Back to Journals » Nature and Science of Sleep » Volume 13
Relationship Between Obstructive Sleep Apnea and Late Gadolinium Enhancement and Their Effect on Cardiac Arrhythmias in Patients with Hypertrophic Obstructive Cardiomyopathy
Authors Wang S, Cui H, Ji K, Zhu C, Huang X, Lai Y, Wang S
Received 9 July 2020
Accepted for publication 14 September 2020
Published 25 March 2021 Volume 2021:13 Pages 447—456
DOI https://doi.org/10.2147/NSS.S270684
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Steven A Shea
Shengwei Wang,1 Hao Cui,2 Keshan Ji,3 Changsheng Zhu,4 Xiaohong Huang,3 Yongqiang Lai,1 Shuiyun Wang4
1Cardiovascular Surgery Center, Beijing Anzhen Hospital, Capital Medical University, Beijing Institute of Heart, Lung and Blood Vascular Diseases, Beijing, People’s Republic of China; 2Department of Cardiovascular Surgery, Mayo Clinic, Rochester, MN, USA; 3Special Medical Treatment Center, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 4Department of Cardiovascular Surgery, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Yongqiang Lai
Cardiovascular Surgery Center, Beijing Anzhen Hospital, Capital Medical University, Beijing Institute of Heart, Lung and Blood Vascular Diseases, No. 2, Anzhen Road, Chaoyang District, Beijing, 100029, People’s Republic of China
Email [email protected]
Shuiyun Wang
Department of Cardiovascular Surgery, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 167, Beilishi Road, Xicheng District, Beijing, 100037, People’s Republic of China
Email [email protected]
Purpose: Obstructive sleep apnea (OSA) and myocardial fibrosis are associated with cardiac arrhythmia. The purpose of this study was to explore the relationship between OSA and myocardial fibrosis, as well as their impact on cardiac arrhythmia in hypertrophic obstructive cardiomyopathy (HOCM) patients.
Methods: We prospectively studied 151 consecutive patients with a confirmed diagnosis of HOCM at the Fuwai Hospital between September 2017 and 2018. Polysomnography, Holter electrocardiography, and cardiac magnetic resonance imaging were performed on all patients. Myocardial fibrosis was reflected by late gadolinium enhancement (LGE), detected using cardiac magnetic resonance imaging.
Results: Myocardial fibrosis, measured using LGE%, was found to increase with increasing OSA severity [6.8% (3.6– 12.9%), 6.1% (3.4– 10.0%), 9.6% (5.5– 14.5%), and 15.5% (9.3– 20.0%) for no-OSA, mild OSA, moderate OSA, and severe OSA, respectively; p=0.003]. LGE% correlated with the New York Heart Association functional classifications (p=0.018), septal thickness (p=0.026), and apnea-hypopnea index (AHI) (p< 0.001). The prevalence of isolated premature ventricular contraction (PVC) (p=0.028), paired PVC (p=0.036), ventricular bigeminy (p=0.005)/trigeminy (p< 0.001), non-sustained ventricular tachycardia (NSVT) (p=0.001), isolated premature atrial contraction (PAC) (p=0.032), and supraventricular tachycardia (p=0.029) was significantly higher in patients with OSA. Additionally, LGE% and AHI were independent risk factors for isolated PVC (OR: 1.04, p=0.001 and OR: 1.07, p=0.039, respectively), ventricular bigeminy (OR: 1.04, p=0.003 and OR: 1.26, p=0.002, respectively)/trigeminy (OR: 1.07, p=0.040 and OR: 1.06, p=0.001, respectively), and NSVT (OR: 1.17, p< 0.001 and OR: 1.08, p< 0.001, respectively) after adjustment for age, sex, and other parameters.
Conclusion: Both OSA and LGE% were associated with a greater likelihood and increased frequency of ventricular arrhythmias (including NSVT) in patients with HOCM. Thus, the severity of OSA was independently associated with more severe myocardial fibrosis in patients with HOCM.
Keywords: hypertrophic obstructive cardiomyopathy, obstructive sleep apnea, cardiac arrhythmia
Introduction
Hypertrophic cardiomyopathy (HCM) is a common genetic heart disease occurring in 1 in 500 individuals within the general population and is one of the most common causes of sudden cardiac death in young adults.1,2 Obstructive sleep apnea (OSA) is the most common sleep disorder; with a prevalence ranging between 32% and 71%, OSA is even more common in HCM patients,3 where it is independently associated with atrial fibrillation and non-sustained ventricular tachycardia (NSVT).4,5 Late gadolinium enhancement (LGE), detected using cardiac magnetic resonance imaging, reflects myocardial fibrosis.6 Myocardial fibrosis creates a potentially arrhythmogenic substrate, increasing susceptibility to cardiac arrhythmia, especially ventricular arrhythmia.7 A recent study reported that sleep disordered breathing was associated with a more than two-fold increase in the risk of myocardial fibrosis in patients with subclinical myocardial infarction;8 however, whether there is a link between OSA and LGE, as well as their relationship with arrhythmia in patients with hypertrophic obstructive cardiomyopathy (HOCM), remains unclear. Therefore, this study aimed to explore the relationship between OSA and LGE, and their impact on cardiac arrhythmia in patients with HOCM.
Patients and Methods
Study Population
This prospective observational study included 151 consecutive patients with HOCM who were referred to Fuwai Hospital (Beijing, China) between September 2017 and 2018. Inclusion criteria were (1) age >18 years; (2) agreement to undergo cardiac magnetic resonance imaging, polysomnography (PSG), and Holter electrocardiography; and (3) agreement to participate in the study. According to the AHA and ACC guidelines,1,2 the diagnostic criteria for HOCM included an unexplained septal hypertrophy with a thickness >15 mm, as well as a left ventricular outflow tract gradient >50 mm Hg at rest or with provocation. A fasting blood sample was obtained from all patients on the second day of hospitalization; glucose, total cholesterol, and brain natriuretic peptide levels were concurrently monitored.
The study was approved by the Ethics Committee of Fuwai Hospital. All patients provided informed consent before enrollment, and all patient testing was conducted in accordance with the ethical principles stated in the Declaration of Helsinki.
Polysomnography
Standard polysomnography (Embletta, Embla, UK) was performed on all patients; no patient received continuous positive airway pressure therapy. The investigation involved monitoring of the electroencephalogram, electrooculogram, submental electromyogram, electrocardiographic thoracoabdominal excursions, oronasal airflow using an airflow pressure transducer, and arterial oxygen saturation using pulse oximetry; respiratory events and data were scored by an experienced scorer. Hypopnea was defined as a 50% reduction in oronasal airflow for 10 seconds, associated with a 4% decrease in oxygen saturation. Apnea was defined as cessation of oronasal airflow for ≥10 seconds (whether central, obstructive, or mixed in nature). Obstructive apnea was defined as apnea in the presence of out-of-phase thoracoabdominal effort; >80% of apnea events were obstructive. Therefore, OSA was specifically characterized by the presence of thoracic efforts. The total apnea-hypopnea index (AHI) representing the number of respiratory events per hour of sleep was calculated using the total recording time as the denominator; additionally, oxygen desaturation index, lowest SpO2, time ratio of SpO2, and total sleep time were also recorded during sleep. Mild, moderate, and severe OSA were respectively defined as an AHI >5 events/h, ≥15 events/h, and ≥30 events/h.9
Holter Electrocardiogram Monitoring
A minimum of one 24-hour Holter electrocardiogram monitoring (BI9800, Biomedical Instruments Co., Ltd., Osaka, Japan) session was performed on all patients. Arrhythmia categories included supraventricular arrhythmia, ventricular arrhythmia, and conduction delay arrhythmia. Supraventricular arrhythmias included (1) premature atrial contraction (PAC) (defined as ≥5 PAC events per hour in the absence of atrial fibrillation); (2) bigeminy/trigeminy; (3) atrial fibrillation or flutter (occurring intermittently or continuously); (4) supraventricular tachycardia (SVT); and (5) a composite variable of combined supraventricular tachycardia. Ventricular arrhythmias included (1) isolated premature ventricular contractions (PVC) (defined as ≥5 events per hour); (2) bigeminy/trigeminy; (3) non-sustained ventricular tachycardia (NSVT: defined as an episode of three consecutive ventricular beats at a rate of at least 100 beats/min (bpm), and a maximum episode length of 30 seconds); and (4) a composite variable of complex ventricular ectopy (bigeminy, trigeminy, quadrigeminy, or NSVT, excluding isolated PVC).10–12 Data were scored by an experienced electrophysiologist.
Echocardiography and Cardiac Magnetic Resonance Imaging
Echocardiographic examinations were performed on patients by the same experienced physician using a GE LOGIQ E9 ultrasound system. Diameters of the cardiac chambers were expressed as the maximum value of the anteroposterior diameter detected during the cardiac cycle, whereas the thicknesses of the interventricular septum and ventricular wall were determined during diastole. Aside from the maximum thickness, a representative thickness of the interventricular septum was also recorded; the left ventricular outflow tract (LVOT) gradient was calculated using a simplified Bernoulli equation. Pulmonary hypertension was defined as a pulmonary artery systolic pressure ≥35 mm Hg, while measurements of left ventricular ejection fraction (LVEF) were determined following recommendations from the American Society of Echocardiography.13
Cardiac magnetic resonance imaging was performed using a 1.5 Tesla cardiac magnetic scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany), while cine scans in cardiac short- and long-axis views were acquired by applying true fast imaging with steady-stage precession sequence (TrueFISP). Image analysis was completed using a commercial imaging workstation (Siemens Medical Systems). LVEF, as well as indexed left ventricular mass and volume, were measured by analyzing the short-axis cine image. Inner and outer myocardial edges were manually delineated, while LGE was determined semi-automatically as a percentage of total myocardium, defined as having an intensity >6 standard deviations above the normal myocardium as defined in a previous study.14 In this study, LGE% was defined as the percentage of LV mass containing LGE.
Statistical Analysis
The results were expressed as mean ± standard deviation, median (interquartile range), or percentage. The χ2 or Fisher’s exact tests were used to compare nominal variables, whereas differences among the four groups were compared using one-way ANOVA or the Kruskal–Wallis H-test, as appropriate. Prior to linear regression analysis, Spearman’s rank correlation analysis was performed to select correlated parameters associated with LGE; significant parameters further entered a stepwise multivariate linear regression model. Univariable and stepwise multivariate logistic regression analyses were used to determine factors associated with various arrhythmias. Variables with p<0.10 on univariate analysis were entered into a multivariate analysis; all reported probability values were two-tailed, and a p-value <0.05 was considered statistically significant. SPSS version 24.0 software (IBM) and R 3.5.0 (R foundation for Statistical Computing, Vienna, Austria) were used for calculations and illustrations, respectively.
Results
Baseline Patient Characteristics
Baseline characteristics of the entire patient population and of subgroups according to the severity of OSA are described in Table 1. Increasing age, levels of fasting blood glucose, and norepinephrine were associated with increasing OSA severity. The prevalence of a family history of HCM or sudden cardiac death was lower, whereas syncope was higher in patients with more severe OSA. Additionally, as described in Table 1, the polysomnography parameters had significantly changed with the severity of OSA; however, the total sleep time was similar among the four groups. Imaging data of the study population are shown in Table 2. The left ventricular end-diastolic diameter increased with the severity of OSA; left atrial diameter and LVOT obstruction at rest also tended to be higher in patients with OSA. Moreover, cardiac magnetic resonance parameters, including LGE%, all increased with increasing severity of OSA; LGE% was significantly higher in patients with than in those without OSA (7.5±6.2 vs 10.1±7.3, p=0.042).
 |
Table 1 Baseline Characteristics of the Study Population |
 |
Table 2 Imaging Parameters of the Study Population |
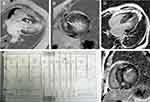
Representative LGE images from patients with a higher LGE% and higher prevalence of ventricular arrhythmia are shown in Figure 1. The association between LGE% and demographic, clinical, and echocardiographic parameters is presented in Table 3. The multivariate linear regression model (adjusted R2=0.264) indicated that LGE% positively correlated with interventricular septal thickness (β=0.163, p=0.026), New York Heart Association class (β=0.174, p=0.018), and AHI (β=0.286, p<0.001) after adjusting for age, sex, body mass index, and other relevant variables.
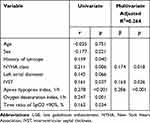 |
Table 3 Clinical Variables Associated with LGE% from Univariate and Multivariate Analysis |
Holter Electrocardiographic Data
The prevalence of cardiac arrhythmia according to the severity of OSA is presented in Table 4; almost all ventricular arrhythmias were associated with the severity of OSA, including isolated PVC, paired PVC, ventricular bigeminy/trigeminy, and NSVT. By contrast, only a few atrial arrhythmias were associated, including isolated PAC, SVT, atrial fibrillation, and a trend for atrial bigeminy. Characteristics (including numbers, frequency, durations, and rate fastest run) of various arrhythmias among different groups according to the severity of OSA are shown in Supplemental Table 1. Additionally, LGE% was significantly higher in most patients with ventricular arrhythmias (Figure 2).
 |
Table 4 Relationship Between Arrhythmias and OSA |
Clinical Data Associated with Ventricular and Atrial Arrhythmia
As shown in Table 5, after adjusting for age, sex, body mass index, and other relevant covariates (variables with p<0.10 on univariate analysis), the association between AHI, LGE%, and cardiac arrhythmia persisted for some, but not all types of arrhythmias. For example, AHI and LGE% were independent risk factors for isolated PVC, ventricular bigeminy/trigeminy, and NSVT; however, only AHI was associated with isolated PAC and atrial fibrillation, instead of SVT (Table 5).
 |
Table 5 Adjusted Logistic Regression Among Arrhythmias and Significant Variables from Univariate Analysis |
Discussion
This study evaluated the association between OSA, LGE, and arrhythmias in a large series of consecutive patients with HOCM, making several new findings. First, LGE% was significantly higher in patients with OSA; the severity of OSA was independently associated with an increased LGE%. Second, almost all ventricular arrhythmias increased with increasing severity of OSA, including isolated PVC, paired PVC, bigeminy/trigeminy, and NSVT; conversely, only isolated PAC and SVT were associated with the severity of OSA. Third, after adjusting for age, sex, and other relevant covariates, the association between AHI, LGE%, and cardiac arrhythmia persisted for some, but not all types of arrhythmias, primarily ventricular arrhythmia.
There is a paucity of studies on the relationship between OSA and LGE and their impact on cardiac arrhythmias in patients with HOCM. A previous study reported that sleep disordered breathing (SDB) was independently associated with nocturnal cardiac arrhythmias, and that an increasing severity of SDB was associated with an increasing risk of any cardiac arrhythmia.10 In addition, SDB was also associated with atrial fibrillation, NSVT, and ventricular tachycardia in patients with HCM.4,15,16 In our previous study, we found that the severity of OSA was an independent risk factor for NSVT in patients with HOCM;5 however, the relationship between OSA and LGE and their impact on other cardiac arrhythmias were not clarified in that study. As described in previous studies, LGE detected using CMR in patients with HCM represents intramyocardial fibrosis,17 is strongly associated with arrhythmia, and is significantly associated with subsequent SCD and/or ICD discharge after controlling for other variables in patients with HCM.18 A recent study indicated that LGE also has a high diagnostic value for ventricular arrhythmias,19 while another study reported that SDB (including mild) was independently associated with a two-fold increase in LGE in patients with subclinical myocardial infarction.8 Nevertheless, the relationship between OSA and LGE and their effect on cardiac arrhythmias in HOCM patients remains unclear.
In this study, we found that the extent of LGE% was significantly associated with the presence and severity of OSA in patients with HOCM. LGE on CMR primarily represents myocardial fibrosis, which provides a substrate for ventricular arrhythmias, and is associated with an increased risk of NSVT in patients with HCM.20 LGE, presumably representing the consequences of longstanding microvascular ischemia, results in myocyte death and ultimately replacement fibrosis as a repair process.21 Intermittent hypoxemia, with associated hypercapnia caused by OSA via the chemoreflexes, results in increased sympathetic activation even during daytime normoxic wakefulness. Furthermore, increased sympathetic activation results in myocardial oxygen demand when oxygen saturation is at its lowest, a situation that may lead to myocardial ischemia.22 Moreover, OSA is characterized by repetitive forced inspiration against a closed upper airway, which generates substantial negative pressures - to levels approaching −65 mmHg - in the chest cavity; this negative intrathoracic pressure might cause the impaired diastolic function, which is also associated with myocardial fibrosis.23 This is consistent with our study’s results, indicating that AHI is an independent risk factor for LGE% after adjustment for age, sex, body mass index, and other relevant variables. In addition, HOCM itself is usually accompanied by subendocardial ischemia; therefore, we speculate that the presence of OSA may aggravate myocardial ischemia in patients with HOCM through the above mechanisms, ultimately leading to myocardial fibrosis.
In our study, we also found that both AHI and LGE% were independent risk factors for most ventricular arrhythmias, including isolated PVC, paired PVC, ventricular bigeminy/trigeminy, and NSVT, in patients with HOCM. By contrast, most atrial arrhythmias were primarily associated with left atrial diameter, instead of AHI and LGE%. Ventricular arrhythmia primarily emanated from regions of structurally abnormal myocardium (including areas of organized architecture and myocardial fibrosis). In patients with HOCM complicated with OSA, chronic hypoxemia and aggravation of myocardial ischemia both increase the severity of myocardial fibrosis and might contribute to the development of ventricular arrhythmia.
Cardiac arrhythmias, especially ventricular tachyarrhythmia, is associated with a poor quality of life and adverse cardiovascular events in patients with HOCM. In this study, we found that the number of ventricular bigeminy, paired PVC, NSVT, trigeminy, and other relevant arrhythmias increased with the severity of OSA, which obviously affected patients’ quality of life. While NSVT is also a risk factor for sudden cardiac death and cardiovascular death in patients with HCM,24 patients with ventricular and atrial arrhythmia in this study had a higher LGE%, which was an independent risk factor for all-cause death, cardiovascular death, and sudden cardiac death in patients with HCM.25,26 Therefore, OSA might promote arrhythmias by exacerbating the myocardial fibrosis reflected by LGE on CMR, impacting the quality of life and clinical outcomes in this special cohort.
Previous studies have reported that continuous positive airway pressure treatment can reduce the frequency of PVC for heart failure and termination of ventricular tachycardia;27,28 however, whether continuous positive airway pressure is also suitable for patients with HOCM, can further improve their quality of life, and can reduce the risk of adverse cardiovascular events remains unclear. Therefore, a prospective, multicenter, long-term study regarding the treatment effects of continuous positive airway pressure on HOCM patients complicated with OSA should be conducted in the future to clarify the impact of OSA on myocardial fibrosis and arrhythmias in these patients.
Limitations
There are several limitations to our study. First, this was a single-center study; thus, the number of patients was relatively small. A larger, multicenter study is needed to confirm our results. Furthermore, a retrospective analysis of HCM patients with treated OSA versus HCM patients with untreated OSA would shed light on important clinical outcomes for this specialized patient population. Ultimately, a collaborative, multicenter registration study regarding the treatment effects of continuous positive airway pressure is warranted. Second, not all patients underwent dynamic electrocardiographic monitoring more than once; longer Holter electrocardiographic monitoring would have been more useful, and likely informative. Finally, due to the cross-sectional nature of this study, we could not conclude that OSA and higher LGE% are independently associated with future cardiovascular complications in patients with HOCM.
Conclusions
In conclusion, both the severity of OSA and LGE% were independently associated with a greater likelihood and an increased frequency of ventricular arrhythmias (including NSVT). Furthermore, the severity of OSA was independently associated with increased LGE%, reflecting increased myocardial fibrosis. Our study adds to the current understanding of the increased risk of cardiac arrhythmia associated with OSA and LGE in patients with HOCM. Therefore, we should pay more attention to the importance of OSA in patients with HOCM and follow the therapeutic measures to avoid a series of complications.
Data Sharing Statement
According to the Fuwai Hospital system, we are not allowed to share original study data publicly. Therefore, the datasets generated and/or analyzed during the current study are not publicly available.
Ethics Approval and Consent to Participate
Approval of the Ethics Committee of Fuwai Hospital was obtained before the start of the work, and each participant signed written consent form.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 81570276) and Beijing Postdoctoral Research Foundation.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. American College of Cardiology Foundation/American Heart Association Task Force on P, American Association for Thoracic S, American Society of E, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2011;142(6):e153–e203.
2. Elliott PM, Anastasakis A, Borger MA, et al; Authors/Task Force m. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733–2779.
3. Nerbass FB, Pedrosa RP, Danzi-Soares NJ, Drager LF, Arteaga-Fernandez E, Lorenzi-Filho G. Obstructive sleep apnea and hypertrophic cardiomyopathy: a common and potential harmful combination. Sleep Med Rev. 2013;17(3):201–206. doi:10.1016/j.smrv.2012.06.006
4. Konecny T, Brady PA, Orban M, et al. Interactions between sleep disordered breathing and atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2010;105(11):1597–1602. doi:10.1016/j.amjcard.2010.01.023
5. Wang S, Cui H, Song C, et al. Obstructive sleep apnea is associated with nonsustained ventricular tachycardia in patients with hypertrophic obstructive cardiomyopathy. Heart Rhythm. 2019;16(5):694–701. doi:10.1016/j.hrthm.2018.12.017
6. Chan RH, Maron BJ, Olivotto I, et al. Significance of late gadolinium enhancement at right ventricular attachment to ventricular septum in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2015;116(3):436–441. doi:10.1016/j.amjcard.2015.04.060
7. Adabag AS, Maron BJ, Appelbaum E, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51(14):1369–1374. doi:10.1016/j.jacc.2007.11.071
8. Shah NA, Reid M, Kizer JR, et al. Sleep-disordered breathing and left ventricular scar on cardiac magnetic resonance: results of the Multi-Ethnic Study of Atherosclerosis(MESA). J Clin Sleep Med. 2020;16(6):855–862. doi:10.5664/jcsm.8340
9. Quan SF, Gillin JC, Littner MR, Shepard JW. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of sleep medicine task force. Sleep. 1999;22(5):667–689.
10. Selim BJ, Koo BB, Qin L, et al. The association between nocturnal cardiac arrhythmias and sleep-disordered breathing: the DREAM Study. J Clin Sleep Med. 2016;12(6):829–837. doi:10.5664/jcsm.5880
11. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67(13):e27–e115.
12. Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol. 2018;72(14):e91–e220.
13. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi:10.1016/j.echo.2005.10.005
14. Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5(4):370–377. doi:10.1016/j.jcmg.2011.11.021
15. Pedrosa RP, Drager LF, Genta PR, et al. Obstructive sleep apnea is common and independently associated with atrial fibrillation in patients with hypertrophic cardiomyopathy. Chest. 2010;137(5):1078–1084. doi:10.1378/chest.09-2335
16. Konecny T, Somers VK. Sleep-disordered breathing in hypertrophic cardiomyopathy: challenges and opportunities. Chest. 2014;146(1):228–234. doi:10.1378/chest.14-0084
17. Soler R, Rodríguez E, Monserrat L, Méndez C, Martínez C. Magnetic resonance imaging of delayed enhancement in hypertrophic cardiomyopathy: relationship with left ventricular perfusion and contractile function. J Comput Assist Tomogr. 2006;30(3):412–420. doi:10.1097/00004728-200605000-00011
18. Rubinshtein R, Glockner JF, Ommen SR, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3(1):51–58. doi:10.1161/CIRCHEARTFAILURE.109.854026
19. Hennig A, Salel M, Sacher F, et al. High-resolution three-dimensional late gadolinium-enhanced cardiac magnetic resonance imaging to identify the underlying substrate of ventricular arrhythmia. Europace. 2018;20(FI2):f179–f191. doi:10.1093/europace/eux278
20. Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart. 2015;101(17):1406–1411. doi:10.1136/heartjnl-2015-307682
21. Maron MS, Maron BJ, Harrigan C, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54(3):220–228. doi:10.1016/j.jacc.2009.05.006
22. Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62(7):610–616. doi:10.1016/j.jacc.2013.04.080
23. Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi:10.1016/j.jacc.2008.05.002
24. Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKenna WJ. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;42(5):873–879. doi:10.1016/S0735-1097(03)00827-1
25. O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56(11):867–874. doi:10.1016/j.jacc.2010.05.010
26. Mentias A, Raeisi-Giglou P, Smedira NG, et al. Late gadolinium enhancement in patients with hypertrophic cardiomyopathy and preserved systolic function. J Am Coll Cardiol. 2018;72(8):857–870. doi:10.1016/j.jacc.2018.05.060
27. Seyis S, Usalan AK, Rencuzogullari I, Kurmus O, Gungen AC. The effects of continuous positive airway pressure on premature ventricular contractions and ventricular wall stress in patients with heart failure and sleep apnea. Can Respir J. 2018;2018:2027061. doi:10.1155/2018/2027061
28. Shimada YJ, Sato K, Hanon S, et al. Termination of recurrent ventricular tachycardia by continuous positive airway pressure ventilation. Ann Noninvasive Electrocardiol. 2009;14(4):404–406. doi:10.1111/j.1542-474X.2009.00331.x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.