantly between Babesia-positive and Babesia-negative dogs, which suggests that these parameters are not sensitive indicators for monitoring canine babesiosis, however, pulse and heart rates appear to be positive and sensitive indicators. Hematological tests that determine PCV and WBC counts are often performed in the diagnosis of canine babesiosis. In this study, both PCV and WBC counts are not sensitive indicators for monitoring the progression and prognosis of babesiosis in dogs.
Disclosure
The authors report no conflicts of interest in this work.
References
Di Cicco MF, Birkenheuer AJ. Canine babesiosis. Infectious disease/parasitology. Consultants on Call/NAVC Clinician’s Brief; 2012. | ||
Lobetti R. Hematological changes associated with tick-borne diseases (infectious/parasitic diseases). In: World Small Animal Veterinary Association World Congress Proceedings. October 6–9, 2004, for 29th WSAVA Congress Proceedings. | ||
Jacobson LS, Lobetti R. Glucose, lactate and pyruvate concentrations in dogs with babesiosis. Am J Vet Res. 2005;66(2):244–250. | ||
Zygner W, Gojska-Zygner O, Dlugosz E, Wedrychowicz H. Liver enzyme activity in dogs infected with Babesia canis. Bull Vet Inst Pulawy. 2011;55(3):423–427. | ||
Ogo NI, Lawal AI, Okubanjo OO, Kamani J, Ajayi OO. Current status of canine babesiosis and the situation in Nigeria: a review. Niger Vet J. 2011;32(2):69–78. | ||
Sasaki M, Omobowale O, Tozuka M, et al. Molecular survey of Babesia canis in dogs in Nigeria. J Vet Med Sci. 2007;69(11):1191–1193. | ||
Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am. 2008;22(3):469–488,viii–ix. | ||
Martinod S, Laurent N, Moreau Y. Resistance and immunity of dogs against Babesia canis in an endemic area. J Vet Parasitol. 1986;19(3–4):245–254. | ||
Johnston LAY, Leatch G, Jones PN. The duration of latent infection and functional immunity in Droughmaster and Hereford cattle following natural infection with Babesia argentina and Babesia bigemina. Aust Vet J. 1978;56(1):14–18. | ||
Levy MG, Clabaugh G, Ristic M. Age resistance in bovine babesiosis: role of blood factors in resistance to Babesia bovis. Infect Immun. 1982;37(3):1127–1131. | ||
Salem NY, Farag HS. Clinical, hematologic and molecular findings in naturally occurring Babesia canis vogeli in Egyptian dogs. Vet Med Int. 2014;2014(2014). | ||
Breitschwerdt EB, Malone JB, MacWilliams P, Levy MG, Quails CW,Prudich MJ. Babesiosis in the Greyhound. J Am Vet Med Assoc. 1983;182(9):978–982. | ||
Yamane I, Gardener I, Ryan C, Levy M, Urrico J, Conrad P. Survey of Babesia canis, Babesia gibsoni and Ehrlichia canis in pound dogs in California, USA. Prev Vet Med. 1994;18(4):293–304. | ||
Birkenheuer AJ, Correa MT, Levy MG, Breitschwerdt EB. Geographical distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003). J Am Vet Med Assoc. 2005;227(6):942–947. | ||
Macintire DK, Bourdreaux MX, West GD, Bournec C, Wright JC, Conrad PA. Babesia gibsoni infection among dogs in the Southeastern United States. J Am Vet Med Assoc. 2002;220(3):325–329. | ||
Birkenheuer AJ, Levy MG, Stebbins M, Poore M, Breitschwerdt EB. Serosurvey of anti-Babesia antibodies in stray dogs and American pit bull terriers and American Staffordshire terriers from North Carolina. J Am Anim Hosp Assoc. 2003;39(6):551–557. | ||
Bashir IN, Chaudhry ZI, Ahmed S, Saeed MA. Epidemiological and vector identification studies on canine babesiosis. Pakistan Vet J. 2009;29(2):51–54. | ||
Lobetti GR. Canine babesiosis. In: Day MJ, Littelwood JD, Mackin A, editors. Manual of Canine and Feline Haematology and Transfusion Medicine. Cheltenham: British Small Animal Veterinary Association; 2000:85–91. | ||
Vaughan-Scott T. Serum Concentration of Tumor Necrosis Factor in Dogs Naturally Infected with Babesia canis and Its Relation to Severity of Disease. [master’s thesis]. Cape Town: University of Pretoria; 2001. | ||
Böhm M. The Comparative Assessment of Capillary and Venous Babesia rossi Parasitaemias on Thin Blood Smears and Their Association with Disease Manifestation [master’s thesis]. Pretoria: University of Pretoria; 2006. | ||
Ayoob AL, Hackner SG, Prittie J. Clinical management of canine babesiosis. J Vet Emerg Crit Care. 2010;20(1):77–89. | ||
Torbica G, Bedrica L, Samardzija M, et al. Canine babesiosis treatment with three different medicines. Acta Vet (Beograd). 2013;63(2–3):279–290. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.