Back to Journals » Neuropsychiatric Disease and Treatment » Volume 12
Relationship between neutrophil/lymphocyte ratio with oxidative stress and psychopathology in patients with schizophrenia
Authors Kulaksizoglu B, kulaksizoglu S
Received 13 April 2016
Accepted for publication 10 June 2016
Published 12 August 2016 Volume 2016:12 Pages 1999—2005
DOI https://doi.org/10.2147/NDT.S110484
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Roger Pinder
Burak Kulaksizoglu,1 Sibel Kulaksizoglu2
1Psychiatry Department, Antalya Education and Research Hospital, 2Biochemistry Department, Antalya Education and Research Hospital, Antalya, Turkey
Introduction: The aim of our study was to evaluate the relationship between neutrophil/lymphocyte ratio (NLR) and total antioxidant status (TAS), total oxidative status (TOS), oxidative stress index (OSI), paraoxonase, and total thiol (T.thl) in schizophrenic patients compared to healthy control group and investigate the relationship between these parameters and psychopathological symptoms.
Methods: The study population consisted of 61 healthy control subjects and 64 volunteer patients monitored in the outpatient clinics of psychiatry of Antalya Education and Research Hospital. Hemograms were determined by using a fully automated hematology analyzer (Beckman Coulter LH780). Serum TOS, TAS, paraoxonase, and T.thl were measured using a novel automated colorimetric measurement method developed by Erel. Sociodemographic data forms were completed by the participants. The Positive and Negative Syndrome Scale (PANSS) was used to assess the patients.
Results: Neutrophils, NLR, TAS, and TOS significantly increased, whereas lymphocytes, T.thl, and T.thl/OSI ratio were significantly lower in the schizophrenia patient group compared to the control group. A statistically significant positive relationship was found between PANSS positive subscale with leukocytes and significantly negative relationships were found between PANSS positive subscale with lymphocytes and T.thl/OSI ratio. Significant positive relationships were found between PANSS total subscale with leukocytes and NLR. Statistically significant negative relationships were found between PANSS total subscale with lymphocytes and T.thl/OSI ratio. In the group of patients with schizophrenia, a significant negative correlation was found between NLR with T.thl/OSI. In the group of patients with schizophrenia, a significant positive correlation was found between NLR with TOS and OSI.
Conclusion: By measuring NLR, which is simple, inexpensive, and suitable for routine use, we can obtain information about oxidative stress and psychopathological symptoms in patients with schizophrenia. Inflammation and oxidative stress are important in the pathogenesis of schizophrenia and are closely related with the patients’ clinical symptoms.
Keywords: oxidative stress, antioxidant status, PANSS, schizophrenia
Introduction
“Schizophrenia is still one of the most mysterious and costliest mental disorders in terms of human suffering and societal expenditure.”1 As schizophrenia is a chronic psychotic disease, emerging oxidant molecules make toxic effects to neurons over the years.2 In the pathogenesis of schizophrenia, two important processes are inflammation and oxidative stress. Inflammation is a complicated response to deleterious stimuli and is mediated by cytokines cascades and oxidative factors.3 There are various parameters showing the inflammation. A new, easy, and cheap option, suitable for routine use to show inflammation, is measurement of the neutrophil/lymphocyte ratio (NLR).4 NLR is associated especially with malignant diseases, cardiovascular diseases, pancreatitis, inflammatory bowel syndrome, and traumas.5–8 Assessing the relationship between schizophrenia and NLR, the studies are limited and there is a contradiction in data.9,10 According to these studies, NLR was found to be significantly higher in patients with schizophrenia than in controls.
In schizophrenic patients, there are various studies showing increment or decrement levels of antioxidant enzyme.11 The failure of antioxidant defense mechanisms causes damage on cell membranes, and it might have a negative effect on neurotransmission and the clinical course of schizophrenia.12 Inflammation is one of the causes of oxidative stress. Myeloperoxidase enzyme released from neutrophils plays an important role in inflammation and causes increased production of reactive oxygen metabolites. Free radicals generated as a result of inflammation kill neurons and cause neuronal toxicity.3 There is only one study assessing together the oxidative stress and inflammation in patients with schizophrenia, and in the same study C-reactive protein, fibrinogen, total oxidative status (TOS), and oxidative stress index (OSI) values were higher in schizophrenic patients than controls.13 In the literature, there are no studies evaluating the relationship between NLR and oxidative stress parameters in patients with schizophrenia.
Based on these data, the aim of our study was to evaluate the relationship between NLR and total antioxidant status (TAS), TOS, OSI, paraoxonase (PON1), and total thiol (T.thl) in schizophrenic patients compared to a healthy control group and investigate the relationship between these parameters and psychopathological symptoms.
Methods
The study complies with the Declaration of Helsinki and was approved by the institutional ethics committee of Antalya Education and Research Hospital. In all, 64 schizophrenic patients and 61 healthy controls aged between 18 and 65 years old participated in the study. Informed consent was obtained from all participants. Healthy controls were assessed through a semistructured psychiatric interview. The schizophrenic patients had been diagnosed according to the diagnostic criteria of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision and were followed for at least 2 years in the psychiatry clinic of Antalya Education and Research Hospital.14
Patients were excluded from the study if they met one or more of the following criteria: alcohol and substance abuse or dependence, hypertension, heart disease, diabetes mellitus, hepatic or renal failure, autoimmune diseases, active infection, active or chronic inflammatory diseases, heavy smoking (>15 cigarettes per day), obesity (body mass index >30 kg/m2), and treatment with antiinflammatory, antioxidant, or immunosuppressive medications. Sociodemographic data forms were filled by the participants. Positive and Negative Syndrome Scale (PANSS) scale which was developed by Kay et al was applied to the patients.15
Antecubital vein blood was taken after 12 hours of fasting from the participants for laboratory analysis. For complete blood counts, ethylenediamine tetraacetic acid anticoagulated tubes and for other parameters gel tube was used. Serum was separated by centrifugation for 10 minutes at 3,000 rpm and serum fractions were stored at −80°C and used to analyze PON1, TOS, TAS, and T.thl concentrations. Hemograms were determined by using a fully automated hematology analyzer (Beckman Coulter LH780; Beckman Coulter, Brea, CA, USA).
TOS, TAS, PON1, and T.thl were measured using a novel automated colorimetric measurement method developed by Erel.16
In the TOS method, oxidants present in the serum oxidize the ferrous ion–chelator complex to ferric ion. The ferric ion makes a colored complex, which can be measured spectrophotometrically.17
In the TAS method, antioxidants in the serum reduce the dark blue-green colored 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) radical to a colorless reduced form. The antioxidative effect of the serum against the potent free radical reactions initiated by the produced hydroxyl radical is measured.16
The ratio of TOS level to TAS level was accepted as OSI.
The OSI value was calculated according to the following formula: OSI (arbitrary unit) = TOS (micromolar hydrogen peroxide equivalent per liter)/TAS (micromolar trolox equivalent per liter).17
The PON1 activity measurement method consists of two different sequential reagents. The first reagent is an appropriate Tris buffer and also contains calcium ion. Absorbance of P-nitrophenol which is produced from paraoxon, increases linearly. Hydrolysis of paraoxon was subtracted from the total rate of hydrolysis.18
Serum T.thl concentration was measured by the method described by Ellman19 and modified by Hu.20
Statistical analysis
Continuous variables are presented as mean ± standard deviation, while categorical variables are given as percentages. The Kolmogorov–Smirnov test was used to verify the normality of the distribution of continuous variables. Statistical analysis of clinical data between two groups consisted of unpaired t-tests for parametric data and Mann–Whitney U-test analysis for nonparametric data. Correlations were assessed with the Pearson/Spearman correlation coefficient and the chi-square/Fisher’s exact test was used for categorical variables.
Receiver operating characteristic (ROC) curve analysis was used to determine the optimum cut-off levels of PON1, T.thl, TAS, TOS, and OSI. Using the ROC curve, the responsiveness is described in terms of sensitivity and specificity. Values for sensitivity and for false-positive rates (1 – specificity) are plotted on the y- and the x-axes of the curve and the area under the curve represents the probability a measure correctly classifies patients as improved or unchanged. Analyses were performed with PASW 18 (SPSS Inc., Chicago, IL, USA) software and a P-value <0.05 was considered statistically significant.
Results
In terms of sociodemographic data, patient and control groups are consistent with each other (Table 1).
Neutrophils, NLR, TAS, and TOS significantly increased whereas the lymphocytes, T.thl, and T.thl/OSI ratio were significantly lower in the schizophrenia patient group compared to the control group. There was no statistical difference in PON1 and OSI values between groups (Table 2).
Between PANSS positive subscale with leukocytes a significant positive relationship, and between PANSS positive subscale with lymphocytes and T.thl/OSI ratio significant negative relationships were found. Between PANSS total subscale (PANSS-T) with leukocytes and NLR significant positive relationships, and between PANSS-T with lymphocytes and T.thl/OSI ratio significant negative relationships were found (Table 3).
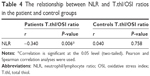
In the group of patients with schizophrenia, between NLR and T.thl/OSI a significant negative correlation was found. There was no significant correlation in the control group (Table 4).
In the group of patients with schizophrenia, a significant positive correlation was found between NLR with TOS and OSI (Table 5).
ROC curves of all parameters are seen in Figure 1.
ROC analysis of area under the curve, cut-off levels, and sensitivity and specificity values are given in Table 6.
Discussion
Studies are still continuing today to clarify the psychopathology of schizophrenia. Inflammation and oxidative stress processes are closely associated with each other.3 Chronic inflammation is associated with many diseases. In schizophrenic patients, chronic inflammation is associated with reactive oxygen radicals. As inflammation triggers oxidative stress, oxidative stress is also known to induce inflammation. There is only one study assessing the oxidative stress and inflammation in the same time in patients with schizophrenia, and in this study C-reactive protein, fibrinogen, TOS, and OSI levels were significantly higher compared to controls.13 In the literature, there are studies assessing NLR and level of oxidative stress as the parameters of inflammation.9–11 Our study is the first evaluating the relationship between oxidative stress and NLR in schizophrenia at the same time.
NLR is an important indicator of inflammation and it is cheap, practical, and suitable for routine use.4 There are very few studies assessing the relationship between schizophrenia and NLR.9,10 In these studies, NLR was found higher in schizophrenic patients than controls. In our study, we have also found that NLR was significantly higher in the group of patients with schizophrenia than controls. Our patient group was taking antipsychotic drugs. In one study, antipsychotic drugs did not change the NLR.9 In another study, NLR was significantly higher in first episode schizophrenic patients who were not taking antipsychotic medication.10
In our study, NLR increase is due to the significant increase in the number of neutrophils and decrease in the number of lymphocyte counts. Similar findings are also compatible with the study carried out with 156 schizophrenic patients by Semiz et al.9 In literature, there are studies that found a relative increase in leukocytes or direct decrease in lymphocyte levels in patients with schizophrenia.21,22 According to our results, consistent with these data, NLR increase as an important parameter of inflammation in patients with schizophrenia plays a significant role in the pathogenesis of schizophrenia.
PANSS is commonly used to detect psychopathological symptoms in patients with schizophrenia.15 In our study, a positive meaningful correlation between PANSS-T and NLR shows us that NLR is associated with not only the pathogenesis but also the clinic activity of schizophrenia. To the best of our knowledge, there is no published study comparing the PANSS with NLR in the literature until now. In just two studies, the relationship between NLR with BPRS score was examined and no relationship was found.9,10 The cut-off value of NLR found in our study was 1.98. Especially above the level of 1.98, NLR may be very practical and effective in the assessment of psychopathological status and clinical monitoring of schizophrenia patients. If NLR can be used at follow-up and clinical assessment of the schizophrenic patients, further studies are needed on this subject.
According to the meta-analysis results in schizophrenia, while the levels of systemic oxidative mediators, such as malondialdehyde and nitric oxide, increased; the levels of antioxidants, such as superoxide dismutase, catalase, and glutathione, decreased; and lipid peroxidation in erythrocytes was found higher.23
In the literature, different data are available about oxidant and antioxidant systems in patients with schizophrenia.24–26 We found that TAS and TOS values were significantly higher in the patient group in our study. In another study which was performed in 50 patients with schizophrenia, there were no significant differences in the mean values of TAS, TOS, and OSI compared to the control group.24 In 30 male schizophrenic patients, TOS and OSI values were found to be significantly higher and no significant difference in TAS and PON1 levels was found compared to the control group.13 In our study, a significant increase in TOS values showed us that oxidative stress plays a role in the pathophysiology of schizophrenia. Especially measuring TOS values over the cut-off value of 5.9 may show a significant increase of oxidative stress in patients with schizophrenia.
TAS values give information about the antioxidant capacity of the organism and in the studies with schizophrenic patients, TAS values have been found lower or normal compared to the control group.13,25 In a study including a total of 64 schizophrenia patients (38 with symptomatic remission and 26 without symptomatic remission), the TOS, OSI, and 8-hydroxydeoxiguanine levels were significantly higher in nonremission schizophrenic patients than in the controls. TOS and OSI levels were found to be significantly high compared to 80 healthy control subjects. In the same study, TAS values were found to be significantly higher in the nonremission group compared to remission group.27 In another study, TAS levels were significantly lower in the first episode schizophrenic patients.28 As shown in some studies, TAS values were low at the beginning of the disease and by the activation of compensatory mechanisms, TAS values were found higher in the chronic phase.28 This situation can be interpreted as the oxidative stress is more effective in the early stages in patients with schizophrenia.
In our study, the patients were using antipsychotic drugs; this may be a limitation of our study, but it was reported in the studies that antipsychotic medications used in the treatment of schizophrenia did not affect the antioxidant capacity.13,29
Cysteine has a role in the defensive protein mechanism of the body, and its functional thiol group plays an important role in preventing oxidative damage. Protection of cells against oxidative damage is carried out with low molecular weight of thiol and cysteine.30 There is no study assessing the T.thl in schizophrenic patients. In our study, T.thl levels were found significantly lower compared to the control group. According to our study, especially the levels of T.thl under 496.85 may show the low activity in the antioxidant defense systems of the schizophrenic patients. Our study is the first that measures the T.thl value using a different method in patients with schizophrenia.
The most striking finding in our study was the significant negative correlation between NLR and T.thl/OSI values. The absence of this relationship in the control group makes our findings more meaningful.
In our study, T.thl/OSI value under 137.46 means that the patients with schizophrenia were more exposed to oxidative stress and especially in this case we believe that PANSS positive subscale and PANSS-T results must be evaluated more carefully as they reflect the clinical situation. According to our findings, a decrease in the T.thl/OSI value resulted in the increase of psychopathological symptoms in schizophrenia patients.
Conclusion
By measuring NLR, which is simple, inexpensive, and suitable for routine use, we can have information about oxidative stress and psychopathological symptoms in patients with schizophrenia. Inflammation and oxidative stress are important in the pathogenesis of schizophrenia and closely related with the patients’ clinical symptoms. With the progress of the studies done in this regard, antioxidant medication effectiveness and its place in the treatment of schizophrenia will be better understood.
Disclosure
The authors report no conflicts of interest in this work.
References
Os JV, Kapur S. “Schizophrenia”. Lancet. 2009;374(9690):635–645. | ||
Fendri C, Mechri A, Khiari G, Othman A, Kerkeni A, Gaha L. Oxidative stress involvement in schizophrenia pathophysiology: a review. Encéphale. 2006;32(Pt 1):244–252. | ||
Altamura AC, Buoli M, Pozzoli S. Role of immunological factors in the pathophysiology and diagnosis of bipolar disorder: Comparison with schizophrenia. Psychiatry Clin Neurosci. 2014;68(1):21–36. | ||
Zahorec R. Ratio of neutrophil to lymphocyte counts rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14. | ||
Ayhan SS, Oztürk S, Erdem A, et al. Relation of neutrophil/lymphocyte ratio with the presence and severity of coronary artery ectasia. Turk Kardiyol Dern Ars. 2013;41(3):185–190. | ||
Azab B, Jaglall N, Atallah JP, et al. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11(4):445–452. | ||
Szkandera J, Absenger G, Liegl-Atzwanger B, et al. Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients. Br J Cancer. 2013;108(8):1677–1683. | ||
Fowler AJ, Agha RA. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography–the growing versatility of NLR. Atherosclerosis. 2013;228(1):44–45. | ||
Semiz M, Yildirim O, Canan F, et al. Elevated neutrophil/lymphocyte ratio in patients with schizophrenia. Psychiatr Danub. 2014;26(3):220–225. | ||
Varsak N, Aydin M, Eren I. Evaluation of neutrophil-lymphocyte ratio in first-episode psychosis. Bull Clin Psychopharmacol. 2015;25:7–9. | ||
Yao JK, Reddy R. Oxidative stress in schizophrenia: pathogenetic and therapeutic implications. Antioxidants Redox Signal. 2011;15(7):1999–2002. | ||
Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400–409. | ||
Yegin A, Ay N, Aydin O, Yargici N, Eren E, Yilmaz N. Increased oxidant stress and inflammation in patients with chronic schizophrenia. Int J Clin Med. 2012;3(5):368–376. | ||
First MB, Pincus HA. The DSM-IV Text Revision: rationale and potential impact on clinical practice. Psychiatr Serv. 2002;53(3):288–292. | ||
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–275. | ||
Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37(4):277–285. | ||
Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–1111. | ||
Eckerson HW, Wyte MC, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983;35(6):1126–1138. | ||
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. | ||
Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380–385. | ||
Steiner J, Jacobs R, Panteli B, et al. Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci. 2010;260(7):509–518. | ||
Mazzarello V, Cecchini A, Fenu G, et al. Lymphocytes in schizophrenic patients under therapy: serological, morphological and cell subset findings. Ital J Anat Embryol. 2004;109(3):177–188. | ||
Grignon S, Chianetta JM. Assessment of malondialdehyde levels in schizophrenia: a meta-analysis and some methodological considerations. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):365–369. | ||
Pazvantoglu O, Selek S, Okay T, et al. Oxidative mechanisms in schizophrenia and their relationship with illness subtype and symptom profile. Psychiatry Clin Neurosci. 2009;63(5):693–700. | ||
Ustundag B, Atmaca M, Kirtas O, Selek S, Metin K, Tezcan E. Total antioxidant response in patients with schizophrenia. Psychiatry Clin Neurosci. 2006;60(4):458–464. | ||
Reddy R, Sahebarao MP, Mukherjee S, Murthy JN. Enzymes of the antioxidant system in chronic schizophrenic patients. Biol Psychiatry. 1991;30(4):409–412. | ||
Copoglu US, Virit O, Kokacya MH, et al. Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res. 2015;229(1–2):200–205. | ||
Li XF, Zheng YL, Xiu MH, Chen DC, Kosten TR, Zhang XY. Reduced plasma total antioxidant status in first-episode drug-naive patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):1064–1067. | ||
Padurariu M, Ciobica A, Dobrin I, Stefanescu C. Evaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychotics. Neurosci Lett. 2010;479(3):317–320. | ||
Wlodek L. Beneficial and harmful effects of thiols. Pol J Pharmacol. 2002;54(3):215–223. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.