Back to Journals » International Journal of Women's Health » Volume 14
Relationship of Placental and Serum Lipoprotein-Associated Phospholipase A2 Levels with Hypertensive Disorders of Pregnancy
Authors Wang J, Dong X, Wu HY, Bu WH, Cong R, Wang X, Shang LX, Jiang W
Received 10 February 2022
Accepted for publication 9 June 2022
Published 17 June 2022 Volume 2022:14 Pages 797—804
DOI https://doi.org/10.2147/IJWH.S361859
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Jing Wang,1 Xing Dong,2 Hong-Yan Wu,1 Wen-Hua Bu,1 Rong Cong,1 Xin Wang,1 Li-Xin Shang,1 Wen Jiang1
1Department of Obstetrics and Gynecology, The Seventh Medical Center of Chinese PLA General Hospital, Beijing, People’s Republic of China; 2Department of General Surgery, The First Medical Center of Chinese PLA General Hospital, Beijing, People’s Republic of China
Correspondence: Wen Jiang, Email [email protected]
Background: A series of studies has shown that lipoprotein-associated phospholipase A2 (Lp-PLA2) is closely associated with abnormal lipid metabolism and vascular endothelial cell injury, but its role in hypertensive disorders of pregnancy (HDP) remains unclear. This study aims to determine the relationship between placental and serum LP-PLA2 levels and HDP, and to provide a feasible method for predicting HDP.
Methods: The placental and serum Lp-PLA2 levels of 63 patients with HDP (20, 25, and 18 cases with gestational hypertension, mild preeclampsia, and severe preeclampsia, respectively) and 20 women with normal pregnancies (control group) were measured via a combination of tissue microarray and immunohistochemistry, real-time quantitative RT-PCR and enzyme-linked immunosorbent assay (ELISA).
Results: 1) The gene and protein expression levels of placental LP-PLA2: the HDP group had significantly higher levels than those of the control group (P < 0.05). The mild preeclampsia group had significantly higher levels than those of the control group (P < 0.05); the severe preeclampsia group had significantly higher levels than those of the mild preeclampsia group (P < 0.05). 2) Serum levels of Lp-PLA2: the HDP group had significantly higher levels than those of the control group (P < 0.05). The Lp-PLA2 levels increased gradually with the progression of the HDP; there were significant differences in the four groups using pair-wise comparisons (P < 0.05). 3) Serum levels of LP-PLA2 were positively correlated with placental LP-PLA2 levels in the HDP group (r = 0.435, P < 0.05).
Conclusion: Elevated Lp-PLA2 levels may be associated with the occurrence of HDP, and changes of Lp-PLA2 levels in the maternal blood may be regarded as a monitoring indicator for this disease.
Keywords: hypertensive disorders of pregnancy, preeclampsia, lipoprotein-associated phospholipase A2, placenta, serum
Introduction
Hypertensive disorders of pregnancy (HDP) refers to a group of diseases in which pregnancy and hypertension coexist, mainly manifesting with hypertension and proteinuria after 20 weeks of pregnancy, with secondary eclampsia, brain edema, cerebral hemorrhage, multiple organ dysfunction, HELLP (hemolysis, elevated liver enzymes and low platelet count syndrome), placental abruption, fetal growth restriction, and other serious fetal and maternal complications. HDP is the leading cause of maternal and fetal morbidity and mortality. It has negative implications for the long-term prognosis of the parturients and offspring.1 Currently, there is a poor understanding of the etiology and pathogenesis of HDP, as well as few effective treatment recommendations to guide care.2,3 Thus, it is very important to study the pathogenesis of HDP and find effective predictors and treatment methods.4
Recent studies have shown that disorders of lipid metabolism are involved in the inflammatory factors secreted by the endothelial cells under the action of oxidative stress or lipid peroxide, followed by cellular damage, which is one of the basic pathological changes in HDP.5,6 Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a vascular-specific inflammatory enzyme secreted by macrophages, mast cells, and T cells, and it combines with low-density lipoprotein (LDL) to form a complex that is stored in the body.7 Lp-PLA2 can form pro-inflammatory medium oxidized free fatty acids and hemolytic lecithin via the proteolysis of the intracellular oxidation of lecithin in a calcium-independent manner. Lp-PLA2 stimulates inflammatory factors and adhesion molecules, promotes the chemotaxis of monocytes and macrophages in the intima of blood vessels, and induces vascular endothelial damage,8,9 all of which are possibly closely associated with the occurrence of HDP.10
In this study, placental and serum LP-PLA2 levels in patients with HDP were detected to determine the role of Lp-PLA2 in the pathogenesis of HDP, and investigate whether LP-PLA2 can be regarded as a new marker for the prediction and treatment of HDP.
Materials and Methods
Ethics Statement
The study was approved by the clinical research ethics committee of the Seventh Medical Center of PLA General Hospital (2017–93), and informed written consent was obtained from all subjects. The study complies with the Declaration of Helsinki.
Study Subjects
A total of 63 patients with HDP were selected from Jan. 2018 to Jan. 2020 as the HDP group, including 20 cases of patients with gestational hypertension, 25 cases of patients with mild preeclampsia, and 18 cases of patients with severe preeclampsia. Twenty pregnant women with normal blood pressure who delivered at the same time were randomly selected as the control group. The inclusion criteria were as follows: (1) gestational hypertension: hypertension in the gestation period, systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, negative urinary protein; mild preeclampsia: SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, with random urine protein ≥ 1 + (30 mg/dl) or ≥ 0.3 g/24 h after 20 weeks of gestation; severe preeclampsia: presenting beyond 20 weeks of gestation with SBP ≥ 160 mmHg, DBP ≥ 110 mmHg, and random urine protein ≥ 3 + (300 mg/dl) or ≥ 5 g/24 h, or those with HELLP syndrome.11 (2) All cases were primipara with singleton births. Exclusion criteria were as follows: (1) chronic hypertension, diabetes, and serious heart, kidney, or liver diseases; (2) a history of alcoholism, smoking, or mental illness; (3) immune system diseases.
Sample Collection
Four milliliters of fasting venous blood was taken from all subjects in the morning (blood collection before treatment in the HDP group), and was centrifuged at 2500 rpm for 15 min at 4 °C. The supernatant was collected and stored at −70 °C.
Immediately after delivery, two pieces of placental tissue (approximately 1.0 cm × 1.0 cm × 1.0 cm) were taken from the central area of the maternal surface of the placenta from the HDP group and control group, avoiding infarction and calcification. One was rapidly fixed in 10% neutral formaldehyde for the preparation of the tissue microarray (TMA), and the other was quickly stored at −80 °C for real-time quantitative PCR.
Tissue Microarray Combined with Immunohistochemistry
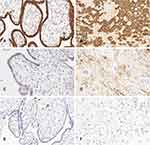
Construction of the TMA was performed as described previously.12 The expression of placental LP-PLA2 was analyzed by the streptavidin-biotin-peroxidase complex method. The rabbit anti-human Lp-PLA2 antibody (diluted 1:100) and the immunohistochemical kit were from Origene Technologies (Origene, USA; TA325117). The experiment was carried out while following the manufacturer’s instructions. The determination of the immunohistochemical staining results was as follows: positive cells had clear brownish-yellow granules in the cytoplasm, and negative cells were without coloring or had staining consistent with the background color. Ten high-magnification fields were selected for each tissue slice, and 100 cells were counted in each field and scored according to the percentage of the number of positive cells and their staining intensity relative to the total number of cells.13 (1) The score according to the number of positive cells was recorded as 0 points when the number of positive cells was < 5%, 1 point when the number of positive cells ranged from 5% to 25%, 2 points when the number of positive cells ranged from 26% to 50%, and 3 points when the number of positive cells was > 50%. (2) The score according to staining intensity was recorded as 0 points for colorless, 1 point for light yellow, 2 points for pale brown, and 3 points for brown. (3) When the score for the number of positive cells was multiplied by the score for the staining intensity, a result of ≥ 4 points was (+) and 0–3 points was (−).
Real-Time Quantitative RT-PCR
Total RNA was extracted from placental tissues while following the TRIzol Reagent user guide. RNA was transcribed to cDNA using the RT first-strand synthesis kit (Takara Biomedical Technology Co., Ltd., Beijing, China). Reverse transcription was performed as follows: 30 °C for 5 min, 42 °C for 60 min, 99 °C for 5 min and 5 °C for 5 min, in 20 µL of the reaction mixture. The expression level of the Lp-PLA2 was examined using real-time PCR according to the protocol of the Real-time SYBR Green PCR Master Mix (BioRad). GAPDH expression was used as an internal reference. Briefly, real-time PCR amplification was performed as follows: pre-denaturation at 94 °C for 5 min; then 30 cycles of 94 °C for 30s, 55 °C for 30s, and 72 °C for 40s; followed by an extension at 72 °C for 10 min. The primers used were: GAPDH forward and reverse, 5’-ATCAAGAAGGTGGTGAAGCAGG-3’ and 5’-AGGTGGAAGAGTGGGAGTTGCT-3’, respectively. Lp-PLA2 forward and reverse, 5’-TCATCAGCATGGGTCAACAAA-3’ and 5’-CCAAAGGGTGTCAAGGCGAT-3’, respectively. After amplification, Ct values for each group were obtained and standard curves were drawn. The ΔΔCt-based method was used for a relative quantitative analysis with a difference of gene expression of –2ΔΔCt, and ΔΔCt = ΔCt sample – ΔCt as an internal reference.
Enzyme-Linked Immunosorbent Assay (ELISA)
The Lp-PLA2 levels were measured with an ELISA kit (R&D Systems China Co. Ltd., Shanghai, China). The sensitivity was 0.01 ng/mL. Operations were carried out while following the kit instructions.
Statistical Analysis
All data analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). Results were presented as the mean ± SD. The clinical data between the groups were compared by Student’s t-test. The mean serum and placental gene expression levels for the Lp-PLA2 among the groups were compared by Mann Whitney test. The mean placental Lp-PLA2 protein expression levels were compared using the Chi-square test. Spearman correlation coefficient analyses were performed to assess possible relationships. P < 0.05 was considered statistically significant.
Results
Comparisons of the General Conditions of Pregnant Women and Their Newborns Between Groups
No significant differences in age, gestational week, pregnancy times, BMI before pregnancy, weight gain during pregnancy, or neonatal gender were found between the two groups (P > 0.05). There were statistically significant differences in birth weights and body lengths between the two groups (P < 0. 05, Table 1).
 |
Table 1 Comparison of General Conditions of Pregnant Women and Their Newborns Between Groups |
Comparisons of the Placental Expression Levels of LP-PLA2 Protein
LP-PLA2 was mainly expressed in the membrane and cytoplasm of the placental villous trophoblasts and decidual cells (Figure 1A–F). The placental expression level of LP-PLA2 protein was significantly higher in the HDP group than in the control group (P < 0.01). The expression level of LP-PLA2 was significantly higher in the mild preeclampsia group than in the control group (P < 0.05), and placental LP-PLA2 was significantly higher in the severe preeclampsia group than in the mild preeclampsia group (P < 0.05). The expression level of LP-PLA2 protein was higher in the gestational hypertension group than in the control group, but without statistical significance (P > 0.05, Table 2).
 |
Table 2 Comparison of Lp-PLA2 Expression Levels in the Placenta Among Groups |
Comparisons of the Placental Expression Levels of LP-PLA2 mRNA
The relative placental expression of the LP-PLA2 mRNA levels was significantly higher in the tissues of the HDP group (2.443 ± 0.389) than in those of the control group (1.018 ± 0.281) (P < 0.01). A pair-wise comparison showed that the relative expression of LP-PLA2 mRNA was significantly higher in the mild preeclampsia group (2.321 ± 0.357) than in the control group (P < 0.01), and in the severe preeclampsia group (3.839 ± 0.406) versus the mild preeclampsia group (P < 0.01), but it was not significantly higher in the gestational hypertension group (1.217 ± 0.340) versus the control group (P > 0.05) (Figure 2).
Comparisons of Serum LP-PLA2 Levels
The serum levels of Lp-PLA2 were significantly higher in the HDP group compared to the control group (P < 0.01). With the aggravation of the disease, the levels of Lp-PLA2 gradually increased. There were significant differences in the pair-wise comparisons between the control, gestational hypertension, mild preeclampsia, and severe preeclampsia groups (P < 0.05, Table 3).
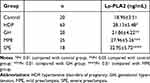 |
Table 3 Comparison of Lp-PLA2 Levels in the Serum Among Groups |
Correlation Study
There was a positive correlation between placental and serum LP-PLA2 levels in the HDP group (r =0.441, P < 0.05), which was a medium strength of association. There was also a positive correlation between the placental and serum LP-PLA2 levels in the control group (r = 0.423, P < 0.05), which was a medium strength of association too.
Discussion
The etiology and pathogenesis of HDP are still not well elucidated. Recent studies have suggested that abnormal lipid metabolism, inflammatory injury, and dysfunction of the vascular endothelial cells may be centrally linked to HDP.5,6 Lp-PLA2 is a member of the phospholipase A2 (PLA2) superfamily, with the human gene located on chromosome 6p12-21.1 and consisting of 441 amino acids.14 It was found that Lp-PLA2 can promote the release of inflammatory mediators, cause an inflammatory reactions, and damage vascular endothelial cells.15 Lp-PLA2 can cause platelet aggregation and lead to thrombosis,16 and plays an important role in stabilizing cell membranes, reconstructing phospholipids, transmitting cell signals, and lipid metabolism.17–19 In vivo, T lymphocytes, mature macrophages, and other inflammatory cells can synthesize and secrete LP-PLA2 into circulation, using apolipoprotein B as the carrier associated with LDL to participate in the regulation of lipid metabolism.20 Because of the role of Lp-PLA2 in inflammation responses and lipid metabolism, it has been speculated that Lp-PLA2 may be closely associated with the development and progression of HDP.
Balc et al21 found that the levels of LP-PLA2 and C-reactive protein were higher in patients with preeclampsia than in those in a control group, and LP-PLA2 and C-reactive protein were positively correlated, suggesting that changes in LP-PLA2 levels lead to inflammatory responses in the endothelial cells, followed by preeclampsia. In this study, the protein and mRNA expression levels of placental LP-PLA2 were significantly higher in the HDP group compared with those of the control group. On the other hand, the protein and mRNA expression levels of placental LP-PLA2 were significantly higher in the mild preeclampsia group compared with the control group, and in the severe preeclampsia group compared with the mild preeclampsia group, suggesting that rising levels of LP-PLA2 are related to the development and progress of HDP. The inhibition of LP-PLA2 expression may provide a new target for the treatment of HDP. The protein and mRNA expression levels of LP-PLA2 were similar in the gestational hypertension group and the control group, possibly reflecting mild changes related to HDP.
The possible mechanisms of HDP are as follows: (1) The combination of Lp-PLA2 and LDL may cause a lipid metabolism disorder, leading to the atherosclerosis of the uterine and placental arteries and reduced placental function, and finally resulting in HDP. (2) Lp-PLA2 can hydrolyze and oxidize lecithin to produce free fatty acids and hemolytic lecithin, both of which promote inflammatory reactions. By activating cytokines and adhesion factors, vascular endothelial cells are damaged and become dysfunctional, leading to the development and progression of HDP.22 (3) Lp-PLA2 contributes to a vascular inflammatory reaction by promoting the release of IL-6, further promoting collagen deposition and fibroblast proliferation at the inflammatory reaction site, as well as platelet aggregation, leading to vascular endothelial cell damage and the occurrence of HDP.23
Güngör et al24 enrolled 200 pregnant women and divided them into a control group (normal blood pressure), early-onset preeclampsia group (average gestational weeks of 28.7 ± 3.0), and late-onset preeclampsia group (average gestational weeks of 36.0 ± 1.4). Their study showed that serum Lp-PLA2 levels were significantly higher in the early-onset and late-onset preeclampsia groups compared with those of the control group, suggesting that elevated Lp-PLA2 levels may be associated with preeclampsia and postpartum cardiovascular diseases, and may be used as a predictor of HDP. In this study, we found that serum Lp-PLA2 levels were significantly higher in the HDP group compared with the control group. The levels of LP-PLA2 gradually increased with the progression of the disease. There were significant differences in pair-wise comparisons between the control, gestational hypertension, mild preeclampsia, and severe preeclampsia groups. In addition, serum levels of Lp-PLA2 were positively correlated with placental LP-PLA2 levels, suggesting that the changes in serum Lp-PLA2 levels reflects the severity of the HDP. Therefore, LP-PLA2 measurements may have important significance in the monitoring of HDP and may provide a novel clinical predictor.
In conclusion, Lp-PLA2 levels were closely associated with the development and progress of HDP. The detection of serum Lp-PLA2 levels in pregnant women may provide a novel indicator for predicting the occurrence of HDP. Effectively inhibiting the expression of Lp-PLA2 in the placental tissue may provide a unique avenue for the prevention and treatment of HDP.
Acknowledgments
We thank Mrs. Li-juan Sun and Miss Gui-ju Cai for helping with recruiting patients for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tarca AL, Romero R, Benshalom-Tirosh N, et al. The prediction of early preeclampsia: results from a longitudinal proteomics study. PLoS One. 2019;14(6):e0217273. doi:10.1371/journal.pone.0217273
2. Jia K, Ma LJ, Wu SY, et al. Serum levels of complement factors C1q, Bb, and H in normal pregnancy and severe pre-eclampsia. Med Sci Monit. 2019;25(9):7087–7093. doi:10.12659/MSM.915777
3. Tomimatsu T, Mimura K, Matsuzaki S, et al. Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int J Mol Sci. 2019;20(17):4246–4264. doi:10.3390/ijms20174246
4. Olaoye T, Oyerinde OO, Elebuji OJ, et al. Knowledge, perception and management of pre-eclampsia among health care providers in a maternity hospital. Int J MCH AIDS. 2019;8(2):80–88. doi:10.21106/ijma.275
5. Alahakoon TI, Medbury HJ, Williams H, et al. Lipid profiling in maternal and fetal circulations in preeclampsia and fetal growth restriction-a prospective case control observational study. BMC Pregnancy Childbirth. 2020;20(1):61–71. doi:10.1186/s12884-020-2753-1
6. Tenório MB, Ferreira RC, Moura FA, et al. Cross-talk between oxidative stress and inflammation in Preeclampsia. Oxid Med Cell Longev. 2019;2019(11):8238727–8238753. doi:10.1155/2019/8238727
7. Otsuka F, Zhao X, Trout HH, et al. Community-based statins and advanced carotid plaque: role of CD163 positive macrophages in lipoprotein-associated phospholipase A2 activity in atherosclerotic plaque. Atherosclerosis. 2017;267(12):78–89. doi:10.1016/j.atherosclerosis.2017.10.014
8. Huang FB, Wang K, Shen JH. Lipoprotein-associated phospholipase A2: the story continues. Med Res Rev. 2020;40(1):79–134. doi:10.1002/med.21597
9. Jackisch L, Kumsaiyai W, Moore JD, et al. Differential expression of Lp-PLA2 in obesity and type 2 diabetes and the influence of lipids. Diabetologia. 2018;61(5):1155–1166. doi:10.1007/s00125-018-4558-6
10. Passaro A, Vigna GB, Romani A, et al. Distribution of paraoxonase-1 (PON-1) and lipoprotein phospholipase A2(Lp-PLA2) across lipoprotein subclasses in subjects with type 2 diabetes. Oxid Med Cell Longev. 2018;2018(11):1752940. doi:10.1155/2018/1752940
11. Xie X. Obstetrics and Gynecology.
12. Yan DW, Li DW, Yang YX, et al. Ubiquitin D is correlated with colon cancer progression and predicts recurrence for stage II-III disease after curative surgery. Br J Cancer. 2010;103(7):961–969. doi:10.1038/sj.bjc.6605870
13. Chen BF, Deng Y, Xu X, et al. Effect of selective thrombus aspiration on serum lipoprotein-associated phospholipase A2 in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention with high thrombus burden. Acta Cardiol Sin. 2018;34(3):233–241. doi:10.6515/ACS.201805_34(3).20170227A
14. Siddiqui MK, Kennedy G, Carr F, et al. Lp-PLA2 activity is associated with increased risk of diabetic retinopathy: a longitudinal disease progression study. Diabetologia. 2018;61(6):1344–1353. doi:10.1007/s00125-018-4601-7
15. Li JW, Wang H, Tian J, et al. Change in lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndrome. Medicine. 2018;97(28):e11517. doi:10.1097/MD.0000000000011517
16. Zhang Y, Zhang M, Yu F, et al. Karyopherin alpha 2 is a novel prognostic marker and a potential therapeutic target for colon cancer. J Exp Clin Cancer Res. 2015;34(1):145–156. doi:10.1186/s13046-015-0261-3
17. Tian Y, Jia H, Li S, et al. The associations of stroke, transient ischemic attack, and/or stroke-related recurrent vascular events with Lipoprotein-associated phospholipase A2: a systematic review and meta-analysis. Medicine. 2017;96(51):e9413. doi:10.1097/MD.0000000000009413
18. Cao J, Hsu YH, Li S, et al. Structural basis of specific interactions of Lp-PLA2 with HDL revealed by hydrogen deuterium exchange mass spectrometry. J Lipid Res. 2013;54(1):127–133. doi:10.1194/jlr.M030221
19. Paik JK, Kim M, Kim M, et al. Circulating Lp-PLA2 activity correlates with oxidative stress and cytokines in overweight/obese postmenopausal women not using hormone replacement therapy. Age. 2015;37(2):32–42. doi:10.1007/s11357-015-9770-4
20. He S, Chousterman BG, Fenn A, et al. Lp-PLA2 antagonizes left ventricular healing after myocardial infarction by impairing the appearance of reparative macrophages. Circ Heart Fail. 2015;8(5):980–987. doi:10.1161/CIRCHEARTFAILURE.115.002334
21. Balc EÖ, Ekmekçi H, Güngör Z, et al. Evaluation of Lp-PLA2 mass, vitronectin and PAI-1 activity levels in patients with preeclampsia. Arch Gynecol Obstet. 2015;292(1):53–58. doi:10.1007/s00404-014-3601-1
22. Hong Y. Study on the relationship between Lp-PLA2 and pregnancy induced hypertension and its clinical significance. Jilin: Jilin University, 2015:1–31.
23. Dhamoon MS, Cheung YK, Moon YP, et al. Interleukin-6 and lipoprotein-associated phospholipase A2 are associated with functional trajectories. PLoS One. 2019;14(4):e0214784. doi:10.1371/journal.pone.0214784
24. Gungör ZB, Tuten A, Ekmekçi H, et al. Possible effects of lipoprotein-associated phospholipase A2 single-nucleotide polymorphisms on cardiovascular risk in patients with preeclampsia. J Matern Fetal Neonatal Med. 2018;31(23):3119–3127. doi:10.1080/14767058.2017.1365125
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.