Back to Journals » Therapeutics and Clinical Risk Management » Volume 12
Relationship between the iceA gene of Helicobacter pylori and clinical outcomes
Authors Huang X, Deng Z, Zhang Q, Li W, Wang B, Li M
Received 8 March 2016
Accepted for publication 4 May 2016
Published 6 July 2016 Volume 2016:12 Pages 1085—1092
DOI https://doi.org/10.2147/TCRM.S107991
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deyun Wang
Xiaojun Huang,1,2 Zhaomin Deng,1 Qiang Zhang,1 Wanyi Li,1 Baoning Wang,1 Mingyuan Li1,3
1Department of Microbiology, West China School of Preclinical and Forensic Medicine, Sichuan University, Chengdu, People’s Republic of China; 2Department of Microbiology, School of Medicine, Hubei University for Nationalities, Enshi, People’s Republic of China; 3State Key Laboratory of Oral Diseases, Chengdu, People’s Republic of China
Background: The complex pathogenesis of Helicobacter pylori (H. pylori) and the features of the host influence the diverse clinical outcomes. A mass of studies about virulence genes have accelerated the exploration of pathogenesis of H. pylori infection. Induced by contact with epithelium gene A (iceA) is one of the biggest concerned virulence genes. In this study, we explored the relationship between iceA and the magnitude of the risk for clinical outcomes and the prevalence of iceA-positive H. pylori in People’s Republic of China and other countries.
Methods: We searched the electronic databases of PubMed, Embase, CNKI, VIP, and Wanfang by literature search strategy. The studies conforming to the inclusion criteria were assessed. With these data, we systematically analyzed the relationship between the iceA gene of H. pylori and clinical outcomes.
Results: Nineteen articles with 22 studies, a total of 2,657 cases, were involved in the study. The iceA1 gene was significantly associated with peptic ulcer disease (odds ratio =1.28, 95% confidence interval =1.03–1.60; P=0.03), especially in People’s Republic of China (odds ratio =1.40, 95% confidence interval =1.07–1.83; P=0.01). Moreover, the prevalence of iceA1 was significantly higher than iceA2 in People’s Republic of China (P<0.0001). The prevalence of both iceA1 and iceA2 was significantly different (P<0.0001) in People’s Republic of China and in other countries.
Conclusion: The system analysis showed that infection with the iceA1-positive H. pylori significantly increased the overall risk for peptic ulcer disease, especially in People’s Republic of China. The iceA2 gene status and clinical outcome of H. pylori infection have no significant correlation. H. pylori iceA1 genotype is the major epidemic strain in People’s Republic of China.
Keywords: Helicobacter pylori, iceA, gastritis, peptic ulcer disease, gastric carcinoma
Introduction
Helicobacter pylori (H. pylori) is a gram-negative microaerophilic spiral bacterium tenaciously colonizing the gastric mucosa of approximately half the human population in the world. A minority of the infected population will suffer from chronic gastritis and peptic ulcer disease (PUD), and some even progress to gastric carcinoma (GC) and gastric mucosa-associated lymphoid tissue lymphoma. The International Agency for Research on Cancer confirmed that H. pylori infection was the most significant risk factor for gastric cancer and that the eradication of H. pylori can reduce the risk of gastric cancer in 2012.1 H. pylori, the host, and environment factors influence the diverse clinical outcomes. In particular, many virulence genes of H. pylori play an important role.2–5 Different genotypes of H. pylori produce different virulence factors. Urea enzymes, adhesins, cagA,6 and vacA7 are conclusively associated with severe gastroduodenal diseases. Some other virulence genes have been found, one of which is induced by contact with epithelium gene A (iceA), which is independent of cagA and vacA.4,8–10
The iceA gene was identified in the H. pylori isolated from PUD and gastritis patients. There are at least two alleles of iceA, iceA1, and iceA2.11 The relationship between H. pylori iceA and clinical outcomes is controversial. Some studies have suggested that iceA (iceA1/iceA2) may be significantly associated with diseases of digestive system,10,12,13 whereas others showed contrary findings.14–17 In this study, the relationship between the genetic status of iceA (iceA1 and iceA2) and gastritis, nonulcer dyspepsia (NUD), PUD, and GC were systematically assessed. We also evaluated the distribution difference of iceA status in People’s Republic of China and other countries.
Materials and methods
Literature search strategy
A literature search was performed using PubMed, Embase, CNKI, VIP, and Wanfang databases for articles assessing the relationship between iceA gene and clinical outcomes in H. pylori-infected populations. All included studies were retrieved using the search terms: 1) “iceA” or “iceA1”or “iceA2” and 2) “Helicobacter pylori” or “H. pylori” or “Hp”. Papers written in English or Chinese and published before October 2014 were selected for this study.
Inclusion criteria
The criteria applied to select papers were as follows: 1) studies exploring the relationship between iceA gene (iceA1 or iceA2) status and clinical outcomes; 2) fully published case–control studies; 3) studies with case groups including gastritis or NUD, PUD (gastric ulcer or duodenal ulcer), and GC defined by upper gastric endoscopy and histological examination; 4) studies in which genomic DNA was extracted using isolated H. pylori colony; 5) studies in which the presence/absence of iceA gene was examined by polymerase chain reaction; and 6) studies written in English or Chinese.
Exclusion criteria
Studies were excluded if they were reviews or conference proceedings, did not present integrated raw data, included only children or adults and if DNA was extracted from mucosal biopsy specimens of the antrum/corpus. When overlapping data existed, only the largest and latest study was selected.
Quality evaluation and data extraction
Assessment of all the included articles and extraction of raw data were performed by two investigators independently. The following information was extracted from each study: first author’s name, year of publication, country of the study population, iceA1 and iceA2 status according to clinical outcomes (gastritis/NUD, PUD, and GC), and the total number of cases and controls, respectively. Disagreements were resolved by discussion, and all the entered data were consistent finally.
Statistical analysis
Statistical analysis was carried out using RevMan software (Version 5.3.0, The Cochrane Collaboration, Copenhagen, Denmark). The strength of association between the presence/absence of iceA gene and gastritis/NUD, PUD, or GC was evaluated by odds ratios (ORs) and corresponding 95% confidence intervals (CIs). Heterogeneity among the included studies was measured by χ2-based Q and I2 statistics. When the heterogeneity was not considered significant (P≥0.1 for Q test and I2<50%), a fixed-effects model was applied to calculate the pooled OR. Otherwise, a random-effects model was used. To exclude any possible influence of a single study, a sensitivity analysis was performed to evaluate the substantially altered or statistical results of the summary estimate. In addition, publication bias was evaluated qualitatively by funnel plots. Two-sided P-values were assessed in the meta-analysis, and P<0.05 was considered as statistically significant.
Results
Characteristics of selected studies
According to the literature search strategy, a total of 123 potentially relevant records were retrieved, and 37 studies were excluded after duplicate checking. Sixty-seven studies were further excluded because they were irrelevant, abstracts, or reviews, or because they did not conform to the inclusion criteria. Finally, only 19 articles met the inclusion criteria (Figure 1). One article reported by Yamaoka et al14 investigated the correlation of iceA gene with clinical outcomes in four countries, so the data were treated separately. Finally, a total of 22 independent studies were considered for the systematic analyses. Twenty-two studies (with 2,657 patients) assessed the association between iceA1 and infection outcomes, but eight studies did not involve GC patients. Nineteen articles with 2,281 patients showing the relationship between iceA2 and infection outcomes were included, but seven studies did not involve GC patients. Thirteen of the 22 studies were related to the Chinese population. The main characteristics of the studies included in the systematic analyses are summarized in Table 1.
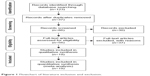  | Figure 1 Flowchart of literature inclusion and exclusion. |
Association between the iceA1 status and clinical outcomes
There were 22 studies, comprising 1,005 cases and 1,247 controls, that assessed the distribution difference of iceA1 status between patients with PUD and gastritis or NUD controls. The prevalence of iceA1 ranged from 10.64% to 96.97% in PUD patients and from 25.42% to 90.91% in gastritis or NUD patients. The general prevalence of iceA1 was 67.66% (680/1,005) in PUD patients and 62.95% (785/1,247) in controls. In the pooled estimate for PUD, the summary OR in the fixed-effects model was 1.23 (95% CI =1.01–1.50, P=0.04), but moderate heterogeneity existed (I2=35%, P=0.06). Sensitivity analysis was conducted, and four studies,10–13 that showed significant differences in the prevalence of iceA1 compared with other studies were removed. The summary OR was 1.28 (95% CI =1.03–1.60, P=0.03), and significant heterogeneity no longer existed among the studies (I2=0%, P=0.92). Subgroup analysis was also performed to explore the correlations with iceA1 status in People’s Republic of China and other countries. The overall prevalence of iceA1 in People’s Republic of China was 70.74% (394/557) in PUD cases and 64.21% (461/718) in gastritis or NUD controls, while in other countries it was 69.93% (200/286) and 62.68% (179/286), respectively. A statistical relationship between iceA1 and PUD was observed in People’s Republic of China subgroup (OR =1.40, 95% CI =1.07–1.83; P=0.01), but no significant association was found in the subgroup of other countries (OR =1.07, 95% CI =0.73–1.57; P=0.73). There was no significant heterogeneity among the subgroups (I2=23.7%, P=0.25) (Figure 2; Table 2).
There were 14 studies, comprising 405 cases and 823 controls, that studied the distribution difference of iceA1 status between patients with GC and gastritis or NUD controls. The overall prevalence of iceA1 was 61.98% (251/405) in GC cases and 58.44% (481/823) in controls. In the pooled estimate for GC, the summary OR in the fixed-effects model was 1.05 (95% CI =0.78–1.40, P=0.75), and there was no significant heterogeneity among the studies (I2=25%, P=0.19). Subgroup analysis was also performed to explore the correlations between iceA1 status in People’s Republic of China and other countries. The overall prevalence of iceA1 in People’s Republic of China was 68.02% (134/197) in GC cases and 62.37% (368/590) in gastritis or NUD controls, while in other countries it was 56.25% (117/208) and 48.50% (113/233), respectively. No significant association was found in People’s Republic of China (OR =1.30, 95% CI =0.89–1.89; P=0.17) and other countries (OR =0.74, 95% CI =0.46–1.20; P=0.22) (Table 2, Figure S1).
We also analyzed the distribution difference of iceA1 status between patients with GC and PUD controls. In the pooled estimate for GC, the summary OR in the fixed-effects model was 0.88 (95% CI =0.64–1.19, P=0.40), and there was no significant heterogeneity among the studies (I2=3%, P=0.42). Subgroup analysis was also performed to explore the correlations with iceA1 status in People’s Republic of China and other countries. The overall prevalence of iceA1 in People’s Republic of China was 68.02 % (134/197) in GC cases and 70.10% (293/418) in PUD controls, while in other countries it was 56.25% (117/208) and 52.74% (125/237), respectively. No significant association was found in People’s Republic of China (OR =0.85, 95% CI =0.57–1.26; P=0.42) and other countries (OR =0.92, 95% CI =0.56–1.49; P=0.73). There was no significant heterogeneity among the subgroups (I2=0%, P=0.80) (Table 2, Figure S2).
Association between the iceA2 status and clinical outcomes
There were 19 studies, comprising 840 cases and 1,070 controls, that examined the distribution difference of iceA2 status between patients with PUD and gastritis or NUD controls. In the pooled estimate for PUD, the summary OR in the fixed-effects model was 1.16 (95% CI =0.94–1.44, P=0.17), and significant heterogeneity existed among these studies (I2=42%, P=0.03). Exploring the sources of heterogeneity showed that Yamaoka et al14 (study in the USA) and Ashour et al15 reported quite a high prevalence of iceA2 in PUD patients (100% and 97.87%, respectively). In addition, the study by Smith et al17 demonstrated an extremely low prevalence of iceA2 in PUD patients (5.26%). After omitting these studies with abnormal results, the heterogeneity was not significant any more (I2=27%, P=0.15), but the analysis still showed there was no significant association between iceA2 and PUD compared with gastritis or NUD controls (OR =1.07, 95% CI =0.85–1.33; P=0.58). Subgroup analysis was also performed to explore the correlations with iceA2 status between People’s Republic of China and other countries. No significant association was found in People’s Republic of China (OR =1.19, 95% CI =0.90–1.58; P=0.21) and other countries (OR =0.87, 95% CI =0.60–1.26; P=0.48). There was no significant heterogeneity among the subgroups (I2=42.6%, P=0.19) (Table 3, Figure S3).
There were 12 studies, comprising 371 cases and 754 controls, that examined the distribution difference of iceA2 status between patients with GC and gastritis or NUD. In the pooled estimate for GC, the summary OR in the fixed-effects model was 0.91 (95% CI =0.67–1.24, P=0.57), and moderate heterogeneity existed among these studies (I2=42%, P=0.06). Exploring the sources of heterogeneity showed that Zhang et al12 and Wei et al13 reported quite a low prevalence of iceA2 in GC patients (10% and 22.64%, respectively). After omitting these studies with abnormal results, the heterogeneity was no longer significant (I2=0%, P=0.65), but the analysis still showed that there was no significant association between iceA2 and GC compared with gastritis or NUD controls (OR =1.27, 95% CI =0.89–1.82; P=0.19). Subgroup analysis was also performed to explore the correlations with iceA2 status between People’s Republic of China and other countries. No significant association was found in People’s Republic of China (OR =1.24, 95% CI =0.70–2.18; P=0.46) and other countries (OR =1.29, 95% CI =0.81–2.05; P=0.28). There was no significant heterogeneity among the subgroups (I2=0%, P=0.91) (Table 3, Figure S4).
In the pooled estimate for GC compared with PUD, the summary OR in the fixed-effects model was 0.79 (95% CI =0.58–1.09, P=0.15), and moderate heterogeneity was observed (I2=43%, P=0.06). Exploring the sources of heterogeneity showed that Zhang et al12 reported quite a low prevalence of iceA2 in GC patients (10%) compared with controls (65.31%). After omitting these data, the heterogeneity was no longer significant (I2=0%, P=0.74), but the analysis still showed there was no significant association between iceA2 and GC compared with PUD controls (OR =0.97, 95% CI =0.69–1.36; P=0.86). Subgroup analysis was also performed to explore the correlations with iceA2 between People’s Republic of China and other countries. No significant association was found in People’s Republic of China (OR =0.92, 95% CI =0.55–1.53; P=0.74) and other countries (OR =1.01, 95% CI =0.65–1.59; P=0.95). There was no significant heterogeneity among the subgroups (I2=0%, P=0.77) (Table 3, Figure S5).
Difference in the prevalence of iceA in People’s Republic of China and other countries
The overall prevalence of iceA was 51.95% (1,784/3,434) in People’s Republic of China and 52.77% (992/1,880) in other countries. There was no significant difference in the prevalence of iceA in People’s Republic of China and other countries (P=0.57). The overall prevalence of iceA1 was 69.25% (1,189/1,717) in People’s Republic of China and 56.06% (527/940) in other countries. The iceA1 status was significantly different in People’s Republic of China than in other countries (P<0.01). Meanwhile, the prevalence of iceA2 was 34.65% (595/1,717) in People’s Republic of China and 49.47% (465/940) in other countries, and such a difference was significant (P<0.01). The iceA1 and iceA2 status in People’s Republic of China was also significantly different (P<0.01), as in other countries (P=0.04).
Publication bias analysis
Publication bias was qualitatively estimated by funnel plots. No significant publication bias was observed for the meta-analyses of association between iceA status and the clinical outcome of H. pylori infection (Figures S6–S11).
Discussion
This meta-analysis included 19 articles, 22 studies, and 2,657 cases and systematically analyzed the association between the iceA gene status and clinical outcomes. The analysis showed that the prevalence of iceA1 significantly increased the risk of PUD compared with gastritis or NUD controls. This significant correlation is particularly significant in Chinese population but not in patients from other countries, suggesting the effect of geographical difference on the relationship between iceA1 and PUD. No significant risk association between iceA1 status and GC was observed in any country population, possibly due to the relatively small sample size of GC cases compared to PUD or gastritis. H. pylori iceA1 affects the immune response of gastric mucosa epithelial cells, and iceA1-positive strains produce more inflammatory cytokines (IL-6, IL-8, etc) than iceA1-negative strains. Meanwhile, it facilitates neutrophil infiltration in gastric mucosa, aggravating mucosal inflammation and PUD.7,11,18,19 Moreover, iceA1-postive strains perhaps disrupt the microecological balance in gastrointestinal tract and accelerate the development of disease. These processes are the potential pathogenic mechanisms of diseases. The finding that there was no significant association between iceA2 and clinical outcomes in People’s Republic of China and other countries is consistent with nearly all the original studies.14–18,20–31 iceA2 gene expression may be more influenced by the gene structure, which has a repeated protein structure but has no homology to known genes, and the function of the iceA2 product remains unclear.20,32
The analysis results showed that the overall prevalence of iceA1 is higher than iceA2 (64.58% and 39.89%, respectively), which is significantly different (P<0.01), and agrees with previous studies.13,16,17,20,27,31 Interestingly, the analysis showed that the prevalence of iceA1 and iceA2 is significantly different in People’s Republic of China (P<0.01), as in other countries (P=0.04). Also, the prevalence of both iceA1 and iceA2 is significantly different between People’s Republic of China and other countries (P<0.01). In People’s Republic of China, H. pylori iceA1 is the major epidemic strain, which is consistent with previous studies showing that H. pylori iceA1 subtype infection is more frequently found in People’s Republic of China, Japan, and Korea.2,4,12 The information about geographical difference in iceA1 gene suggests that it could be used as a potential biomarker for distinguishing PUD from other digestive diseases in People’s Republic of China and reveals a phylogenetic difference of H. pylori strains between People’s Republic of China and other countries. Presumably, damage to the iceA1 gene should reduce the incidence of PUD, which could be a testable hypothesis for future studies.
The pathogenicity of microorganism is not based on one single factor. Virulence genes have many factors that influence the diverse clinical outcomes of bacterial infection, and factors such as the invasion of the bacteria, protein activity, host susceptibility and immune response, age, sex, ethnicity, region, diet habits, concomitant microenvironment, treatment of the host, and collaborative pathogenic mixed infection should not be ignored.33–36
Urea enzymes, cagA, and vacA are conclusively associated with severe gastroduodenal diseases, but the relationship of iceA with clinical outcome is ambiguous. Multiple genes in the H. pylori strain, other unclear subtypes of iceA, detection methods of iceA status, and sample size may influence the analysis results. To confirm the significance of iceA, it is better to carry out a multivariate analysis involving iceA status and other factors. However, it is difficult to obtain the raw data from sufficient studies, which is a caveat of the current analysis. Moreover, children suffer from peptic ulcer or gastric cancer to a lesser extent. So, when a system analysis is performed, this difference between children and adults should be taken into consideration.
The study also had some potential limitations. First, the original literature was insufficient. We only screened papers written in English or Chinese from the electronic databases of PubMed, Embase, CNKI, VIP, and Wanfang. Second, the included studies involved only a few countries. The number of GC patient was relatively small as compared to PUD or gastritis. Third, many factors influence the diverse clinical outcomes of H. pylori infection, such as the geographical distribution of H. pylori strains, host susceptibility and age, sex, ethnicity, region, diet habits, treatment of the host, and collaborative pathogenic mixed infection. These factors may have influenced the results.
Conclusion
In conclusion, the results of this study show that infection with iceA1-positive H. pylori significantly increases the overall risk for PUD, especially in People’s Republic of China. The correlation between iceA2 and clinical outcome of H. pylori infection is not significant. H. pylori iceA1 genotype is the major epidemic strain in People’s Republic of China.
Acknowledgments
This research was supported by Technology Project, Science & Technology Department of Sichuan Province (2014SZ0036, 2015SZ0021).
Disclosure
The authors report no conflicts of interest in this work.
References
International agency for research on cancer. Helicobatcer pylori Eradication as a Strategy for Preventing Gastric Cancer, IARC Working Group Reports [M]. Vol 8, Lyon: IARC, 2013. Available from: http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/Helicobacter_pylori_Eradication.pdf. Accessed June 14, 2016. | ||
Shiota S, Suzuki R, Yamaoka Y. The significance of virulence factors in Helicobacter pylori. J Dig Dis. 2013;14:341–349. | ||
Vannarath S, Vilaichone RK, Rasachak B, et al. Virulence genes of Helicobacter pylori in gastritis, peptic ulcer and gastric cancer in Laos. Asian Pac J Cancer Prev. 2014;15:9027–9031. | ||
Yamaoka Y, Graham DY. Helicobacter pylori virulence and cancer pathogenesis. Future Oncol. 2014;10:1487–1500. | ||
Talebi Bezmin Abadi A, Taghvaei T, Mohabbati Mobarez A, Vaira G, Vaira D. High correlation of babA2-positive strains of Helicobacter pylori with the presence of gastric cancer. Intern Emerg Med. 2013;8:497–501. | ||
Hanada K, Uchida T, Tsukamoto Y, et al. Helicobacter pylori infection introduces DNA double-strand breaks in host cells. Infect Immun. 2014;82:4182–4189. | ||
Sgouras DN, Trang TT, Yamaoka Y. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2015;20(Suppl 1):8–16. | ||
Ben Mansour K, Fendri C, Zribi M, et al. Prevalence of Helicobacter pylori vacA, cagA, iceA and oipA genotypes in Tunisian patients. Ann Clin Microbiol Antimicrob. 2010;9:10. | ||
Taghvaei T, Talebi Bezmin Abadi A, Ghasemzadeh A, Naderi BK, Mohabbati Mobarez A. Prevalence of horB gene among the Helicobacter pylori strains isolated from dyspeptic patients: first report from Iran. Intern Emerg Med. 2012;7:505–508. | ||
van Doorn LJ, Figueiredo C, Sanna R, et al. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. | ||
Yakoob J, Abbas Z, Khan R, et al. Helicobacter pylori: correlation of the virulence marker iceA allele with clinical outcome in a high prevalence area. Br J Biomed Sci. 2015;72:67–73. | ||
Zhang CF, Lin ZH, Wu F, et al. Relationship between iceA gene of Helicobacter pylori and chronic gastritis, peptic ulcer, gastric carcinoma. World Chin J Digestol. 2005;13:685–687. | ||
Wei GC, Chen J, Liu AY, et al. Prevalence of Helicobacter pylori vacA, cagA and iceA genotypes and correlation with clinical outcome. Exp Ther Med. 2012;4:1039–1044. | ||
Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274–2279. | ||
Ashour AA, Collares GB, Mendes EN, et al. iceA genotypes of Helicobacter pylori strains isolated from Brazilian children and adults. J Clin Microbiol. 2001;1746–1750. | ||
You JF, Fang PC, Ye SJ, et al. cagA, vacA and iceA genotypes of Helicobacter pylori isolated in Zhejiang. Chin J Microbiol Immunol. 2003;23:111–112. | ||
Smith SI, Kirsch C, Oyedeji KS, et al. Prevalence of Helicobacter pylori vacA, cagA and iceA genotypes in Nigerian patients with duodenal ulcer disease. J Med Microbiol. 2002;51:851–854. | ||
Wang XF, Cui YB, Wang KX, Wangke X, Li CH. Investigation on the distribution of iceA1 and babA2 genes and its cell immunity function from Helicobacter pylori in Huainan area of Anhui province. Chin J Zoonoses. 2007;23:161–164. | ||
Ciftci IH, Uslan I, Dilek FH, Aşik G, Ozgür MA, Dilek ON. Investigation of Helicobacter pylori iceA1 and iceA2 genes in patients with chronic gastritis and gastric cancer. Mikrobiyol Bul. 2011;45: 228–233. | ||
Chomvarin C, Namwat W, Chaicumpar K, et al. Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA and babA2 genotypes in Thai dyspeptic patients. Int J Infect Dis. 2008;12:30–36. | ||
Chen J, Fang PC, Tao R, et al. Study on predominant genotypes of Helicobacter pylori in Zhejiang Province. Zhejiang Prev Med. 2006;18:1–3, 10. | ||
Chen HJ, Zhang YL, Tang W. Relationship of clinical gastric diseases and the genes, iceA1 and babA2 of Helicobacter pylori isolated. J Jiangsu Univ. 2009;19:173–175, 178. | ||
Gong YH, Liu YE, Sun LP, Dong NN, Yuan Y. Relationship between Helicobacter pylori infection and associated gastric diseases in Liaoning province of China. World Chin J Digestol. 2007;15: 3462–3467. | ||
Han YH, Liu WZ, Zhu HY, Xiao SD. Clinical relevance of iceA and babA2 genotypes of Helicobacter pylori in a Shanghai population. Chin J Dig Dis. 2004;5:181–185. | ||
Ito Y, Azuma T, Ito S, et al. Sequence analysis and clinical significance of the iceA gene from Helicobacter pylori strains in Japan. J Clin Microbiol. 2000;38:483–488. | ||
Li DH, Liu L, Liu YL, Zhou L, Zhuping C, Qinghua T. The characteristics of dominant genotype of pathogenic Helicobacter pylori in a hospital of Guizhou province. Chongqing Med. 2013;42:504–507. | ||
Liu YQ, Su BZ, Song JZ. Distribution of Helicobacter pylori iceA gene in Inner Mongolia. Inner Mongolia Med J. 2008;40:1426–1429. | ||
Miciuleviciene J, Calkauskas H, Jonaitis L, et al. Helicobacter pylori genotypes in Lithuanian patients with chronic gastritis and duodenal ulcer. Medicina (Kaunas). 2008;44:449–454. | ||
Wang F, Kang PP, Wu XJ, Chen ZH. Helicobacter pylori urea, cagA, vacA and iceA status of Guiyang area and relationship to clinical outcomes. Chin J Zoonoses. 2011;27:918–920. | ||
Zheng PY, He WL, Duan FL. Investigation of cagA, iceA gene in Helicobacter pylori strains in the patients with gastric carcinoma and peptic ulcer. Chin J Gastroenterol Hepatol. 1999;8:110–112. | ||
Zhuang K, Zhang J, Zhang LX, Zhang L, Zhang JZ. Relationship between iceA1, iceA2 and babA2 genes of Hp in Xi’an and gastroduodenal diseases. Chin J Cell Mol Immunol. 2007;23:520–522. | ||
Shiota S, Watada M, Matsunari O, Iwatani S, Suzuki R, Yamaoka Y. Helicobacter pylori iceA, clinical outcomes, and correlation with cagA: a meta-analysis. PLoS One. 2012;7:e30354. | ||
Wen S, Moss SF. Helicobacter pylori virulence factors in gastric carcinogenesis. Cancer Lett. 2009;282:1–8. | ||
Moyat M, Velin D. Immune responses to Helicobacter pylori infection. World J Gastroenterol. 2014;20:5583–5593. | ||
Whitmore SE, Lamont RJ. Tyrosine phosphorylation and bacterial virulence. Int J Oral Sci. 2012;4:1–6. | ||
Almeida N, Donato MM, Romãozinho JM, et al. Correlation of Helicobacter pylori genotypes with gastric histopathology in the central region of a South-European country. Dig Dis Sci. 2015;60:74–85. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




