Back to Journals » Clinical Interventions in Aging » Volume 17
Relationship Between Preoperative Hypoalbuminemia and Postoperative Pneumonia Following Geriatric Hip Fracture Surgery: A Propensity-Score Matched and Conditional Logistic Regression Analysis
Authors Tian Y, Zhu Y, Zhang K, Tian M, Qin S, Li X
Received 6 December 2021
Accepted for publication 28 March 2022
Published 13 April 2022 Volume 2022:17 Pages 495—503
DOI https://doi.org/10.2147/CIA.S352736
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Zhi-Ying Wu
Yunxu Tian,1 Yanbin Zhu,1,2 Kexin Zhang,1 Miao Tian,1 Shuhui Qin,2 Xiuting Li1
1Department of Orthopaedic Surgery, The 3rd Hospital of Hebei Medical University, Shijiazhuang, 050051, Hebei, People’s Republic of China; 2Hebei Bone Research Institute, Key Laboratory of Biomechanics of Hebei Province, Shijiazhuang, 050051, Hebei, People’s Republic of China
Correspondence: Yanbin Zhu; Xiuting Li, Department of Orthopaedic Surgery, The 3rd Hospital of Hebei Medical University, Shijiazhuang, 050051, Hebei, People’s Republic of China, Email [email protected]; [email protected]
Background: Pneumonia is a devastating complication following geriatric hip fracture surgery, and preoperative hypoalbuminemia may be a potentially modifiable factor leading to improved outcome. This study aimed to quantify the relationship between preoperative hypoalbuminemia and postoperative pneumonia.
Methods: We retrospectively reviewed the medical records of elderly patients (≥ 60 years) who underwent surgeries for hip fracture in a tertiary referral center between 2016 and 2020. According to the preoperative serum albumin level, they were divided into two groups: < 35 g/ L and ≥ 35 g/ L. To reduce potential confounding, propensity score matching (PSM) in a 1:1 ratio was performed, with the caliper value set as 0.002; and further conditional logistic regression analysis was used to control the other potential confounders to determine the association strength.
Results: Among 3,147 eligible patients included, PSM yielded 1,318 matched patients, with 659 in each respective group, suggesting significantly improved balance in most variables (standardized mean deviation improvement range, 20.7% to 99.1%), except for basophil count. The conditional logistic regression analysis, adjusted for PS and other intra- or post-operative variables, showed 6.18-fold (relative ratio, 6.18; 95% CI, 3.15– 11.98; P< 0.001) increased risk of postoperative pneumonia associated with preoperative hypoalbuminemia.
Conclusion: Preoperative hypoalbuminemia was identified to be independently and highly associated with development of postoperative pneumonia in elderly patients undergoing hip fracture surgeries. However, whether the patients who had such condition may benefit from preoperative targeted nutritional support needs to be clarified by more prospective studies.
Keywords: hip fracture, geriatric population, hypoalbuminemia, pneumonia
Introduction
Pneumonia is one of the most prevalent complications in geriatric patients following surgeries for hip fracture, with a reported range of 5.1% to 14.9%.1–3 Its rapid progression can lead to complications in other parts of the lung, such as pleural effusion and atelectasis, and in some, particularly morbid patients, multiple organ dysfunction may be complicated leading to deterioration of the condition.4–6 In spite of the standard and multimodal therapy in medical care, elderly patients who have developed pneumonia after surgeries for hip fracture have an increased 3.05-fold risk of 30-day mortality, prolonged hospital-stay by 1.6-fold, and increased risk of readmission by 79.1%.4,7,8 Substantial evidence has suggested that hypoalbuminemia, a remarkable serum indicator for malnutrition, has a significant effect on prognosis, as it can impair immune system and cardiopulmonary functions.9 Furthermore, in elderly patients who generally have morbid conditions or poor functional ability, perioperative malnutrition can occur in 17.4%-50%.10–12 Therefore, a clear and adequate understanding of perioperative serum albumin level and its relation to postoperative complications should better inform the perioperative management of hip fracture in elderly patients.
Review of literature showed multiple attempts in investigation of the relationship between perioperative serum albumin level and complications as a composite or individually, following surgeries for hip fracture.13–16 However, several shortcomings might have limited the acquisition of the true association or the association magnitude, such as small sample size, relatively few clinical variables to include, and insufficient adjustment for confounders. In fact, confounding is one of the greatest risks to the scientificity and objectivity of a clinical study, because of the complexity and variability relating to a patient’s medical condition and clinician practice. As is known, in various surgical subspecialties (like thoracic and gastrointestinal surgery et al), preoperative serum albumin has been identified to be associated with postoperative pneumonia.17–19 However, extrapolation of these results to hip fracture orthopedics is not appropriate.
Based on the previous findings, we hypothesize that there is a correlative relationship between preoperative hypoalbuminemia and postoperative pneumonia following geriatric hip fracture surgery.
Thus, we designed this study, with the aim to determine the “true” relationship between preoperative hypoalbuminemia and postoperative pneumonia in a geriatric cohort of patients undergoing surgeries for hip fracture.
Materials and Methods
This retrospective cohort study was conducted in a single-center tertiary referral hospital (the 3rd Hospital of Hebei Medical University) between January 2016 and December 2020. The inclusion criteria were patients aged 60 or older presenting with acute hip fracture caused by low-energy injury mechanism and definitely undergoing orthopedic surgery by arthroplasty or osteosynthesis. Exclusion criteria were medium- or high-energy fractures, old fractures (≥21 days from initial injury to surgery), pathological fractures, multiple fractures or polytrauma, conservative treatment, revision surgery, re-operations for any reason, chronic usage of immunosuppressants such as corticosteroids, preoperative existence of pneumonia or respiratory tract inflammation, pre-fracture hip joint functional dependence, death due to any cause during hospitalization or patients with incomplete data.
Exposure
According to the literature documentation and the reference range for clinical evaluation of hypoalbuminemia, patients were classified into two groups with a preoperative serum albumin level < 35 g/ L or ≥ 35 g/ L.20,21 When one patient had multiple time measurements of serum albumin concentration before operation, the one closest to the time of operation was used for classification.
Outcome (Postoperative Pneumonia)
Pneumonia was defined in accordance with the American Thoracic Society guidelines for healthcare-associated pneumonia,22 and confirmed if a patient was documented to have developed pneumonia after hip fracture surgery during the hospital stay. Clinically, pneumonia was diagnosed when at least one of the following criteria was satisfied: presence of new and/or progressive and persistent respiratory symptoms, including coughing and purulent secretions, fever or hypothermia (body temperature >38°C or body temperature <36°C), presence of lung consolidation and/or moist rale, lab examination suggesting leukocytosis or leukopenia (white cell count >10×109/L or white cell count <4×109/L), positive blood cultures or sputum sample.
Covariables
The variables of interest collected from electronic medical records were based on five aspects, sociodemographics, comorbidities, injury, operation, and laboratory tests. These variables included sex, age, body mass index (BMI, categorized as 18.5–23.9, <18.5, 24.0–27.9 or ≥28.0kg/m2), hypertension, diabetes mellitus, respiratory disease, heart disease, cerebrovascular disease, liver cirrhosis, kidney disease, tumors, cigarette smoking, fracture type (femoral neck or intertrochanteric), any previous operation, time to surgery, surgical duration, intraoperative bleeding, intraoperative blood transfusion, procedure (arthroplasty or osteosynthesis), American Society of Anesthesiologists (ASA, categorized as either I-II or III-IV) classification, anesthesia (general or local), preoperative albumin (ALB), white blood cell (WBC), hemoglobin (HGB), hematocrit (HCT), red blood cell (RBC), platelet (PLT), neutrophil count (NEUT), eosinophils (EOS), and basophilic (BAS). When a patient had multiple measurements of laboratory indexes before operation, those closest to the time of operation were used for analyses.
Statistical Analysis
Data were expressed as means and standard deviation for continuous variables which were normally distributed, otherwise as median and interquartile (IQR), with use of Shapiro–Wilk normality test to evaluate the normality status. t-test or Whitney U test was used to evaluate the between-group difference, as appropriate. Categorical variables were expressed as number and percentages and compared using Chi-squared or Fisher’s exact test.
To minimize the confounding effects arising from between-group distribution differences, propensity score matching (PSM) in a ratio 1:1 based on the propensity score for one patient was performed, using a logistic regression model. Patients who had preoperative hypoalbuminemia were matched to those who did not, using calipers of 0.002, via the greedy matching method (nearest neighbor without replacement in this study). The covariates that were included in the propensity score analyses involved demographics, comorbidities, injury, and laboratory testing results. We did not include operative variables such as bleeding, blood transfusion, surgical duration, procedure type, anesthesia and postoperative antibiotics, because these are more likely to be affected by preoperative malnutrition. In addition, considering the importance of liver cirrhosis and chronic kidney disease associated with hypoalbuminemia, we included them as covariables in the conditional logistic regression analysis. The standardized mean differences (SMD) for all covariates were used to assess the balance before and after PSM, with ≥0.10 indicative of imbalance.
For further investigation of whether the operative variables will affect the outcome or exploring which variable may mediate the relationship, we used conditional logistic regression analysis with hypoalbuminemia, propensity score and the previously-mentioned operative variables entered for adjustment. The relative ratio (RR) and 95% confidence interval (95% CI) were calculated to indicate the association magnitude. P < 0.05 was set as the statistical significance level. SPSS 26.0 (IBM, Armonk, NY, USA) and R software version 3.4.2 were used to perform all the analyses.
Results
In the study period, 4,692 patients (≥60) were diagnosed with hip fracture and 1,545 patients were excluded, due to medium- or high-energy fractures (321), old fractures (172), pathological fractures (non-osteoporotic fractures) (31), multiple fractures or polytrauma (129), conservative treatment (293), revision or secondary surgery (102), chronic usage of immunosuppressants (82), preoperative existence of pneumonia (44), pre-fracture hip joint functional dependence (112), death due to any cause during hospitalization (17), and incomplete clinical data (242), leaving 3,147 for data analysis and 182 (5.8%) developed postoperative pneumonia (Figure 1).
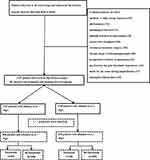 |
Figure 1 The flowchart illustrates the selection of the participants for this research. |
Table 1 showed the baseline characteristics of patients before PSM. Compared to those who did not have hypoalbuminemia (n=1582), patients who had preoperative hypoalbuminemia (n=1383) were older (78.6 ± 8.1 vs 73.2 ±8.6), less likely to smoke (11.2% vs 15.9%), more likely to have history of respiratory disease (5.6% vs 2.9%), heart disease (19.5% vs 9.1%) and cerebrovascular disease (21.1% vs 13.9%), intertrochanteric fracture (67.4% vs 30.3%), and longer preoperative stay (5.8 ± 4.1 vs 5.0 ± 3.9) (Table 1). The incidence rate of postoperative pneumonia was and among them 182 (5.8%) developed postoperative pneumonia.
 |
Table 1 Comparison of the Baseline Characteristics of the Patients with and without Preoperative Hypoalbuminemia |
After PSM, a total of 1,318 patients were identified, with 659 in each respective group, and the SMD of all included covariates was <0.10, indicating a good balance (Table 1). The univariate analyses also showed the non-significant differences between groups after PSM (all P>0.05).
Conditional logistic regression analyses revealed non-significance for all the operative variables: liver cirrhosis (RR, 2.68; 95% CI, 1.58–3.08; P=0.032), kidney disease (RR, 1.87; 95% CI, 0.95–2.32; P=0.138), intraoperative bleeding (RR, 1.03; 95% CI, 0.98–1.05; P=0.908), intraoperative blood transfusion (RR, 1.29; 95% CI, 0.73–2.15; P=0.368), surgical duration (RR, 1.02; 95% CI, 0.97–1.15; P=0.332), procedure type (RR, 1.13; 95% CI, 0.63–1.95; P=0.663), anesthesia technique type (RR, 1.32; 95% CI, 0.89–2.13; P=0.265), postoperative prophylactic antibiotic use (RR, 2.50; 95% CI, 0.79–7.95; P=0.121) (Table 2). However, preoperative hypoalbuminemia was associated with 6.18-fold increased risk (adjusted RR, 6.18; 95% CI, 3.15–11.98) of postoperative pneumonia, showing slight change compared to the crude RRs derived from both raw data (RR, 6.26; 95% CI, 4.12 to 9.35) and the “before-adjustment” data (RR, 5.78; 95% CI, 3.01 to 11.05) (Table 3).
 |
Table 2 Risk for Postoperative Pneumonia |
 |
Table 3 Comparison of the RR Value in Different Settings |
Discussion
Our study showed that the incidence rate of postoperative in-hospital pneumonia following surgeries for elderly hip fractures was 5.8%, and preoperative hypoalbuminemia was identified as an independent predictor that had 6.18-fold increased risk of postoperative pneumonia, even after PSM and conditional logistic regression analysis.
The relationship identified between preoperative hypoalbuminemia and postoperative pneumonia can be explained by the following potential mechanisms. First, hypoalbuminemia itself reflects reduced organ preserving functional ability to protect these patients having undergone major trauma orthopedic surgery from infectious events, especially in those elderly patients often with underlying conditions or diseases. Second, hypoalbuminemia has been proven to impair the immune function of the body, reduce the number of lymphocytes, weaken phagocytosis, and reduce the enzyme activity of antibody synthesis, thus increasing the chance of lung infection.23 Third, hypoalbuminemia destroys vascular endothelial growth factor, leading to transcapillary albumin leakage and interstitial edema, further impairing lung function.24 Fourth, the low free radical scavenging rate associated with hypoalbuminemia also contributes to the increased risk of postoperative pneumonia.25 In addition, patients with preoperative hypoalbuminemia have poor functional outcomes after hip fracture surgery which could be an important aspect in postsurgical pneumonia.26 These damaging effects by hypoalbuminemia on pneumonia, presumably, would have been aggravated in the setting of hip fracture in such a fragile cohort of elderly patients.
Our findings were consistent with previous studies of different postoperative complications, in different settings or surgical fields. In studies of hip or knee joint arthroplasty, researchers have found patients suffering preoperative hypoalbuminemia had a 1.9 to 2.0-fold increased risk of any postoperative adverse events and 4-fold increased risk of blood transfusion, respectively.27,28 Seong-Eun et al29 retrospectively analyzed 519 elderly patients with hip fracture and found that the incidence of preoperative malnutrition in patients with aspiration pneumonia was significantly higher than that in the control group (36.8% vs 9.1%). Toshihiro et al30 investigated 426 elderly patients undergoing surgery for hip fracture and found postoperative hypoalbuminemia was associated with 5.8-fold increased risk of postoperative pneumonia. Shin et al2 investigated 1,155 elderly patients undergoing surgery for hip fracture and found postoperative hypoalbuminemia was associated with 2.4-fold increased risk of postoperative pneumonia. In their study, multivariate regression analysis was also used, similar to ours and the cut-off value for albumin level was determined as 3.0g/L and 3.5g/L. Compared to theirs, we further adjusted for the intraoperative variables in the after-PSM cohort using the conditional logistics regression model, thus minimizing the confounding effects.
It is reassuring that serum albumin level not only has the potential for predicting the occurrence of adverse events, but can also be a factor for allowing modification. Substantial evidence has shown that early nutritional optimization can supplement calories and protein in a timely manner, regulate immune function, and maintain normal cell metabolism.31–33 Yokoyama et al34 reported that through preoperative nutrition, the outcomes of patients at risk of postoperative infection-related complications following femoral neck fracture surgery were significantly improved. In another study, Malafarina et al35 demonstrated that oral nutritional supplements prior to surgery could prevent the aggravation of operative complications in older adults with hip fracture. Similarly, in a clinical trial involving 80 elderly patients following hip fractures, daily intake of the optimal dose of nutritional supplements before surgery demonstrated enhanced immunity and reduced the incidence of postoperative complications.36 As a consequence, considering the higher amount of consumption of nutrition and overall fragility in such elderly trauma patients, we should pay attention to preoperative nutrition management.
The advantages of this study included the large sample, use of PSM for balancing the between-group differences, and the conditional logistic regression analysis for further adjustment. We think the result is robust, more closely reflecting the true correlation. However, several limitations should be mentioned. First, despite the fact that the data were extracted from a prospectively collected database, many variables were determined by patients’ self-reports, especially the underlying comorbidities. This might have induced recall bias which can compromise the accuracy of data. Second, this was a single-center study in a University-affiliated and tertiary-referral institution, and thus the resultant selection bias might have limited the generalizability of our finding. Third, the analyses in the present study revealed correlative relationships, not causality. The “real world study” seems a practical method to address clinical issues like causation, but the results should still be treated in the context of observational designs. Fourth, primary result of postoperative pneumonia was only limited to those cases occurring during hospitalization period and the follow-up of patients after discharge was not conducted, potentially leading to underestimation of the incidence of postoperative pneumonia.
Conclusion
In summary, this study concluded that preoperative hypoalbuminemia is associated with 6.18-fold increased risk of postoperative pneumonia in elderly patients following surgeries for hip fracture. But further studies are necessary to confirm whether the patients who had such condition may benefit from preoperative targeted nutritional support.
Abbreviations
BMI, body mass index; ASA, American Society of Anesthesiologists; ALB, preoperative albumin; WBC, white blood cell; HGB, hemoglobin; HCT, hematocrit; RBC, red blood cell; PLT, platelet; NEUT, neutrophil count; EOS, eosinophils; BAS, basophilic; SMD, standardized mean difference; RR, relative ratio; 95% CI, 95% confidence interval; PSM, propensity score matching.
Data Sharing Statement
All the data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of the 3rd Hospital of Hebei Medical University (2014-015-1) and informed consent was waived due to its retrospective nature. All the data were analyzed anonymously to safeguard patient privacy. The study was in compliance with the Helsinki Declaration.
Consent for Publication
We have obtained consent for publication from all participants.
Acknowledgments
We are grateful to Xinqun Cheng of the Department of Statistics and Applications for his generous help with statistical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors have received no external funding in order to support this project.
Disclosure
All authors declare that they do not have a conflict of interest in this work.
References
1. Lo I, Siu C, Tse H, Lau T, Leung F, Wong M. Pre-operative pulmonary assessment for patients with hip fracture. Osteoporos Int. 2010;21:S579–86. doi:10.1007/s00198-010-1427-7
2. Shin K, Kim J, Son S, Hwang K, Han S. Early postoperative hypoalbuminaemia as a risk factor for postoperative pneumonia following hip fracture surgery. Clin Interv Aging. 2020;15:1907–1915. doi:10.2147/cia.S272610
3. Xiang G, Dong X, Xu T, et al. A nomogram for prediction of postoperative pneumonia risk in elderly hip fracture patients. Risk Manag Healthc Policy. 2020;13:1603–1611. doi:10.2147/rmhp.S270326
4. Chatterton BD, Moores TS, Ahmad S, Cattell A, Roberts PJ. Cause of death and factors associated with early in-hospital mortality after hip fracture. Bone Joint J. 2015;97-B(2):246–251. doi:10.1302/0301-620X.97B2.35248
5. Lv H, Yin P, Long A, et al. Clinical characteristics and risk factors of postoperative pneumonia after hip fracture surgery: a pr ospective cohort study. Osteoporos Int. 2016;27(10):3001–3009. doi:10.1007/s00198-016-3624-5
6. Salarbaks AM, Lindeboom R, Nijmeijer W. Pneumonia in hospitalized elderly hip fracture patients: the effects on length of hospital-stay, in-h ospital and thirty-day mortality and a search for potential predictors. Injury. 2020;51(8):1846–1850. doi:10.1016/j.injury.2020.05.017
7. Tarazona-Santabalbina F, Belenguer-Varea A, Rovira-Daudi E, et al. Early interdisciplinary hospital intervention for elderly patients with hip fractures: functional outcome and mortality. Clinics. 2012;67(6):547–556. doi:10.6061/clinics/2012(06)02
8. Bohl DD, Sershon RA, Saltzman BM, Darrith B, Della Valle CJ. Incidence, risk factors, and clinical implications of pneumonia after surgery for geriatric hip fract ure. J Arthroplasty. 2018;33(5):1552–1556.e1. doi:10.1016/j.arth.2017.11.068
9. Kamath A, Nelson C, Elkassabany N, Guo Z, Liu J. Low albumin is a risk factor for complications after revision total knee arthroplasty. J Knee Surg. 2017;30(3):269–275. doi:10.1055/s-0036-1584575
10. Koren-Hakim T, Weiss A, Hershkovitz A, et al. Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clinical Nutrition. 2017;36(3):912. doi:10.1016/j.clnu.2017.01.018
11. Lee H, Chang Y, Jean Y, Wang H. Importance of serum albumin level in the preoperative tests conducted in elderly patients with hip fracture. Injury. 2009;40(7):756–759. doi:10.1016/j.injury.2009.01.008
12. Velanovich V. The value of routine preoperative laboratory testing in predicting postoperative complications: a multivariate analysis. Surgery. 1991;109:236–243.
13. Koren-Hakim T, Weiss A, Hershkovitz A, et al. The relationship between nutritional status of hip fracture operated elderly patients and their functioning, comorbidity and outcome. Clinical Nutrition. 2012;31(6):917–921. doi:10.1016/j.clnu.2012.03.010
14. Patterson B, Cornell C, Carbone B, Levine B, Chapman D. Protein depletion and metabolic stress in elderly patients who have a fracture of the hip. J Bone Joint Surg Am. 1992;74(2):251–260. doi:10.2106/00004623-199274020-00011
15. Bohl D, Shen M, Hannon C, Fillingham Y, Darrith B, Della Valle C. Serum albumin predicts survival and postoperative course following surgery for geriatric hip fracture. J Bone Joint Surg Am. 2017;99(24):2110–2118. doi:10.2106/jbjs.16.01620
16. Wang Y, Li X, Ji Y, et al. Preoperative serum albumin level as a predictor of postoperative pneumonia after femoral neck fractur e surgery in a geriatric population. Clin Interv Aging. 2019 14:2007–2016.
17. Ai S, Sun F, Liu Z, et al. Change in serum albumin level predicts short-term complications in patients with normal preoperative serum albumin after gastrectomy of gastric cancer. ANZ J Surg. 2019;89:E297–E301. doi:10.1111/ans.15363
18. Wierdak M, Pisarska M, Kuśnierz-Cabala B, et al. Changes in plasma albumin levels in early detection of infectious complications after laparoscopic colorectal cancer surgery with ERAS protocol. Surg Endosc. 2018;32(7):3225–3233. doi:10.1007/s00464-018-6040-4
19. Li P, Li J, Lai Y, et al. Perioperative changes of serum albumin are a predictor of postoperative pulmonary complications in lung cancer patients: a retrospective cohort study. J Thorac Dis. 2018;10(10):5755–5763. doi:10.21037/jtd.2018.09.113
20. Bicciré F, Pastori D, Tanzilli A, et al. Low serum albumin levels and in-hospital outcomes in patients with ST segment elevation myocardial infarction. Nutr Metab Cardiovasc Dis. 2021;31:2904–2911. doi:10.1016/j.numecd.2021.06.003
21. Akirov A, Masri-Iraqi H, Atamna A, Shimon I. Low albumin levels are associated with mortality risk in hospitalized patients. Am J Med. 2017;130(12):
22. Kalil A, Metersky M, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis. 2016;63(5):e61–e111. doi:10.1093/cid/ciw353
23. Franch-Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clinical Nutrition. 2001;20(3):265–269. doi:10.1054/clnu.2001.0438
24. Kim S, McClave S, Martindale R, Miller K, Hurt R. Hypoalbuminemia and clinical outcomes: what is the mechanism behind the relationship? Am Surg. 2017;83(11):1220–1227. doi:10.1177/000313481708301123
25. Ballmer PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr. 2001;20(3):271–273. doi:10.1054/clnu.2001.0439
26. Sim SD, Sim YE, Tay K, et al. Preoperative hypoalbuminemia: poor functional outcomes and quality of life after hip fracture surgery. Bone. 2021;143:115567. doi:10.1016/j.bone.2020.115567
27. Hur E, Bohl D, Della Valle C, Villalobos F, Gerlinger T. Hypoalbuminemia predicts adverse events following unicompartmental knee arthroplasty. J Knee Surg. 2021. doi:10.1055/s-0041-1739146
28. Tan Y, Jiang L, Liu H, Pan Z, Wang H, Chen L. The effect of preoperative hypoalbuminemia on complications after primary hip arthroplasty. J Orthop Surg Res. 2021;16(1):562. doi:10.1186/s13018-021-02702-0
29. Byun S, Shon H, Kim J, Kim H, Sim Y. Risk factors and prognostic implications of aspiration pneumonia in older hip fracture patients: a multicenter retrospective analysis. Geriatr Gerontol Int. 2019;19(2):119–123. doi:10.1111/ggi.13559
30. Higashikawa T, Shigemoto K, Goshima K, et al. Risk factors for the development of aspiration pneumonia in elderly patients with femoral neck and trochanteric fractures: a retrospective study of a patient cohort. Medicine. 2020;99(7):e19108. doi:10.1097/md.0000000000019108
31. Weimann A, Braga M, Carli F, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36(3):623–650. doi:10.1016/j.clnu.2017.02.013
32. Lee S, Yeom S, Kim C, Kim H. Effect of preoperative immunonutrition on outcomes of colon cancer surgery: study protocol for a randomized controlled trial. Trials. 2020;21(1):628. doi:10.1186/s13063-020-04544-3
33. Aida T, Furukawa K, Suzuki D, et al. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreatoduodenectomy. Surgery. 2014;155(1):124–133. doi:10.1016/j.surg.2013.05.040
34. Yokoyama K, Ukai T, Watanabe M. Effect of nutritional status before femoral neck fracture surgery on postoperative outcomes: a retrospective study. BMC Musculoskelet Disord. 2021;22(1):1027. doi:10.1186/s12891-021-04913-2
35. Malafarina V, Reginster J, Cabrerizo S, et al. Nutritional status and nutritional treatment are related to outcomes and mortality in older adults with hip fracture. Nutrients. 2018;10(5):555. doi:10.3390/nu10050555
36. Eneroth M, Olsson UB, Thorngren KG. Nutritional supplementation decreases hip fracture-related complications. Clin Orthop Relat Res. 2006;451:212–217. doi:10.1097/01.blo.0000224054.86625.06
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
