Back to Journals » Psychology Research and Behavior Management » Volume 16
Relationship Between Physical Exercise and Cognitive Impairment Among Older Adults with Type 2 Diabetes: Chain Mediating Roles of Sleep Quality and Depression
Authors Zhang H , Zhang Y , Sheng S, Xing Y, Mou Z, Zhang Y , Shi Z, Yu Z, Gao Q, Cai W, Jing Q
Received 6 January 2023
Accepted for publication 10 March 2023
Published 17 March 2023 Volume 2023:16 Pages 817—828
DOI https://doi.org/10.2147/PRBM.S403788
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Igor Elman
Han Zhang,1– 3 Yefan Zhang,1– 3 Sen Sheng,1,2 Yang Xing,4 Zhongchen Mou,5 Yanqiu Zhang,1– 3 Zhixue Shi,1– 3 Zhenjie Yu,1,2 Qianqian Gao,1– 3 Weiqin Cai,1– 3 Qi Jing1– 3
1School of Management, Weifang Medical University, Weifang, Shandong, People’s Republic of China; 2“Health Shandong” Collaborative Innovation Center for Severe Social Risk Prediction and Governance, Weifang, People’s Republic of China; 3China Academy of Rehabilitation and Health, Weifang Medical University, Weifang, People’s Republic of China; 4Weifang People’s Hospital, Weifang, People’s Republic of China; 5School of Psychology, Weifang Medical University, Weifang, People’s Republic of China
Correspondence: Weiqin Cai; Qi Jing, School of Management, Weifang Medical University, No. 7166 Baotongxi Street, Weifang, 261053, People’s Republic of China, Tel +8618106369128, Email [email protected]; [email protected]
Objective: Although physical exercise has been shown to boost physical, psychological, and psychiatric conditions in older adults, there is a relative lack of research on the mechanisms involved in this process for older adults with type 2 diabetes mellitus (T2DM). We thus evaluated whether sleep quality and depression mediated the relationship between physical exercise and cognitive impairment in older adults with T2DM by focusing on the exercise–physiology–psychology and psychiatry connection.
Methods: Self-reported data were collected from 2646 older adults with T2DM in Weifang, Shandong, China. Regression and bootstrap analyses were conducted to explore the chain mediator model including physical exercise, cognitive impairment, sleep quality, and depression.
Results: Engaging in physical exercise (coefficient = − 0.6858, p < 0.001), high levels of sleep quality (coefficient = − 0.3397, p = 0.015), and low levels of depression (coefficient = 0.3866, p < 0.001) were significantly associated with a low level of cognitive impairment. Sleep quality and depression mediated the chain effect between physical exercise and cognitive impairment (total effect = − 1.0732, 95% CI [− 1.3652, − 0.7862]; direct effect = − 0.6858, 95% CI [− 0.9702, − 0.3974]; indirect effect = − 0.3875, 95% CI [− 0.5369, − 0.2521]).
Conclusion: Physical exercise may improve sleep quality in older adults with T2DM, alleviating depression and delaying the development of cognitive impairment. Physical exercise can enhance patients’ ability to resist depression and cognitive impairment, and creating comfortable sleep environments can also reinforce the effects of this process. These findings have important implications for promoting healthy aging in older adults with T2DM.
Keywords: physical exercise, cognitive impairment, older adults, depression, sleep quality, type 2 diabetes
Introduction
The 10th edition of the Global Diabetes Map shows that, in 2021, an estimated 537 million adults between the ages of 20 and 79 years old had diabetes. In the same year, 6.7 million deaths resulted from diabetes and cost at least $966 billion in health expenditures. Type 2 diabetes mellitus (T2DM) accounts for 90–95% of global diabetes cases,1 and the prevalence increase with age.2 Statistics from the UN Population Division show that 21.6% of the global population will be aged 65 or older in 2020. As the world faces the challenge of an aging population, T2DM has become an increasingly prevalent disease in older people.3
To worsen matters, older adults with T2DM often experience psychiatric complications such as cognitive impairment (COI), and approximately more than 20% of older T2DM patients may experience dementia.4 COI refers to a difficulty in an individual’s learning, perception, memory, and other functions. These impairments also cause problems with executive functions, which makes it harder to participate in activities, complete certain actions or tasks, or access events in daily life.5 The most impaired cognitive domain in older adults with T2DM is delayed memory, which often makes them struggle to make healthcare appointments for healthcare treatments, blood glucose management, and disease care.6 Thus, COI may lead to poor glycemic control and greater susceptibility to complications such as diabetic nephropathy and peripheral neuropathy, which can in turn lead to cognitive decline.7 The negative bidirectional effect between the two ultimately leads to increased mortality in older adults with T2DM.8
Physical exercise (PE) is a key part of the treatment regimen for patients with T2DM. It is an effective way for patients to manage their blood glucose by moderating insulin resistance, and good glycemic control facilitates the prevention of T2DM complications and comorbidities.9 In addition, PE is a non-pharmacological strategy that can alleviate cognitive decline in patients with T2DM.10 PE has been shown to be effective in improving memory function, visuospatial function, and daily activity of older people11,12 and can prevent the development of COI. PE can therefore benefit older patients with T2DM as part of their disease treatment and glycemic management. Although PE is widely known for its unique convenience, accessibility, and cost-effectiveness, the mechanisms at play need to be further explored.
There are multiple pathways by which PE affects COI. A randomized controlled study found a significant effect of reduced depression and improved sleep quality on the relationship between motor functions and COI in older adults.13 It has also been found that regular PE improved sleep quality and alleviated depression,14 which in turn have been indicated to mitigate the development of COI.13 Studies have also shown that there is a marked negative relationship between sleep quality and depression15 and that low levels of sleep quality can also contribute to psychological and psychiatric disorders. Sleep is an important process for restoring an individual’s functional and mental state. Poor sleep quality indicates that older adults with T2DM lack sufficient resilience to mitigate adverse events caused by the disease, which leads to the development of psychological and psychiatric problems such as depression and COI. Individuals with both sleep disorders and depression may lose cognitive function more quickly and bear a greater burden than individuals without sleep disorders.16 Accordingly, PE may affect COI through sleep quality and depression.
Although PE has been demonstrated to be an effective means of delaying COI among older adults, studies on how PE affects COI in older adults with T2DM are relatively scarce, particularly related to the possible chaining effects. Unlike the general population of older adults, cognitive decline in older patients with T2DM is not only associated with sleep disorders, depression related hippocampus shrinkage, and deficits in prefrontal cortical functionality13 but also strongly associated with T2DM. T2DM exacerbates the effects of these pathways on COI as well as promotes the development of COI through other pathways, such as increased inflammation and insulin resistance.17 Additionally, disorders such as blood glucose management and other comorbidities limit mobility and increase psychological stress in older patients with T2DM, which has a further adverse effect on cognitive function.18 Therefore, exploring the pathway of PE affecting COI among older adults with T2DM is necessary. We aimed to explore the relationship between PE and COI in older patients with T2DM and examine the underlying mechanisms of action from an “exercise–physiology–psychology and psychiatry” perspective. This study hypothesized that PE indirectly delays the development of COI through high levels of sleep quality and mild depression in a serial mediator model. We have combined our previous studies to propose the following more detailed hypothesis (Figure 1).
Hypothesis 1: PE negatively predicts COI in older adults with T2DM (c). Hypothesis 2: Sleep quality mediates the relationship between PE and COI (a1, b1). Hypothesis 3: Depression mediates the relationship between PE and COI (a2, b2). Hypothesis 4: Sleep quality and depression mediates the relationship between the presence or absence of PE and the severity of COI through a chain mediator model (a1, d, b2).
 |
Figure 1 A model of chain mediating effects of sleep quality and depression between physical exercise and cognitive impairment. |
Materials and Methods
Participants
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Weifang Medical University (approval number: 2020YX018). Before conducting the survey, we obtained permission from each community health service center. A cross-sectional survey on the health status of patients with T2DM was conducted from April to June 2021 in Weifang, Shandong Province, China. This study used a multi-stage sampling method. In the first stage, two or three townships under the jurisdiction of 12 counties (cities and districts) in Weifang were selected randomly. In the second phase, patients with T2DM who participated in Weifang’s community diabetes management service were recruited within the jurisdiction of the selected township. All surveys were completed in eight regions (cities and districts), including Hanting, Fangzi, Quiwen, Zhucheng, Shouguang, Changyi, Linqu, and Changle, with 4275 valid questionnaires collected (valid response rate of 97.16%). Eligible participants in this study included people aged 60 years or older with T2DM who had lived in their county for more than a year. Before the survey, the general practitioners informed respondents of the purpose of the study and obtained their verbal informed consent. The total sample included 2646 participants.
Measures
Physical Exercise
In assessing health-related behaviors in patients with T2DM, a single-item was used to measure PE: “Do you regularly participate in physical exercise?” The possible answers were 0 = “no” or 1 = “yes.” In the survey, we defined regular physical exercise as sessions of 30 minutes or longer of exercise performed three or more times per week.
Cognitive Impairment
The Chinese version of the Mini-Mental State Examination (MMSE) was used to assess cognitive function in older adults with T2DM. MMSE is commonly used among older adults in China because of its excellent reliability and effectiveness.19 The scale measures cognitive function in terms of orientation, memory, attention, and executive functions. It contains a total of 30 items, and its highest score is 30 points. Each item has two responses (0 = incorrect/I do not know, 1 = correct), and one point was awarded for each correct response. This study uses reverse scoring, so higher scores suggest a higher risk of developing COI. The Cronbach’s alpha of the MMSE was 0.895.
Sleep Quality
Sleep quality was assessed by the question “How well do you sleep and rest?” (including sleep duration and whether the respondent experiences insomnia, wakefulness, early awakening, etc.). The score ranged from 1 = “poor” to 3 = “good.”
Depression
Depression was measured by the Patient Health Questionnaire (PHQ-9), which is widely used in China.20 Because the mediating variable in this study was sleep quality, to avoid common method bias, we removed the sleep item from this scale and used eight self-reported items (PHQ-8) to measure whether participants experienced depressive symptoms over a two-week period. Responses were given on a four-point frequency scale, ranging from 0 (not at all) to 3 (almost every day). Total scores range from 0 (no depression) to 24 (most severe depression). A PHQ-8 total score ≥ 7 indicates a risk of depression and the need for clinical diagnosis.21 The Cronbach’s alpha of the PHQ-8 was 0.918.
Covariates
We also identified a number of factors that may confound the effect of PE on COI in older patients with T2DM based on established literature and statistical analysis. Age (in years), sex (1 = male, 2 = female), marital status (1 = married, 2 = other), educational level (≤ primary school, junior high school, ≥ senior high school), job status (1 = employed/retired, 2 = unemployed), annual household income (< 20,000 RMB, 20,000–50,000 RMB, 50,000–100,000 RMB, > 100,000 RMB), self-reported health status (from 1 = very healthy to 5 = very unhealthy), number of years with T2DM (in years), and self-reported T2DM control effect (very good, relatively stable, mediocre, little) were all control variables.
Statistical Analyses
Data analyses for this study were performed in SPSS 26.0 (SPSS; IBM, Armonk, NY, USA). First, a descriptive analysis was conducted to report the sample characteristics (mean and standard deviation of study variables). Next, correlations between the study variables were examined. Finally, the PROCESS macro was used to analyze the chain mediation effect. After introducing covariates, we used sleep quality (M1) and depression (M2) as mediating variables, PE or not as independent variables (X), and COI as the outcome variable (Y). We reported effect values for each pathway. To further clarify the mechanism between sleep quality and depression, we also tested a model where M1 was depression and M2 was sleep quality. We used the 10,000 bootstrap resamples confidence intervals (CIs) (with or without including 0) to judge whether the direct/indirect effect and the total effect were significant.
Results
Descriptive Statistics
Table 1 presents basic information about the 2646 older adults with T2DM who participated in the study.
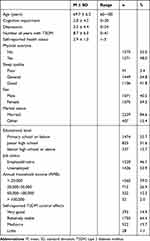 |
Table 1 Descriptive Analysis of Sample Characteristics (n = 2646) |
Table 2 provides correlations of the study variables. PE was significantly negatively associated with depression and COI (p < 0.01) and significantly positively associated with sleep quality (p < 0.01). Sleep quality was significantly negatively associated with depression and COI (p < 0.01). COI was significantly positively associated with depression (p < 0.01).
 |
Table 2 Correlation Analysis of Variables |
Chain Mediation Effect Analysis
Table 3 shows that PE positively predicted sleep quality (coefficient = 0.2968, p < 0.001) but negatively predicted depression (coefficient = −0.5326, p < 0.001). In contrast, depression positively predicted COI (coefficient = 0.3866, p < 0.001). Low levels of sleep quality were significantly associated with high levels of depression (coefficient = −0.7036, p < 0.001) and COI (coefficient = −0.3397, p = 0.017). Because the direct effect of PE was significant in negatively predicting COI (coefficient = −0.6858, p < 0.001), the effect of PE on COI is partially mediated by sleep quality and depression.
 |
Table 3 Regression Analysis of Physical Exercise, Sleep Quality, and Depression on Cognitive Impairment |
Model 6 in PROCESS was used to examine whether sleep quality and/or depression mediated the relationship between PE and COI. Figure 1 and Table 4 display the chain mediated effect model with a significant total effect (Effect = −1.0732, 95% CI [−1.3652, −0.7862]) and direct effect of PE on COI (Effect = −0.6858, 95% CI [−0.9702, −0.3974]), and hypothesis 1 was supported. The total indirect effect of the presence or absence of PE on the severity of COI was significant (Effect = −0.3875, 95% CI [−0.5369, −0.2521]). The sleep quality mediating effect was significant (Effect = −0.1008, 95% CI [−0.1889, −0.0166]), and hypothesis 2 was supported. The indirect effect of the mediating role of depression was significant (Effect = −0.2059, 95% CI [−0.3359, −0.0943]), therefore hypothesis 3 was supported. The chain mediating effect of sleep quality and depression was equally significant (Effect = −0.0807, 95% CI [−0.1171, −0.0469]), and hypothesis 4 was also supported.
 |
Table 4 Analysis of Chain Mediating Effects |
Discussion
As life expectancy and the older adult population have increased, COI and dementia have emerged as one of the greatest challenges to health in the 21st century. In some regions of China, the prevalence of COI in older people is as high as 44.2%.22 When older adults with T2DM develop COI, a greater disease burden is imposed on the nation and society. Approximately 10–20% of patients with T2DM experience COI,23 with 46.9% prevalence in medical institutions.24 T2DM not only increases the risk of developing COI, it also increases the rate at which COI progresses to dementia.25 Therefore, this study aimed to determine the relationship and mechanisms between PE and COI in older adults with T2DM. Specifically, regular PE may improve sleep quality, which may alleviate depressive symptoms and thus delay the development of COI.
Consistent with previous studies, PE was significantly and negatively associated with COI, and performing PE negatively predicted COI.26,27 In the absence of a cure for COI, palliative care such as early diagnosis, counseling, and lifestyle interventions (diet, exercise, etc.) are often used to alleviate the decline in cognitive function.28 During the aging process, older adults experience muscle loss and osteoporosis. These symptoms limit their ability to perform daily activities and reduce opportunities for exercise and socialization, which can lead to both the body and brain not receiving adequate stimulation.29 At the same time, long-term functional decline of the brain and accompanying structural changes further decrease cognitive function, and T2DM can accelerate this process.30 Obesity is also common in patients with T2DM.31 Obesity alters the metabolic profile of adipose tissue and leads to dysregulation of adipokine secretion.32 Adipokines play a crucial role in immune function, and dysregulation of adipokines may trigger immune inflammation.33 Moreover, obesity is also associated with inflammation in the central nervous system, represented by the hippocampus, which is responsible for learning and memory tasks.34 These inflammatory conditions can lead to obesity-induced COI,35,36 and aging accelerates this process.34 Therefore, older patients with T2DM are more likely to develop COI. Another important mechanism, which is very common in patients with T2DM, may be that hypoxia, oxidative stress, and other cerebral microvascular pathological changes lead to cerebral microvascular dysfunction, making it more likely that patients with T2DM will develop COI.37
Engaging in PE has many benefits for the health and correct functioning of both the body and brain. First, PE helps create a mutually reinforcing virtuous cycle, as it contributes to the prevention or reversal of bone loss with age,38 which helps sustain PE for the long-term, promoting physical and mental health. Second, PE may help alleviate the metabolic problems and inflammation associated with obesity and T2DM by improving the secretion of adipokines, which may reduce the occurrence of risk factors for COI, such as dyslipidemia, and thus prevent the development of COI.7,39 Third, PE promotes blood circulation, which is key in ensuring sufficient oxygen is provided to brain cells, which in turn improves the coordination of neural activity in the cerebral cortex and eases tension in the brain, thus delaying negative cerebral structural changes.30 Moreover, PE can effectively stimulate individual executive and cognitive functions, which can delay cognitive decline.40
In contrast to previous studies, our study found a chain mediating effect of sleep quality and depression between PE and COI among older adults with T2DM. First, sleep quality individually mediated the effect of PE on COI. Regular PE can improve sleep quality in older adults.21 By regulating the endocrine system, PE may alleviate the adverse effects of typical symptoms of T2DM, such as nocturia, on sleep quality in older adults.41 Additionally, PE can improve sleep quality by regulating melatonin levels and circadian patterns.42 Poor sleep quality may contribute to the development of neurodegenerative diseases and increased amyloid deposition,43 which is an important biomarker of COI.44 Therefore, PE may delay the development of COI by improving sleep quality.
Second, depression independently mediated the effect of PE on COI. Depression often manifests as depressed mood and sadness, which can lead to a lack interest in life and fewer opportunities to communicate with the outside world and subsequently insufficiently stimulated thinking and memory among older adults with T2DM.40 Some patients who experience depression may develop poor lifestyle habits as a result of self-doubt and boredom, such as smoking, excessive alcohol intake, and consumption of high-fat foods.45 These unhealthy habits are important risk factors for COI and may cause further decline in cognitive function.46 Furthermore, depression puts older adults with T2DM at two to three times higher risk of developing COI than the general population of older adults.47 The possible reason for this is that both T2DM and depression are associated with changes in the brain’s white matter,48 which considerably increases the risk of developing COI. PE may be able to counteract this process by promoting the increase of brain-derived neurotrophic factor, which in turn promotes neuroplasticity, neuronal growth, and differentiation to potentially alleviate depression.49 Not only can PE facilitate the dissipation of adverse emotions, but it can also improve problems related to an unhealthy lifestyle50 and control blood glucose levels to a certain extent,51 thus reducing T2DM-associated symptoms in older adults, which in turn alleviates depression, anxiety, and other adverse states. Thus, PE may delay the development of COI by alleviating depression.
Finally, PE may improve sleep quality, and effective sleep may ease depression and thus delay the development of COI. Sleep quality has been shown to be a significant predictor of depression in older adults,52,53 which supports our study’s findings. Poor sleep quality can contribute to depression and lower motivation to obtain pleasure in older adults with T2DM, which can negatively impact psychological status.54 In addition, it has been found that older adults with T2DM who have poor sleep quality have less psychological resilience to relieve the symptoms of their disease and improve their condition.55 Long-term self-management and accumulated psychological stress can increase physical and mental exhaustion, which exacerbates T2DM-associated pain and makes older adults more prone to depression.56 Depression may also be associated with neuroinflammation in the brain;57 a high level of sleep quality has been shown to contribute to reducing this inflammation.58 Therefore, good sleep quality may alleviate the physical damage caused by T2DM as well as effectively moderate depression and other adverse psychological states. In summary, sleep quality and depression play both a separate mediating role between PE and COI in older adults with T2DM and a chain mediating role.
Implications
In the current context of aging and the prevalence of chronic diseases, new challenges are posed to the healthy aging of older adults with chronic diseases. Previously, people often attributed symptoms such as depressed mood and memory loss in older adults with T2DM to the T2DM itself and ignored the importance of mental health. More importantly, the synergistic effects of T2DM and mental health may accelerate the deterioration caused by the disease59 compared to those without comorbid mental health concerns. Consequently, the mental health of older adults with T2DM should be a major focus of health concerns in the future. To contribute to this body of knowledge, we focused on the mental status of a specific group of older adults with T2DM and explored the mechanism of PE on COI based on the “exercise–physiology–psychology and psychiatry” connection. As an important intervention for patients with chronic diseases, PE has an irreplaceable role in promoting physical and mental health.
Accordingly, we suggest that the community and families should work together to develop appropriate exercise programs, such as tai chi, baduanjin, qigong, and yoga, to improve sleep quality and cognitive function among older adults with T2DM. A comprehensive exercise program not only offers a variety of pleasurable activities to alleviate depression, it can also improve motor and cognitive performance by providing multiple stimulation of thought, attention, and memory,60 which can be more beneficial than a single cognitive or exercise program. We also found that high levels of sleep quality were associated with lower depressive symptoms and lower risk of developing COI. This suggests that creating a favorable sleep environment—for example, more comfortable beds and reduced light and noise—along with using PE to improve sleep are important for older adults with T2DM.
Limitations
This study does have a few limitations. First, the cross-sectional data limit our ability to infer causal relationships between PE, sleep quality, depression, and COI. Future studies will need to use a longitudinal design to provide more robust data. Second, the single-item measures of the independent variables may not adequately reflect the specifics of the participants. This includes, but is not limited to, the effects of PE intensity and frequency according to individual and gender differences, as moderate PE, as opposed to none or excessive PE, has been found to promote physical and mental health.61 The dose–response relationship between PE and COI should also be further investigated to provide a reference for the development of group-specific PE programs. Third, we did not compare older adults with T2DM to the general population of older adults, and the participants in our study were older adults with T2DM who were not explicitly diagnosed with depression or COI. Future studies should include samples of older adults diagnosed with psychiatric disorders such as depression and COI so findings could be generalized to these groups. Finally and most interestingly, previous studies have shown that depression and sleep quality appear to have a two-way effect.62 However, we did not find an outstanding effect value (the chain effect value accounted for less than 1%) when examining the “PE → depression → sleep quality → COI” pathway. Thus, we need to test our results with a broader and more representative sample and to verify the applicability of the findings to other countries.
Conclusions
In summary, our study results suggest that PE in older adults with T2DM delays the development of COI. In addition, we found that sleep quality and depression have a chain mediating effect between PE and COI, that is, PE seems to improve patients’ sleep quality, thus alleviating depression and other adverse moods and ultimately reducing the risk of COI. Above all, we found that developing a PE program and creating a comfortable sleep environment may enhance patients’ ability to resist depression and COI. These findings have positive implications for healthy aging in older adults with T2DM.
Abbreviations
T2DM, type 2 diabetes mellitus; COI, cognitive impairment; PE, physical exercise; PHQ, Patient Health Questionnaire; MMSE, Mini-Mental State Examination.
Data Sharing Statement
The data used to support the conclusions of this study can be obtained from the corresponding authors upon reasonable request.
Compliance with Ethical Standards
This study was conducted in accordance with the Declaration of Helsinki. This study involving human biomedical research was approved by the Ethics Committee of Weifang Medical University (approval number: 2020YX018).
Informed Consent
Under the organization and coordination of the Weifang Municipal Health Commission, we obtained permission from each community health service center to conduct the survey. Before the survey, the general practitioners informed respondents of the purpose of this study and obtained their verbal informed consent, which was approved by the Ethics Committee of Weifang Medical University.
Acknowledgments
We thank all the investigators who participated in this project, the coordinators who made the study successful, and the respondents who cooperated with us.
Author Contributions
All authors made a significant contribution to the study’s conception, design, execution, acquisition of data, or analysis or interpretation, or in all these areas. All authors took part in drafting, revising, and critically reviewing the article and gave their final approval of the version submitted for publication. All authors agreed on the journal for submission and to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China under Grant [72004165, 72104186].
Disclosure
All authors declare that they have no conflicts of interest.
References
1. American Diabetes Association. Standards of medical care in diabetes-2020 abridged for primary care providers. Clin Diabetes. 2020;38(1):10–38. doi:10.2337/cd20-as01
2. Yu Y, Xie K, Lou Q, et al. The clinical characteristics of Chinese elderly patients with different durations of type 2 diabetes mellitus. Front Endocrinol. 2022;13:904347. doi:10.3389/fendo.2022.904347
3. Christiaens A, Henrard S, Zerah L, Dalleur O, Bourdel-Marchasson I, Boland B. Individualisation of glycaemic management in older people with type 2 diabetes: a systematic review of clinical practice guidelines recommendations. Age Ageing. 2021;50(6):1935–1942. doi:10.1093/ageing/afab157
4. Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020;8(6):535–545. doi:10.1016/S2213-8587(20)30118-2
5. Dening KH, Lloyd-Williams M. Minimising long-term effect of COVID-19 in dementia care. Lancet. 2020;396(10256):957–958. doi:10.1016/S0140-6736(20)32024-9
6. Valenza S, Paciaroni L, Paolini S, et al. Mild cognitive impairment subtypes and type 2 diabetes in elderly subjects. J Clin Med. 2020;9(7):2055. doi:10.3390/jcm9072055
7. Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 2015;14(3):329–340. doi:10.1016/S1474-4422(14)70249-2
8. Santos T, Lovell J, Shiell K, Johnson M, Ibrahim JE. The impact of cognitive impairment in dementia on self-care domains in diabetes: a systematic search and narrative review. Diabetes Metab Res Rev. 2018;34(6):e3013. doi:10.1002/dmrr.3013
9. Yun I, Joo HJ, Park YS, Park EC. Association between physical exercise and glycated hemoglobin levels in Korean patients diagnosed with diabetes. Int J Environ Res Public Health. 2022;19(6):3280. doi:10.3390/ijerph19063280
10. Wang J, Niu Y, Tao H, Xue M, Wan C. Knockdown of lncRNA TUG1 inhibits hippocampal neuronal apoptosis and participates in aerobic exercise-alleviated vascular cognitive impairment. Biol Res. 2020;53(1):53. doi:10.1186/s40659-020-00320-4
11. Begde A, Jain M, Hogervorst E, Wilcockson T. Does physical exercise improve the capacity for independent living in people with dementia or mild cognitive impairment: an overview of systematic reviews and meta-analyses. Aging Ment Health. 2022;26(12):2317–2327. doi:10.1080/13607863.2021.2019192
12. Duzel E, van Praag H, Sendtner M. Can physical exercise in old age improve memory and hippocampal function? Brain. 2016;139(Pt3):662–673. doi:10.1093/brain/awv407
13. Song D, Yu DSF. Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: a randomised controlled trial. Int J Nurs Stud. 2019;93:97–105. doi:10.1016/j.ijnurstu.2019.02.019
14. Koevoets EW, Schagen SB, de Ruiter MB, et al. Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: a randomized controlled trial (PAM study). Breast Cancer Res. 2022;24(1):36. doi:10.1186/s13058-022-01530-2
15. Inkelis SM, Ancoli-Israel S, Thomas JD, Bhattacharjee R. Elevated risk of depression among adolescents presenting with sleep disorders. J Clin Sleep Med. 2021;17(4):675–683. doi:10.5664/jcsm.8996
16. Wu C-R, Chen P-Y, Hsieh S-H, et al. Sleep mediates the relationship between depression and cognitive impairment in older men. Am J Mens Health. 2019;13(1):1557988319825765. doi:10.1177/1557988319825765
17. Liu G, Li Y, Xu Y, Type LW. 2 diabetes is associated with increased risk of dementia, but not mild cognitive impairment: a cross-sectional study among the elderly in Chinese communities. Front Aging Neurosci. 2022;14:1004954. doi:10.3389/fnagi.2022.1004954
18. Lin CF, Liu HC, Lin SY. Kidney function and risk of physical and cognitive impairment in older persons with type 2 diabetes at an outpatient clinic with geriatric assessment implementation. Diabetes Metab Syndr Obes. 2022;15:79–91. doi:10.2147/DMSO.S341935
19. Chen L. Self-reported hearing difficulty increases 3-year risk of incident cognitive impairment: the role of leisure activities and psychological resilience. Int J Geriatr Psychiatry. 2021;36(8):1197–1203. doi:10.1002/gps.5511
20. Zhen L, Wang G, Xu G, et al. Evaluation of the paper and smartphone versions of the Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR16) and the Patient Health Questionnaire-9 (PHQ-9) in depressed patients in China. Neuropsychiatr Dis Treat. 2020;16:993–1001. doi:10.2147/NDT.S241766
21. Teusen C, Hapfelmeier A, von Schrottenberg V, et al. Combining the GP’s assessment and the PHQ-9 questionnaire leads to more reliable and clinically relevant diagnoses in primary care. PLoS One. 2022;17(10):e0276534. doi:10.1371/journal.pone.0276534
22. Wang YY, Zhang M, Wang XX, Liu S, Ding H. Correlates of cognitive impairment in the elderly in China: a cross-sectional study. Front Public Health. 2022;10:973661. doi:10.3389/fpubh.2022.973661
23. Xu W, Hu X, Zhang X, Ling C, Wang C, Gao L. Cognitive impairment and related factors among middle-aged and elderly patients with type 2 diabetes from a bio-psycho-social perspective. Diabetes Metab Syndr Obes. 2021;14:4361–4369. doi:10.2147/DMSO.S333373
24. Suain Bon R, Ariaratnam S, Mat Saher Z, Mohamad M, Lee FS. Cognitive impairment and its associated risk factors in the elderly with type 2 diabetes mellitus. Front Psychiatry. 2021;12:669725. doi:10.3389/fpsyt.2021.669725
25. Rawlings AM, Sharrett AR, Albert MS, et al. The association of late-life diabetes status and hyperglycemia with incident mild cognitive impairment and dementia: the ARIC study. Diabetes Care. 2019;42(7):1248–1254. doi:10.2337/dc19-0120
26. Feter N, Dumith SC, Smith EC, et al. Physical activity attenuates the risk for dementia associated with aging in older adults with mild cognitive impairment. findings from a population-based cohort study. J Psychiatr Res. 2021;141:1–8. doi:10.1016/j.jpsychires.2021.06.034
27. Biazus-Sehn LF, Schuch FB, Firth J, Stigger FS. Effects of physical exercise on cognitive function of older adults with mild cognitive impairment: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2020;89:104048. doi:10.1016/j.archger.2020.104048
28. Rondão CAM, Mota MPG, Esteves D. Development of a combined exercise and cognitive stimulation intervention for people with mild cognitive impairment-designing the MEMO_MOVE PROGRAM. Int J Environ Res Public Health. 2022;19:16. doi:10.3390/ijerph191610221
29. Küçükkubaş N, Korkusuz F. What happens to bone mineral density, strength and body composition of ex-elite female volleyball players: a cross sectional study. Sci Sports. 2019;34(4):e259–e269. doi:10.1016/j.scispo.2018.11.006
30. Liu X, Jiang Y, Peng W, et al. Association between physical activity and mild cognitive impairment in community-dwelling older adults: depression as a mediator. Front Aging Neurosci. 2022;14:964886. doi:10.3389/fnagi.2022.964886
31. Scully T, Ettela A, LeRoith D, Gallagher EJ. Obesity, type 2 diabetes, and cancer risk. Front Oncol. 2020;10:615375. doi:10.3389/fonc.2020.615375
32. Kim D-S, Scherer PE. Obesity, diabetes, and increased cancer progression. Diabetes Metab J. 2021;45(6):799–812. doi:10.4093/dmj.2021.0077
33. Taylor EB. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin Sci. 2021;135(6):731–752. doi:10.1042/CS20200895
34. Henn RE, Elzinga SE, Glass E, et al. Obesity-induced neuroinflammation and cognitive impairment in young adult versus middle-aged mice. Immun Ageing. 2022;19(1):67. doi:10.1186/s12979-022-00323-7
35. Sahpolat M, Ayar D, Ari M, Karaman MA. Elevated monocyte to high-density lipoprotein ratios as an inflammation markers for schizophrenia patients. Clin Psychopharmacol Neurosci. 2021;19(1):112–116. doi:10.9758/cpn.2021.19.1.112
36. Sahpolat M, Ari M. Higher prevalence of metabolic syndrome and related factors in patients with first-episode psychosis and schizophrenia: a cross-sectional study in Turkey. Nord J Psychiatry. 2021;75(1):73–78. doi:10.1080/08039488.2020.1815080
37. Teng Z, Feng J, Dong Y, et al. Triglyceride glucose index is associated with cerebral small vessel disease burden and cognitive impairment in elderly patients with type 2 diabetes mellitus. Front Endocrinol. 2022;13:970122. doi:10.3389/fendo.2022.970122
38. Yaprak Y, Küçükkubaş N. Gender-related differences on physical fitness parameters after core training exercises: a comparative study. Prog Nutr. 2020;22(3):e2020028. doi:10.23751/pn.v22i3.9334
39. Geng L, Liao B, Jin L, et al. Exercise alleviates obesity-induced metabolic dysfunction via enhancing FGF21 sensitivity in adipose tissues. Cell Rep. 2019;26(10):2738–2752.e4. doi:10.1016/j.celrep.2019.02.014
40. Huang X, Zhao X, Li B, et al. Comparative efficacy of various exercise interventions on cognitive function in patients with mild cognitive impairment or dementia: a systematic review and network meta-analysis. J Sport Health Sci. 2022;11(2):212–223. doi:10.1016/j.jshs.2021.05.003
41. Fu Z, Wang F, Dang X, Zhou T. The association between diabetes and nocturia: a systematic review and meta-analysis. Front Public Health. 2022;10:924488. doi:10.3389/fpubh.2022.924488
42. Zhou Y, Wu W, Zou Y, et al. Benefits of different combinations of aerobic and resistance exercise for improving plasma glucose and lipid metabolism and sleep quality among elderly patients with metabolic syndrome: a randomized controlled trial. Endocr J. 2022;69(7):819–830. doi:10.1507/endocrj.EJ21-0589
43. Malhotra RK. Neurodegenerative disorders and sleep. Sleep Med Clin. 2018;13(1):63–70. doi:10.1016/j.jsmc.2017.09.006
44. Gabelle A, Gutierrez LA, Jaussent I, et al. Absence of relationship between self-reported sleep measures and amyloid load in elderly subjects. Front Neurol. 2019;10:989. doi:10.3389/fneur.2019.00989
45. Al-Zaru IM, Shahrour G, Mashaal D, Hayajneh AA. Depression and adherence to healthy lifestyle behaviors among patients with coronary artery diseases in Jordan. Heliyon. 2022;8(7):e09752. doi:10.1016/j.heliyon.2022.e09752
46. Teh WL, Abdin E, Vaingankar JA, et al. Prevalence, lifestyle correlates, and psychosocial functioning among multi-ethnic older adults with mild cognitive impairment in Singapore: preliminary findings from a 10/66 population study. Yale J Biol Med. 2021;94(1):73–83. doi:10.1186/alzrt143
47. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi:10.1016/S0140-6736(20)30367-6
48. Chow YY, Verdonschot M, McEvoy CT, Peeters G. Associations between depression and cognition, mild cognitive impairment and dementia in persons with diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2022;185:109227. doi:10.1016/j.diabres.2022.109227
49. Szuhany KL, Otto MW. Assessing BDNF as a mediator of the effects of exercise on depression. J Psychiatr Res. 2020;123:114–118. doi:10.1016/j.jpsychires.2020.02.003
50. Gwinnutt JM, Wieczorek M, Cavalli G, et al. Effects of physical exercise and body weight on disease-specific outcomes of people with rheumatic and musculoskeletal diseases (RMDs): systematic reviews and meta-analyses informing the 2021 EULAR recommendations for lifestyle improvements in people with RMDs. RMD Open. 2022;8(1):e002168. doi:10.1136/rmdopen-2021-002168
51. Ma Y, Liu H, Wang Y, et al. Roles of physical exercise-induced MiR-126 in cardiovascular health of type 2 diabetes. Diabetol Metab Syndr. 2022;14(1):169. doi:10.1186/s13098-022-00942-6
52. Yu J, Rawtaer I, Fam J, et al. Sleep correlates of depression and anxiety in an elderly Asian population. Psychogeriatrics. 2016;16(3):191–195. doi:10.1111/psyg.12138
53. Guan Q, Hu X, Ma N, et al. Sleep quality, depression, and cognitive function in non-demented older adults. J Alzheimers Dis. 2020;76(4):1637–1650. doi:10.3233/JAD-190990
54. Tosun A, Tosun H, Özkaya BO, Erdoğan Z, Gül A. Sleep quality and depression level in nurses in COVID-19 pandemic. Omega. 2022;302228221123159. doi:10.1177/00302228221123159
55. Bonnet MH. Acute sleep deprivation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine.
56. Gunn S, Henson J, Robertson N, et al. Self-compassion, sleep quality and psychological well-being in type 2 diabetes: a cross-sectional study. BMJ Open Diabetes Res Care. 2022;10(5):e002927. doi:10.1136/bmjdrc-2022-002927
57. Xiao H, Zhang Y, Kong D, Li S, Yang N. Social capital and sleep quality in individuals who self-isolated for 14 days during the coronavirus disease 2019 (COVID-19) outbreak in January 2020 in China. Med Sci Monit. 2020;26:e923921. doi:10.12659/MSM.923921
58. Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. doi:10.1152/physrev.00010.2018
59. Thornicroft G, Ahuja S, Barber S, et al. Integrated care for people with long-term mental and physical health conditions in low-income and middle-income countries. Lancet Psychiatry. 2019;6(2):174–186. doi:10.1016/S2215-0366(18)30298-0
60. Venegas-Sanabria LC, Cavero-Redondo I, Martínez-Vizcaino V, Cano-Gutierrez CA, Álvarez-Bueno C. Effect of multicomponent exercise in cognitive impairment: a systematic review and meta-analysis. BMC Geriatr. 2022;22(1):617. doi:10.1186/s12877-022-03302-1
61. Küçükkubaş N, Günay A, Löklüoğlu B, Kakil B. Relationship between body composition, vertical jump, 30 m sprint, static strength and anaerobic power for athletes. Int J Sport Exerc Train Sci. 2019;5(2):68–78. doi:10.18826/useeabd.517037
62. Hu Z, Zhu X, Kaminga AC, Zhu T, Nie Y, Xu H. Association between poor sleep quality and depression symptoms among the elderly in nursing homes in Hunan province, China: a cross-sectional study. BMJ Open. 2020;10(7):e036401. doi:10.1136/bmjopen-2019-036401
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
