Back to Journals » Therapeutics and Clinical Risk Management » Volume 12
Relationships between Th1/Th2 cytokine profiles and chest radiographic manifestations in childhood Mycoplasma pneumoniae pneumonia
Authors Zhao JL , Wang X, Wang YS
Received 9 September 2016
Accepted for publication 18 October 2016
Published 11 November 2016 Volume 2016:12 Pages 1683—1692
DOI https://doi.org/10.2147/TCRM.S121928
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Jiu-ling Zhao,1,2 Xin Wang,1 Yu-shui Wang1
1Department of Pediatrics, Tianjin Nankai Hospital, 2Nankai Clinical School, Tianjin Medical University, Nankai, Tianjin, People’s Republic of China
Background: Mycoplasma pneumoniae pneumonia (MPP) is one of the most common childhood community-acquired pneumonias, and the chest radiograph usually shows bronchial pneumonia, segmental/lobar pneumonia, or segmental/lobar pneumonia with pleural effusion. The imbalance of Th1/Th2 function after Mycoplasma pneumoniae infection is an important immunological mechanism of MPP. In this study, we aimed to evaluate the correlations between Th1/Th2 cytokine profiles and chest radiographic manifestations in MPP children.
Patients and methods: A total of 87 children with MPP were retrospectively reviewed in this study. According to the chest radiographic manifestations, they were divided into the following three groups: bronchial MPP group, segmental/lobar MPP group, and segmental/lobar MPP with pleural effusion group. Clinical features and changes in Th1/Th2 cytokines were further analyzed.
Results: The incidence of tachypnea and cyanosis was higher in children with segmental/lobar MPP with pleural effusion than in those with segmental/lobar or bronchial MPP. The peak body temperature of segmental/lobar MPP was higher than that of bronchial MPP, and the duration of fever and hospitalization was positively correlated with the severity of MPP. MPP children’s chest radiograph showed a relationship with the changes in Th1/Th2 cytokines. Serum interleukin-4, interleukin-10 (IL-10), interferon-γ, and tumor necrosis factor-α (TNF-α) of segmental/lobar MPP were significantly higher than those of bronchial MPP, and serum IL-10 (cutoff value: 27.25 pg/mL) can be used as a diagnostic predictor for segmental/lobar MPP. Serum TNF-α and interleukin-6 of segmental/lobar MPP with pleural effusion were significantly higher than those of segmental/lobar MPP without pleural effusion. Serum TNF-α (cutoff value: 60.25 pg/mL) can be used as a diagnostic predictor for segmental/lobar MPP with pleural effusion.
Conclusion: There were significant correlations between Th1/Th2 cytokine profiles and chest radiographic manifestations in MPP children. Serum IL-10 and TNF-α can be used as an optimal predictor for segmental/lobar MPP and segmental/lobar MPP with pleural effusion, respectively.
Keywords: Mycoplasma pneumoniae pneumonia, Th1/Th2, cytokine, chest radiograph
Introduction
Mycoplasma pneumoniae (MP) has become a prevalent pathogen in respiratory tract infections in children.1 It causes up to 40% of community-acquired pneumonia (CAP) cases in children and as much as 18% of cases requiring hospitalization; these percentages are even higher during epidemics.2–4 The incidence of Mycoplasma pneumoniae pneumonia (MPP) has been increasing in recent years. MPP can lead to many kinds of pulmonary complications, including pleural effusion, asthma, chronic interstitial fibrosis, and acute respiratory distress syndrome.5,6 However, the pathogenesis of MPP is not completely clear. It seems to be primarily caused by the adsorption of MP in the respiratory epithelium, the direct invasion of MP, and the mechanism of immunological pathogenesis.7,8 Among them, Th1/Th2 cytokine profile plays an important role in the immunological response.9 However, studies focusing on Th1/Th2 cytokine profile in childhood MPP with different chest radiographic features have not yet been reported.
Therefore, a retrospective study was carried out from January 2015 to June 2016 in our hospital to examine the clinical features and Th1/Th2 cytokine profile, including interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α), in pediatric patients with MPP, with a special focus on different chest radiographic features.
Patients and methods
Patients
We retrospectively reviewed the electronic medical records of all children admitted between January 2015 and June 2016 with the diagnosis of MPP at the Pediatric Department of Tianjin Nankai Hospital, one of the largest tertiary hospitals of Tianjin City and a teaching hospital of Tianjin Medical University. The data obtained from the records included age, gender, history of present illness, physical examination, laboratory findings, and chest radiographs of all patients. A total of 102 hospitalized children were diagnosed with MPP. Of these patients, 87 children (85.3%, 87/102) with the required complete data were included into this study for further analysis. With the exception of azithromycin, other antibiotics and steroids were not used before hospitalization. All patients received azithromycin, while all patients with pleural effusion and some of those with lobar/segmental MPP received glucocorticoids during the hospitalization period. The study protocol was approved by the ethics committee of Tianjin Nankai Hospital, and patient informed consent was obtained from the patient’s parents.
Study group assignment
According to the performance of chest radiograph, the 87 MPP children were divided into the following three groups: bronchial MPP group (group 1, n=22), segmental/lobar MPP group (group 2, n=36), and segmental/lobar MPP with pleural effusion group (group 3, n=29).
Diagnostic criteria of MPP
In this study, the diagnosis of MPP met the following standards: 1) fever, coughing, and other respiratory tract infection symptoms; 2) chest radiographic examination showed bronchial pneumonia, interstitial pneumonia, segmental or lobar pneumonia, and even pleural effusion; and 3) a single serum anti-Mycoplasma IgM antibody titers of ≥1:160 at the acute phase following admission (in those with no history of respiratory infections in the past 3 months) or more than fourfold higher at the symptomatic recovery phase than at the acute phase or a positive polymerase chain reaction (PCR) test for MP.10 In the present study, the standard for the detection of anti-Mycoplasma IgM antibody titers was the microparticle agglutination method using a commercial kit (Serodia-Myco II, Fujirebio, Tokyo, Japan), and quantitative PCR of MP DNA (cutoff point: >103 copies/mL) was used with a nucleic acid quantitative detection kit (Daan Gene Co., Guangzhou, China).
Exclusion criteria
Patients who were coinfected with other respiratory pathogens, as identified through nasal and throat swabs, were excluded from this study. Patients were tested for influenza A, influenza B, respiratory syncytial virus, human metapneumovirus, adenovirus, parainfluenza 1, parainfluenza 2, and parainfluenza 3 using the D3 FastPoint L-DFA Respiratory Virus Identification Kit (Diagnostic Hybrids, Inc., Athens, OH, USA). Bacterial infections were excluded using throat swab cultures. Patients with known conditions such as congenital immunodeficiencies, allergic diseases, and metabolic diseases were also excluded.
Measurement of serum Th1/Th2 cytokines
Venous blood samples were taken from each subject at admission and centrifuged at 1,000 rpm at 20°C for 20 minutes. Then, the supernatant was collected and the levels of Th1/Th2 cytokines were detected using the BD FACSCalibur flow cytometry (BD Biosciences, San Jose, CA, USA). According to the product instructions, IL-2, IL-4, IL-6, IL-10, IFN-γ, and TNF-α were tested using a Human Th1/Th2 Cytokine Kit II (BD Biosciences). The testing sensitivity was 1.0 pg/mL for these Th1/Th2 cytokines.
Statistical analysis
The statistical evaluation was performed using the Statistical Package of Social Sciences (SPSS) software program, version 17.0 (SPSS Inc., Chicago, IL, USA). Distribution of measurement data in all groups was demonstrated by the Kolmogorov–Smirnov test. The one-way analysis of variance, expressed as mean ± standard deviation, was used to assess the measurement data of normal distribution, and Dunnett’s t-test was used for comparison between groups. The Kruskal–Wallis H test, expressed as medians and interquartile ranges (25th–75th percentiles), was used to assess the measurement data of non-normal distribution. The chi-square test or Fisher’s exact test was used to assess the categorical variables. Receiver operating characteristic (ROC) curve was used to evaluate the value of cytokines in diagnosing segmental/lobar MPP and segmental/lobar MPP with pleural effusion. A two-tailed test was used for all P-values, and a P-value <0.05 was considered as statistically significant.
Results
Comparison of clinical characteristics among the groups
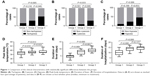
As shown in Table 1, there was no significant difference in the mean age and gender among the three groups. Then the items of tachypnea, cyanosis, hypoxia, peak body temperature, duration of fever, and duration of hospitalization were compared between groups (Figure 1). The incidence of tachypnea and cyanosis was higher in group 3 (segmental/lobar MPP with pleural effusion) than in group 2 (segmental/lobar MPP) and group 1 (bronchial MPP; all P-values <0.05). The incidence of hypoxia was higher in group 3 than in group 1 (P<0.05). There was no significant difference in the incidence of tachypnea, cyanosis, or hypoxia between groups 1 and 2 (all P-values >0.05). Furthermore, there was no statistical difference in the incidence of hypoxia between groups 2 and 3 (P>0.05). Peak body temperature, duration of fever, and duration of hospitalization were significantly higher in group 2 and group 3 than in group 1 (all P-values <0.05), and duration of fever in group 3 was significantly higher than that in group 2 (P<0.05). However, there was no significant difference in peak body temperature and duration of hospitalization between group 2 and group 3 (both P-values >0.05).
Comparison of laboratory findings among the groups
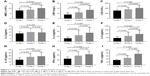
Table 2 shows that there was no significant difference in the percentage of neutrophils (neutrophils%), percentage of lymphocyte (lymphocyte%), hemoglobin, platelet, erythrocyte sedimentation rate, procalcitonin, and IL-2 among the three groups (all P-values >0.05). The comparison between groups was carried out on the statistically significant items (Figure 2). C-reactive protein (CRP), lactate dehydrogenase (LDH), IL-4, IL-10, IFN-γ, and TNF-α in group 2 and group 3 were significantly higher than those in group 1 (all P-values <0.05). In addition, white blood cells (WBCs) and IL-6 in group 3 were significantly higher than those in group 1 (all P-values <0.05). Furthermore, WBC, CRP, LDH, IL-6, and TNF-α in group 3 were significantly higher than those in group 2 (all P-values <0.05).
Diagnostic value of Th1/Th2 cytokine profile in children with segmental/lobar MPP
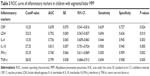
To explore the exact impact of Th1/Th2 cytokine profile levels on the disease progression in children with segmental/lobar MPP, we examined the ROC curves for the biomarkers (CRP, LDH, IL-10, IFN-γ, and TNF-α), which showed a significant difference compared between bronchial MPP group and segmental/lobar MPP group. The results are shown in Figure 3. An IL-10 level of 27.25 pg/mL was the optimal cutoff for predicting segmental/lobar MPP, with an area under the curve (AUC) of 0.813, standard error (SE) of 0.057, and 95% confidence interval (CI) of 0.701–0.924. The diagnostic sensitivity and specificity were 90.0% and 94.5%, respectively, and were significantly higher than other inflammatory markers. The second most useful biomarker was TNF-α, with a cutoff value of 38.05 pg/mL and an AUC of 0.799, SE of 0.058, and 95% CI of 0.684–0.913. The diagnostic sensitivity and specificity were 69.4% and 90.8%, respectively (Table 3).
Diagnostic value of Th1/Th2 cytokine profile in children with segmental/lobar MPP with pleural effusion
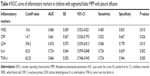
In order to evaluate the effect of Th1/Th2 cytokine profile levels on the disease progression in children with segmental/lobar MPP with pleural effusion, we also carried out the ROC curve analysis for the inflammatory markers, which showed a significant difference compared between segmental/lobar MPP group and segmental/lobar MPP with pleural effusion group. As shown in Figure 4, the ROC curve showed that the TNF-α level of 60.25 pg/mL was the optimal cutoff for predicting segmental/lobar MPP with pleural effusion (sensitivity, 72.4%; specificity, 94.4%; AUC, 0.846; SE, 0.050; 95% CI: 0.748–0.945). It was followed by IL-6, with a cutoff value of 62.80 pg/mL and an AUC of 0.724, SE of 0.064, 95% CI of 0.599–0.848, sensitivity of 72.4%, and specificity of 63.9% (Table 4).
Discussion
MP infection is generally considered a self-limiting disease. However, many cases develop into lower respiratory tract infections or even severe pneumonia. MP is one of the most common causes of CAP in children and can cause serious and life-threatening pulmonary and extrapulmonary complications.6 Over the past few years, macrolide-resistant MP (MRMP) has been reported in the literature, particularly in some Asian countries. The incidence rate of MRMP has been reported to exceed 90% in some areas of China and Japan.11–13 Substantial studies have indicated that, in addition to direct infection by the bacterium,14,15 an excessive immune reaction in the host plays a crucial role in the development of MPP.16 The existing studies show that an imbalance in T-cell cytokines, especially in Th1/Th2 cytokines, is closely involved in the immunopathogenesis of MPP.9,17
Chest radiograph
Chest radiography for MPP can be manifested as bronchial pneumonia, segmental/lobar pneumonia, and pleural effusion.18 To a certain extent, the manifestation of chest radiograph can reflect the severity of the disease and is more conducive to distinguish the disease. In our study, there was no significant difference in age and gender among children with different chest radiographic findings. However, the incidence of tachypnea and cyanosis was higher among children with segmental/lobar MPP with pleural effusion than in those with segmental/lobar MPP without pleural effusion or with bronchial MPP; furthermore, the incidence of hypoxia was higher in the segmental/lobar MPP with pleural effusion group than in the bronchial MPP group. Peak body temperature, duration of fever, and duration of hospitalization in the segmental/lobar MPP with/without pleural effusion group were higher than those in the bronchial MPP group, and duration of fever in the segmental/lobar MPP with pleural effusion group was longer than that in the MPP without pleural effusion group.
WBC and CRP
WBC is one of the most widely used infectious and inflammatory markers, especially in acute bacterial infection. In this study, WBC was increased significantly in patients with segmental/lobar MPP with pleural effusion versus those without pleural effusion. However, compared with other positive inflammatory markers, the sensitivity of WBC was not high.
CRP is a nonspecific inflammatory marker, with a very low content in healthy human blood. When organs or tissues are injured or infected, it can dramatically increase in a short period of time and play an important role in the protection of body’s natural immune process. CRP is correlated with the degree of MPP and plays a role in estimating the severity of the disease. Seo et al19 reported that serum CRP could be a useful marker for predicting the efficacy of macrolides in MRMP. In this study, the levels of CRP were correlated with the severity of chest radiographic manifestations; however, the diagnostic value was poor.
LDH
LDH, which is extensively expressed in body tissues, is a glycolytic enzyme that catalyzes the production of lactic acid from pyruvate. There are five isoforms of LDH: LDH-1, LDH-2, LDH-3, LDH-4, and LDH-5. Among these, LDH-3 is the most abundant in lung tissue. Therefore, the serum LDH-3 level is often increased in patients with pulmonary disease. In addition to lung injury, severe MPP can often cause multiple organ damage in children. LDH levels are higher in patients with severe as compared with mild MPP, and the higher the LDH level, the more severe the MPP. Our finding of significantly higher LDH levels in children with segmental/lobar MPP as compared with bronchial MPP confirms this viewpoint. Kawamata et al20 previously reported that the serum LDH level can be used as an indicator of the severity of MPP and as a marker for initiating corticosteroid therapy in children. Further research has suggested a positive correlation of serum LDH with IL-18 levels.20–22 IL-18 is a pro-inflammatory cytokine that belongs to the IL-1 family. It can be combined with IL-12 to induce high levels of IFN-γ production by T cells. In addition, another report showed that serum LDH can be used as a biomarker to predict refractory MPP in the early stages of hospitalization.23 Therefore, the significance of LDH in MPP remains to be more widely studied.
Th1/Th2 cytokine profiles
Based on the pattern of cytokine secretion, CD4+ cells can be divided into Th1, Th2, Th9, Th17, Th22, Treg, and Tfh cells.24 Among these, Th1/Th2 balance is a basis of the body’s immune response regulation. IL-2, IFN-γ, and TNF-α are secreted from Th1 cells and can promote cell-mediated immune responses to kill intracellular microbial pathogens, while IL-4, IL-6, and IL-10 are secreted from Th2 cells and can promote humoral immunity to kill extracellular microbial pathogens and secrete protective antibodies.25,26 It is generally agreed that Th1/Th2 imbalance in the immune system results in the clinical expression of allergic diseases. Studies have revealed that allergic diseases and respiratory infections can downregulate Th1 cytokines and/or upregulate Th2 cytokines, thereby changing the balance of Th1/Th2 toward Th2 cells.27–29
Separately from allergic diseases and other pathogenic infections, our study showed that, in addition to IL-2, all the other Th1/Th2 cytokines that we investigated (IL-4, IL-6, IL-10, IFN-γ, and TNF-α) increased with the severity of disease on chest X-ray. This suggests that pro-inflammatory and anti-inflammatory cytokines are activated simultaneously in children infected with MP.
IL-2 plays an important role in the immune response, with a wide range of regulatory activities. It is secreted from Th cells by antigen or mitogen stimulation and further influence on effector T cells to differentiate into different functional T-cell subsets, thereby regulating the body’s cellular immune function.30 Thus, IL-2 activity reflects the function of Th cells. In our study, there was no significant difference in the serum IL-2 values among the three MPP groups with different chest radiographic features. The role of IL-2 in MPP remains to be further studied.
IL-4 is mainly secreted by Th2 cells. It can regulate the body’s humoral immune response and is associated with the allergic diseases (such as asthma).31 It is now considered as one of the main factors related to asthma after MP infection.30 Koh et al32 found that the IL-4 values were significantly higher among patients with MPP compared with those in the Streptococcus pneumoniae group and a control group; it was believed that the secretion of cytokines from Th2 cells was increased after MP infection. Our study shows that serum IL-4 levels in segmental/lobar MPP group were significantly higher than those in the bronchial MPP group; however, there was no significant difference between segmental/lobar MPP with and without pleural effusion groups.
IL-6 is an important cytokine and mediator of the acute phase of inflammation.33 In acute inflammatory reaction, IL-6 is mainly manifested in the role of a variety of cells to promote inflammation and the induction of liver cells to synthesize acute phase proteins.34 Our study showed that serum IL-6 levels in segmental/lobar MPP with pleural effusion were significantly higher than those without pleural effusion in pediatric MPP. It suggested that IL-6, as an important Th2 cytokine involved in the pathological process of pulmonary inflammation, may be involved in the infection process of MP and play an important role in the occurrence and development of MPP.
IL-10 is another cytokine produced by Th2 cells. It has a strong anti-inflammatory effect with the function of inhibiting Th1 cells. IL-10 can protect the organs and tissues from the overall pathological changes in the role of inflammatory cytokines. On the other hand, it can also cause immune suppression, thus reducing body’s removal capacity of pathogenic microorganisms and leading to severe infection.35–37 Our study suggested that serum IL-10 levels in segmental/lobar MPP were significantly higher than those in bronchial MPP. As an important anti-inflammatory cytokine, the high level of IL-10 suggests that the immune regulation of MPP is out of control, which may be related to the pathogenesis of MPP. Through the analysis of ROC curve, IL-10 can be used as an inflammatory marker for diagnosis of segmental/lobar MPP (cutoff value of 27.25 pg/mL: sensitivity 90% and specificity 94.5%).
IFN-γ is mainly produced by Th1 cells. It can promote the transformation of Th0 cells to Th1 cells and has an important role in the anti-intracellular pathogen infection. Therefore, IFN-γ plays an important role in the early defense response after infection with pathogens. At present, it is considered that IFN-γ can activate monocytes and macrophages to clear MP and promote cytotoxic T lymphocytes and natural killer cells to exert anti-infection effect.32,37 In this study, serum IFN-γ levels in segmental/lobar MPP were significantly higher than those in bronchial MPP in children, suggesting that an excessive inflammatory reaction can also lead to an increase in IFN-γ.
TNF-α is a polypeptide regulatory factor produced by Th1 cells. Its biologic activity is different according to its concentration. Under normal conditions, it plays a role in the regulation of the immune response and has anti-infection and antitumor activities. It also promotes cell proliferation and differentiation and has other physiological functions with a low concentration. However, high concentration of TNF-α becomes an important inflammatory mediator, with pro-inflammatory effects on eosinophils, neutrophils, T cells, and epithelial cells. TNF-α can cause local inflammation and even multiple organ injury.37,38 In this study, we found that TNF-α levels increased along with the progression of chest inflammation. In addition, TNF-α can be used as an inflammation marker for the diagnosis of segmental/lobar MPP with pleural effusion (cutoff value of 60.25 pg/mL: sensitivity 72.4% and specificity 94.4%).
Limitations
This study still has several limitations: it is a retrospective study, the sample size included in the study is not large enough, and the time point of blood specimen collection is not uniform. Therefore, there may be some bias in this study, and a large sample prospective study remains to be carried out for further assessment.
Conclusion
The incidence of tachypnea and cyanosis was higher in children with segmental/lobar MPP with pleural effusion versus those with segmental/lobar MPP without pleural effusion and bronchial MPP, while hypoxia occurred at a higher incidence in those with segmental/lobar MPP with pleural effusion versus those with bronchial MPP. The peak body temperature of segmental/lobar MPP was higher, and the duration of fever was longer than that of bronchial MPP. The duration of fever and hospitalization was positively correlated with the severity of MPP. Children’s chest radiographs showed a relationship with the changes in Th1/Th2 cytokines. Through statistical analysis, we found that the predictive value of Th1/Th2 cytokine profiles was higher than that of WBC, CRP, and LDH. Serum CRP, LDH, IL-4, IL-10, IFN-γ, and TNF-α in segmental/lobar MPP were significantly higher than those in bronchial MPP, and serum IL-10 (cutoff value: 27.25 pg/mL) can be used as a diagnostic predictor for segmental/lobar MPP. WBC, serum CRP, LDH, TNF-α, and IL-6 in segmental/lobar MPP with pleural effusion were significantly higher than those in segmental/lobar MPP without pleural effusion, and serum TNF-α (cutoff value: 60.25 pg/mL) can be used as a diagnostic predictor for segmental/lobar MPP with pleural effusion.
Acknowledgment
The authors thank Mrs. Xin Ji from School of Medical English and Health Communication of Tianjin Medical University for polishing their use of English in this manuscript.
Author contributions
Jiu-ling Zhao developed the original idea, designed the study, collected the data, wrote the manuscript, and is the guarantor. Xin Wang contributed to data analysis and participated in drafting the manuscript. Yu-shui Wang participated in collecting the data and manuscript writing. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Uehara S, Sunakawa K, Eguchi H, et al. Japanese guidelines for the management of respiratory infectious diseases in children 2007 with focus on pneumonia. Pediatr Int. 2011;53(2):264–276. | ||
Guo L, Liu F, Lu MP, Zheng Q, Chen ZM. Increased T cell activation in BALF from children with Mycoplasma pneumoniae pneumonia. Pediatr Pulmonol. 2015;50(8):814–819. | ||
Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32(6):956–973. | ||
Youn YS, Lee KY, Hwang JY, et al. Difference of clinical features in childhood Mycoplasma pneumoniae pneumonia. BMC Pediatr. 2010;10:48. | ||
Yew P, Farren D, Curran T, Coyle PV, McCaughey C, McGarvey L. Acute respiratory distress syndrome caused by Mycoplasma pneumoniae diagnosed by polymerase chain reaction. Ulster Med J. 2012;81(1):28–29. | ||
Parrott GL, Kinjo T, Fujita J. A compendium for Mycoplasma pneumoniae. Front Microbiol. 2016;7:513. | ||
Moore C, Perry M, Cottrell S. The emerging role of community sentinel surveillance in the understanding of the clinical features and epidemiology of acute Mycoplasma pneumoniae infection. Clin Microbiol Infect. 2014;20(8):O489–O492. | ||
Principi N, Esposito S. Macrolide-resistant Mycoplasma pneumoniae: its role in respiratory infection. J Antimicrob Chemother. 2013;68(3):506–511. | ||
Li W, Liu YJ, Zhao XL, et al. Th1/Th2 cytokine profile and its diagnostic value in Mycoplasma pneumoniae pneumonia. Iran J Pediatr. 2016;26(1):e3807. | ||
Respiratory Branch of Chinese Pediatric Society of Chinese Medical Association, Editorial Board of Chinese Journal of Applied Clinical Pediatrics. Expert consensus on diagnosis and treatment of Mycoplasma pneumoniae pneumonia in children (2015). Chin J Appl Clin Pediatr. 2015;30(17):1304–1308. | ||
Zhao F, Liu G, Wu J. Surveillance of macrolide-resistant Mycoplasma pneumoniae in Beijing, China, from 2008 to 2012. Antimicrob Agents Chemother. 2013;57(3):1521–1523. | ||
Suzuki Y, Itagaki T, Seto J, et al. Community outbreak of macrolide-resistant Mycoplasma pneumoniae in Yamagata, Japan in 2009. Pediatr Infect Dis J. 2013;32(3):237–240. | ||
Yamazaki T, Kenri T. Epidemiology of Mycoplasma pneumoniae infections in Japan and therapeutic strategies for Macrolide-resistant M. pneumoniae. Front Microbiol. 2016;7:693. | ||
Nakane D, Kenri T, Matsuo L, Miyata M. Systematic structural analyses of attachment organelle in Mycoplasma pneumoniae. PLoS Pathog. 2015;11(12):e1005299. | ||
Balish MF. Mycoplasma pneumoniae, an underutilized model for bacterial cell biology. J Bacteriol. 2014;196(21):3675–3682. | ||
Shimizu T. Inflammation-inducing factors of Mycoplasma pneumoniae. Front Microbiol. 2016;7:414. | ||
Fonseca-Aten M, Ríos AM, Mejías A, et al. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am J Respir Cell Mol Biol. 2005;32(3):201–210. | ||
Tanaka H. Correlation between radiological and pathological findings in patients with Mycoplasma pneumoniae pneumonia. Front Microbiol. 2016;7:695. | ||
Seo YH, Kim JS, Seo SC, et al. Predictive value of C-reactive protein in response to macrolides in children with macrolide-resistant Mycoplasma pneumoniae pneumonia. Korean J Pediatr. 2014;57(4):186–192. | ||
Kawamata R, Yokoyama K, Sato M, et al. Utility of serum ferritin and lactate dehydrogenase as surrogate markers for steroid therapy for Mycoplasma pneumoniae pneumonia. J Infect Chemother. 2015;21(11):783–789. | ||
Oishi T, Narita M, Matsui K, et al. Clinical implications of interleukin-18 levels in pediatric patients with Mycoplasma pneumoniae pneumonia. J Infect Chemother. 2011;17(6):803–806. | ||
Inamura N, Miyashita N, Hasegawa S, et al. Management of refractory Mycoplasma pneumoniae pneumonia: utility of measuring serum lactate dehydrogenase level. J Infect Chemother. 2014;20(4):270–273. | ||
Lu A, Wang C, Zhang X, Wang L, Qian L. Lactate dehydrogenase as a biomarker for prediction of refractory Mycoplasma pneumoniae pneumonia in children. Respir Care. 2015;60(10):1469–1475. | ||
Golubovskaya V, Wu L. Different subsets of T cells, memory, effector functions, and CAR-T immunotherapy. Cancers (Basel). 2016;8(3):36. | ||
Eisenstein EM, Williams CB. The Treg/Th17 cell balance: a new paradigm for autoimmunity. Pediatr Res. 2009;65:26R–31R. | ||
Feng P, Yan R, Dai X, Xie X, Wen H, Yang S. The alteration and clinical significance of Th1/Th2/Th17/Treg cells in patients with multiple myeloma. Inflammation. 2015;38(2):705–709. | ||
Al-Daghri NM, Alokail MS, Draz HM, Abd-Alrahman SH, Yakout SM, Clerici M. Th1/Th2 cytokine pattern in Arab children with severe asthma. Int J Clin Exp Med. 2014;7(8):2286–2291. | ||
Gut W, Pancer K, Abramczuk E, et al. RSV respiratory infection in children under 5 y.o. – dynamics of the immune response Th1/Th2 and IgE. Przegl Epidemiol. 2013;67(1):17–22. | ||
Herring AC, Hernández Y, Huffnagle GB, Toews GB. Role and development of TH1/TH2 immune responses in the lungs. Semin Respir Crit Care Med. 2004;25(1):3–10. | ||
Rogala B, Bozek A, Gluck J, Jarzab J. Prevalence of IgE-mediated allergy and evaluation of Th1/Th2 cytokine profiles inpatients with severe bronchial asthma. Postepy Dermatol Alergol. 2015;32(4):274–280. | ||
Walsh GM. Anti-IL-4/-13 based therapy in asthma. Expert Opin Emerg Drugs. 2015;20(3):349–352. | ||
Koh YY, Park Y, Lee HJ, Kim CK. Levels of interleukin-2, interferon-gamma, and interleukin-4 in bronchoalveolar lavage fluid from patients with Mycoplasma pneumonia: implication of tendency toward increased immunoglobulin E production. Pediatrics. 2001;107(3):E39. | ||
Rubini A. Interleukin-6 and lung inflammation: evidence for a causative role in inducing respiratory system resistance increments. Inflamm Allergy Drug Targets. 2013;12(5):315–321. | ||
Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70(1):11–20. | ||
Xu J, Han R, Kim DW, et al. Role of interleukin-10 on nasal polypogenesis in patients with chronic rhinosinusitis with nasal polyps. PLoS One. 2016;11(9):e0161013. | ||
Peñaloza HF, Schultz BM, Nieto PA, et al. Opposing roles of IL-10 in acute bacterial infection. Cytokine Growth Factor Rev. Epub 2016 Jul 25. | ||
Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010. | ||
Walsh GM. Antagonism of cytokine-induced eosinophil accumulation in asthma. Front Pharmacol. 2012;3:197. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.