Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Relationship between nonalcoholic fatty liver disease and bone mineral density in adolescents with obesity: a meta-analysis
Authors Sun Y, Dai W, Liang Y, Yang P, Yang Q, Liang M, Xia N
Received 25 October 2018
Accepted for publication 24 December 2018
Published 30 January 2019 Volume 2019:12 Pages 199—207
DOI https://doi.org/10.2147/DMSO.S192256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Yue Sun,1 Weiran Dai,2 Yuzhen Liang,3 Pijian Yang,1 Qiong Yang,1 Min Liang,4 Ning Xia1,5
1Geriatric Department of Endocrinology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 2Department of Cardiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 3Department of Endocrinology, The Second Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 4Department of Endocrinology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 5Department of Science and Technology Education, Guangxi Zhuang Autonomous Region Health Committee, Nanning, Guangxi, People’s Republic of China
Purpose: Many studies have reported the relationship between nonalcoholic fatty liver disease (NAFLD) and bone mineral density (BMD) among adults. However, fewer studies on this topic have been reported in adolescents. We thus conducted a meta-analysis to show the association between NAFLD and BMD in adolescents with obesity.
Materials and methods: Computer retrieval was carried out via PubMed, Embase, Cochrane Library and the Cochrane Central Register of Controlled Trials from inception to September 2018. Six published case–control studies that assessed the relationship between NAFLD and BMD were included.
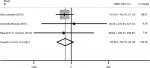
Results: The six studies included 217 obese adolescents with NAFLD and 236 controls. The meta-analysis indicated that obese children with NAFLD had a lower BMD and Z-score than the control group (weighted mean difference [WMD]–0.03, 95% CI [−0.05, –0.02], P=0.000; [WMD] –0.26, 95% CI [−0.37, –0.14], P=0.000). However, we analyzed the factor of bone mineral content, and there was no correlation between the two groups ([WMD]–55.99, 95% CI [−132.16, 20.18], P=0.150).
Conclusion: Obese children with NAFLD are more susceptible to osteoporosis than children with only obesity. Because of the limitations related to the quantity and quality of the included literature, further studies are still needed.
Keywords: nonalcoholic fatty liver disease, NAFLD, bone mineral density, BMD, meta-analysis, obesity, adolescent
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the world. Nearly 25.24% of the overall population has NAFLD.1,2 Moreover, NAFLD is the principal cause of chronic liver disease in children, especially among overweight and obese individuals in industrialized countries.3 On a clinical spectrum, NAFLD ranges from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) and liver cirrhosis and fibrosis.4 The serious outcomes of NAFLD are hepatocellular carcinoma and liver failure.5 Unfortunately, in addition to liver destruction, the effects of NAFLD can also occur in extra hepatic organs and cause type 2 diabetes and cardiovascular disease.6,7
It is well established that the affecting mechanisms of NAFLD are insulin resistance (IR) and chronic inflammation.8 Additionally, a high-calorie diet and a sedentary lifestyle contribute to NAFLD development.9 The incidence rate of NAFLD is coincident with the increase in obesity.10 Therefore, NAFLD has attracted worldwide attention.
Osteoporosis is a group of bone diseases with various causes, including general factors related to aging, obesity and sex steroid deficiency, as well as specific risk factors such as the use of glucocorticoids, reduced bone quality, and disruption of microarchitectural integrity.11 In most cases of osteoporosis, the reduction in bone tissue is mainly due to increased bone resorption. Osteoporosis is characterized by low bone mineral density (BMD), bone pain and easy fracture.12 Osteoporosis is a silent disease until fractures occur with increasing frequency, which can cause important problems and even death.13 In industrialized countries, 9%–38% of women and 1%–8% of men >50 years suffer from osteoporosis.14 Therefore, osteoporosis is not only harmful to health but also increases the financial burden of the impacted countries.15
An epidemiological study from Portugal showed that ~3% of adolescents are obese, and 30% are overweight.16,17 In 2017, the Health Behaviour in School-Aged Children from the WHO showed that, among more than half of the European countries, the incidence of adolescent obesity rapidly increased from 2002 to 2014.18 Many studies have demonstrated that NAFLD is associated with low BMD and osteoporosis,19–21 but most studies have focused on adults, and surveys targeting children and adolescents have been limited.22 Here, we investigated the relationship between NAFLD and BMD in adolescents with obesity through a meta-analysis.
Materials and methods
Research strategy
This meta-analysis was conducted and reported following the PRISMA guidelines.23 All the included studies were filtered through PubMed, Embase, and the Cochrane Database from inception to September 2018. We used the following keywords and terms: (“Non-alcoholic Fatty Liver Disease,” “Non alcoholic Fatty Liver Disease,” “NAFLD,” “Nonalcoholic Fatty Liver Disease,” “Fatty Liver, Nonalcoholic,” “Nonalcoholic Fatty Livers,” “Steatohepatitis, Nonalcoholic”) and (“Bone Density,” “Bone Densities,” “Density, Bone,” “Bone Mineral Density,” “Bone Mineral Content”).
Inclusion criteria
Inclusion factors were: 1) study types including prospective cohort, retrospective cohort, case–control, and cross-sectional studies evaluating the association between NAFLD and BMD; 2) all the participants were adolescents from puberty stage I to V;24 3) NAFLD patients were diagnosed with an ultrasound examination or pathological examination to make a clear and definite diagnosis, and all the participants were obese according to body mass index (BMI); and 4) BMD was measured by dual energy X-ray absorptiometry.
Exclusion criteria
Exclusion factors were 1) other diseases that could cause NAFLD were excluded, such as viral infections, alcohol intake, and the use of drugs and 2) none of the subjects followed specific diets or therapeutic treatments that could influence BMD or liver function.
Data collection
Two investigators abstracted the data from the suitable studies and conformed them to the same criteria, including research topics, the details of the first author, year of publication, study type, number of patients and number in the control group, basic characteristics of participants, and mean values and SDs of BMD.
Quality assessment
The Newcastle Ottawa Scale (NOS) was used to assess the quality of the involved studies.25 Studies with a score of 7–9 points were considered to be of high quality. The score of each study is represented in Table 1.
Statistical analysis
Stata Statistical Software (ver. 12.0; StataCorp LP, College Station, TX, USA) was utilized in the meta-analysis, and P<0.05 was regarded as statistically significant. Continuous variables are presented as weighted mean differences (WMDs). If I2 was more than 50%, heterogeneity was recognized as significant.26 When the heterogeneity was high, a random effects model was used to evaluate the relationship between the two groups. If no obvious heterogeneity existed in the research results, a fixed effects model was used for the meta-analysis.27,28
Results
Literature selection
A total of 116 studies were selected from the databases mentioned at the beginning. After eliminating 32 duplicated studies and screening the titles and abstracts for studies that were not relevant because of the topic or research type, 8 studies were included in the full-text review. Finally, there were six studies included in our meta-analysis. A total of 217 obese adolescents with NAFLD and 236 obese adolescents were included, and the retrieved procedures and excluded details can be found in Figure 1.
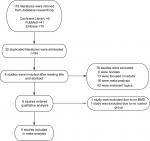  | Figure 1 Flowchart of the studies’ selecting process and results. Abbreviation: BMD, bone mineral density. |
Characteristics of the included studies
The basic characteristics of the six included studies are shown in Table 1. Obese adolescents with NAFLD were included in the case group, whereas obese children without NAFLD were included in the control group. The total number of patients in the case group was 217, whereas the control group included 236 individuals. By scanning the full texts of the six studies, we found that the study types were case–control. Among the included studies, five studies were performed in Europe, and one was performed in Asia. Ultrasound, MRI, and liver biopsy were used to diagnose NAFLD. In addition, the study performed in Asia had three groups: the control group, the simple steatosis group, and the NASH group. We integrated the first two groups into one, which was regarded as the control group in this meta-analysis. The NASH group was included in the case group. In addition, five studies had a high quality score, and the one study conducted in Asia had a lower score.
Results of the meta-analysis
Contrast indicator of BMD (g/cm2)
A total of 173 cases and 192 controls were included. A meta-analysis was conducted using a fixed effects model to evaluate the correlation between the case group and the control group with respect to BMD. The results showed that I2=60.2%, P=0.039 (Figure 2A). Because of significant heterogeneity, we used a random effects model; however, the outcome was the same as that of the above-mentioned model (Figure 2B). After eliminating the study with a low quality score, we performed the analysis using the fixed effects model again, and the result was I2=46.2%, P=0.000 (Figure 3). The meta-analysis indicated that obese adolescents with NAFLD had a higher BMD than those without the illness.
Assessing differences using Z-scores
In this section, six studies were included in the meta-analysis. There were 217 patients in the case group and 236 individuals in the control group. A fixed effects model was used to assess the Z-score relationship between the two groups. The results demonstrated that I2=26.9%, P=0.000 (Figure 4).
The meta-analysis showed that obese adolescents had a positive Z-score; therefore, the Z-scores revealed that obese adolescents with NAFLD were more likely to develop osteoporosis.
Assessing differences using BMC (g)
Three studies contained indicators of BMC. The total number in the case group was 84, whereas in the control group the number was 105. Through a meta-analysis, a fixed effects model showed that there was no significant difference between the NAFLD group and non-NAFLD group (I2=0.0%, P=0.150). The outcome is shown in Figure 5.
Discussion
According to this meta-analysis, we can conclude that obese adolescents with NAFLD have a higher BMD and Z-score than obese adolescents without NAFLD. However, when comparing the BMCs of the two groups, there was no difference. This is the only study to our knowledge to investigate the relationship between BMD and NAFLD in adolescents.
A recent study from Handzlik-Orlik et al29 showed a relationship between NAFLD and osteoporosis. In addition, a retrospective study from People’s Republic of China that involved more than 7,000 men demonstrated that men with NAFLD had a high risk of suffering from an osteoporotic fracture.30 A reduced BMD may be attributed to liver disorder.31,32 Although some clinical studies provided evidence that NAFLD is related to low BMD,19–34 the pathogenesis underlying the correlation between NAFLD and decreased BMD is not clear.
According to current studies, several hypotheses have been proposed, including mechanisms involving tumor necrosis factor (TNF)-α, osteopontin (OPN), osteoprotegerin (OPG), osteocalcin and fetuin-A.35–42 The main factor out of these is TNF-α. A previous study demonstrated that TNF-α could enhance osteoclast activity, inhibit osteoblast differentiation, and increase osteoblastapoptosis.43–45 Additionally, vitamin D plays a significant role in liver pathophysiology and NAFLD.46 These cytokines and regulatory pathways have been associated with the presence of NAFLD.47 Therefore, it has been proposed that the presence of systematic and constant inflammation in NAFLD patients contributes to the correlation between NAFLD and low BMD.45 When focusing on children and adolescents, apart from the mechanisms mentioned above, a study of Hispanic children indicated that NAFLD was associated with obesity and the PNPLA3 gene.48 NAFLD is strongly associated with obesity.49 Childhood obesity can develop into adult obesity.50,51 Simmonds et al52 conducted a meta-analysis, and the results showed that obese children had a 5-fold higher risk of adult obesity than normal-weight children. Therefore, children and adolescents who are overweight and obese are susceptible to NAFLD and problems related to bone metabolism.
Boys had notably higher BMC values than girls older than 14 years old; however, before this age cutoff, there were no sex differences in total body, femoral neck or lumbar spine BMD in a study conducted by Baxter-Jones et al.53 Other experts also demonstrated that within the age range of 9–11, no differences in total BMD were observed between boys and girls.54,55 As for adolescents with NAFLD, research on the prevalence of osteoporosis in different genders is limited. Yu et al56 performed a study on the relationship between bone marrow fat content and hepatic fat content in children with NAFLD. In their study, they found that in boys, bone marrow fat content and hepatic fat content had a significant positive relationship with known or suspected NAFLD, whereas in girls, no such relationship was observed. However, until now, there has been no direct evidence to explain this difference. Genetic factors, biological differences, sample sizes, and other elements may contribute to this phenomenon. As a result, further studies need to explore this relationship between different genders.
However, there are several limitations of this meta-analysis. First, some confounders were not eliminated, and some included studies were missing essential data, such as the number of boys and girls, age, BMI, BMC, and BMD; thus, we did not conduct subgroup analyses. Second, we did not evaluate the severity of NAFLD, and the results we obtained included the range of this disease. Third, because few studies have been performed on this subject, after filtering the studies, only six studies were included in this meta-analysis. If more studies are conducted in the future, we will further study the subgroups. Finally, most studies were from western countries, only one was from Asia, so the results may not be generalizable to some regions; further research needs to be done.
Conclusion
This meta-analysis explored the concept of obese adolescents with NAFLD exhibiting a lower BMD. However, due to the quality and quantity of the included studies, further studies are needed to reveal the relationship between NAFLD and BMD in obese children.
Acknowledgment
This study was supported by the First Affiliated Hospital of Guangxi Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–1609. | ||
Rinella M, Charlton M, Mary R. The globalization of nonalcoholic fatty liver disease: prevalence and impact on world health. Hepatology. 2016;64(1):19–22. | ||
Welsh JA, Karpen S, Vos MB, Saul K. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr. 2013;162(3):496–500. | ||
Burt AD, Lackner C, Tiniakos DG, Burt Alastair D, Carolin L. Diagnosis and assessment of NAFLD: definitions and histopathological classification. Semin Liver Dis. 2015;35(3):207–220. | ||
Nobili V, Svegliati-Baroni G, Alisi A, Miele L, Valenti L, Vajro P. A 360-degree overview of paediatric NAFLD: recent insights. J Hepatol. 2013;58(6):1218–1229. | ||
Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2013;19(4):325–348. | ||
Alkhater SA. Paediatric non-alcoholic fatty liver disease: an overview. Obes Rev. 2015;16(5):393–405. | ||
Méndez-Sánchez N, Arrese M, Zamora-Valdés D, Uribe M, Nahum M-S, Daniel Z-V. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007;27(4):423–433. | ||
Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): updates and future directions. Dig Liver Dis. 2017;49(5):471–483. | ||
Umehara T, Toshihiro U. Nonalcoholic fatty liver disease with elevated alanine aminotransferase levels is negatively associated with bone mineral density: cross-sectional study in U.S. adults. PLoS One. 2018;13(6):e0197900. | ||
Sözen T, Özışık L, Başaran NÇ, Tümay S, Lale Özışık, Çalık BN. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4(1):46–56. | ||
Yamamoto K. [Definition and diagnostic criteria of osteoporosis in Japan]. Clin Calcium. 2001;11(1):19–24. | ||
Cosman F, de Beur SJ, Leboff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. | ||
Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O’Malley CD. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos. 2014;9(1):182. | ||
Cummings SR, Melton LJ, Joseph ML. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–1767. | ||
Videira-Silva A, Sardinha L, Fonseca H, Antonio V-S. Effect of a physical activity consultation in the management of adolescent overweight (the PAC-MAnO project): study rationale, design and methods. BMJ Paediatr Open. 2018;2(1):e000214. | ||
Sardinha LB, Santos R, Vale S, et al. Prevalence of overweight and obesity among Portuguese youth: a study in a representative sample of 10-18-year-old children and adolescents. Int J Pediatr Obes. 2011;6(2-2):e124–e128. | ||
Inchley J, Currie D, Jewell J. Adolescent Obesity and Related Behaviours: Trends and Inequalities in the WHO European Region; 2002–2014. Geneva: World Health Organization. 2017. | ||
Pardee PE, Dunn W, Schwimmer JB. Non-alcoholic fatty liver disease is associated with low bone mineral density in obese children. Aliment Pharmacol Ther. 2012;35(2):248–254. | ||
Purnak T, Beyazit Y, Ozaslan E, Efe C, Hayretci M. The evaluation of bone mineral density in patients with nonalcoholic fatty liver disease. Wien Klin Wochenschr. 2012;124(15–16):526–531. | ||
Cui R, Sheng H, Rui XF, et al. Low bone mineral density in Chinese adults with nonalcoholic fatty liver disease. Int J Endocrinol. 2013;2013:396545. | ||
Poggiogalle E, Donini LM, Lenzi A, Chiesa C, Pacifico L. Non-alcoholic fatty liver disease connections with fat-free tissues: a focus on bone and skeletal muscle. World J Gastroenterol. 2017;23(10):1747–1757. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. | ||
Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51(3):170–179. | ||
Stang A, Andreas S. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. | ||
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. | ||
Shachar M. Meta-analysis: the preferred method of choice for the assessment of distance learning quality factors. IRRODL. 2008;9(3):1–15. | ||
Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. | ||
Handzlik-Orlik G, Holecki M, Wilczyński K, Duława J. Osteoporosis in liver disease: pathogenesis and management. Ther Adv Endocrinol Metab. 2016;7(3):128–135. | ||
Li M, Xu Y, Xu M, et al. Association between nonalcoholic fatty liver disease (NAFLD) and osteoporotic fracture in middle-aged and elderly Chinese. J Clin Endocrinol Metab. 2012;97(6):2033–2038. | ||
Moon SS, Lee YS, Kim SW, Seong-Su M. Association of nonalcoholic fatty liver disease with low bone mass in postmenopausal women. Endocrine. 2012;42(2):423–429. | ||
Guañabens N, Parés A, Núria G, Albert P. Osteoporosis in chronic liver disease. Liver Int. 2018;38(5):776–785. | ||
Ozgur P, Huseyin B, Ismet T. Correlation of insulin sensitivity with bone mineral status in obese adolescents with nonalcoholic fatty liver disease. Clin Endocrinol. 2011;75:189–195. | ||
Campos RMS, de Piano A, da Silva PL, et al. The role of pro/anti-inflammatory adipokines on bone metabolism in NAFLD obese adolescents: effects of long-term interdisciplinary therapy. Endocrine. 2012;42(1):146–156. | ||
Aigner E, Theurl I, Theurl M, et al. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am J Clin Nutr. 2008;87(5):1374–1383. | ||
Manco M, Marcellini M, Giannone G, Nobili V. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;127(6):954–960. | ||
Morimoto J, Kon S, Matsui Y, Uede T, Junko M, Shigeyuki K. Osteopontin; as a target molecule for the treatment of inflammatory diseases. Curr Drug Targets. 2010;11(4):494–505. | ||
Yilmaz Y, Yonal O, Kurt R, et al. Serum levels of osteoprotegerin in the spectrum of nonalcoholic fatty liver disease. Scand J Clin Lab Invest. 2010;70(8):541–546. | ||
Yilmaz Y, Kurt R, Eren F, Imeryuz N. Serum osteocalcin levels in patients with nonalcoholic fatty liver disease: association with ballooning degeneration. Scand J Clin Lab Invest. 2011;71(8):631–636. | ||
Haukeland JW, Dahl TB, Yndestad A, et al. Fetuin A in nonalcoholic fatty liver disease: in vivo and in vitro studies. Eur J Endocrinol. 2012;166(3):503–510. | ||
Yilmaz Y, Yonal O, Kurt R, et al. Serum fetuin A/α2HS-glycoprotein levels in patients with non-alcoholic fatty liver disease: relation with liver fibrosis. Ann Clin Biochem. 2010;47(Pt 6):549–553. | ||
Reinehr T, Roth CL, Thomas R. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93(11):4479–4485. | ||
Kudo O, Fujikawa Y, Itonaga I, Sabokbar A, Torisu T, Athanasou NA. Proinflammatory cytokine (TNFalpha/IL-1alpha) induction of human osteoclast formation. J Pathol. 2002;198(2):220–227. | ||
Gilbert L, He X, Farmer P, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277(4):2695–2701. | ||
Haley B, Pegah G, Younossi Zobair M. Pediatric non-alcoholic fatty liver disease. Children. 2017;4(6). | ||
Yilmaz Y. Review article: non-alcoholic fatty liver disease and osteoporosis – clinical and molecular crosstalk. Aliment Pharmacol Ther. 2012;36(4):345–352. | ||
Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11(2):183–190. | ||
Betancourt-Garcia MM, Arguelles A, Montes J, Hernandez A, Singh M, Forse RA. Pediatric nonalcoholic fatty liver disease: the rise of a lethal disease among Mexican American Hispanic children. Obes Surg. 2017;27(1):236–244. | ||
Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. | ||
Venn AJ, Thomson RJ, Schmidt MD, et al. Overweight and obesity from childhood to adulthood: a follow-up of participants in the 1985 Australian Schools Health and Fitness Survey. Med J Aust. 2007;186(9):458–460. | ||
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. | ||
Simmonds M, Burch J, Llewellyn A, et al. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess. 2015;19:1–336. | ||
Baxter-Jones AD, Mirwald RL, McKay HA, Bailey DA. A longitudinal analysis of sex differences in bone mineral accrual in healthy 8-19-year-old boys and girls. Ann Hum Biol. 2003;30(2):160–175. | ||
Ferretti JL, Capozza RF, Cointry GR, et al. Gender-related differences in the relationship between densitometric values of whole-body bone mineral content and lean body mass in humans between 2 and 87 years of age. Bone. 1998;22(6):683–690. | ||
Maynard LM, Guo SS, Chumlea WC, et al. Total-body and regional bone mineral content and areal bone mineral density in children aged 8-18 Y: the Fels longitudinal study. Am J Clin Nutr. 1998;68(5):1111–1117. | ||
Yu NY, Wolfson T, Middleton MS. Bone marrow fat content is correlated with hepatic fat content in paediatric non-alcoholic fatter liver disease. Clin Radiol. 2017;72(5):e9–425. | ||
Pacifico L , Bezzi M , Lombardo C V , et al. Adipokines and C-reactive protein in relation to bone mineralization in pediatric nonalcoholic fatty liver disease[J]. World Journal of Gastroenterology, 2013, 19(25):4007–4014. | ||
Labayen I,Ruiz J R,Arenaza L et al. Hepatic fat content and bone mineral density in children with overweight/obesity.[J] .Pediatr. Res. 2018, 84: 684–688. | ||
Antonella M, Danilo F, Elenora S, et al. Relationship between nonalcoholic steatohepatitis, PNPLA3 I148M genotype and bone mineral density in adolescents[J]. Liver International. 2018, 38: 2301–2308. | ||
Campos RMS, de Piano A, da Silva PL, et al. The role of pro/antiinflammatory adipokines on bone metabolism in NAFLD obese adolescents: effects of long-term interdisciplinary therapy. Endocrine. 2012;42(1):146–156. | ||
Pirgon O , Bilgin H , Tolu I , et al. Correlation of insulin sensitivity with bone mineral status in obese adolescents with nonalcoholic fatty liver disease[J]. Clinical Endocrinology, 2011, 75(2):189–195. | ||
Jae C E , Yong Y D , Ran Y H . Vitamin D Status and Bone Mineral Density in Obese Children with Nonalcoholic Fatty Liver Disease[J]. Journal of Korean Medical Science, 2015, 30(12):1821–1827. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.