Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Relationship Between Metabolic Syndrome and Bone Health – An Evaluation of Epidemiological Studies and Mechanisms Involved
Authors Chin KY , Wong SK , Ekeuku SO , Pang KL
Received 5 August 2020
Accepted for publication 22 September 2020
Published 13 October 2020 Volume 2020:13 Pages 3667—3690
DOI https://doi.org/10.2147/DMSO.S275560
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Kok-Yong Chin,1,2 Sok Kuan Wong,1 Sophia Ogechi Ekeuku,1 Kok-Lun Pang1
1Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia; 2State Key Laboratory of Oncogenes and Related Genes, Renji-Med X Clinical Stem Cell Research Center, Department of Urology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, People’s Republic of China
Correspondence: Kok-Yong Chin
Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Yaacob Latif, Bandar Tun Razak, 56000 Cheras, Kuala Lumpur, Malaysia
Tel +60 3-9145 9573
Email [email protected]
Abstract: Metabolic syndrome (MetS) and osteoporosis are two medical problems plaguing the ageing populations worldwide. Though seemingly distinctive to each other, metabolic derangements are shown to influence bone health. This review summarises the relationship between MetS and bone health derived from epidemiological studies and explains the mechanistic basis of this relationship. The discourse focuses on the link between MetS and bone mineral density, quantitative sonometric indices, geometry and fracture risk in humans. The interesting sex-specific trend in the relationship, probably due to factors related to body composition and hormonal status, is discussed. Mechanistically, each component of MetS affects the bone distinctly, forming a complex interacting network influencing the skeleton. Lastly, the effects of MetS management, such as pharmacotherapies, exercise and bariatric surgery, on bone, are presented. This review aims to highlight the significant relationship between MetS and bone, and proper management of MetS with the skeletal system in mind could prevent cardiovascular and bone complications.
Keywords: bone mineral density, diabetes, dyslipidemia, fracture, hypertension, obesity
Introduction
The skeleton is a dynamic system responsive to internal and external stimuli to provide support to the body and regulate mineral homeostasis. Deterioration of skeletal mass and microstructure will result in osteoporosis and increase the risk of fragility fracture.1 Apart from primary causes, such as sex hormone deficiency and advanced age, metabolic derangements are significant secondary risk factors of skeletal deterioration.2 Meta-analyses reported that diabetes mellitus increases the total [relative risk (RR) 1.32; 95% confidence interval (CI) 1.17–1.48] and hip fracture risk (RR 1.77; 95% CI 1.56–2.02),3 and obesity increases the upper arm [hazard ratio (HR) 1.60, 95% CI 1.42–1.80] and all osteoporotic fracture risk (HR: 1.16; 95% CI 1.09–1.23) after adjustment for bone mineral density (BMD).4 The increasing aged, obese and diabetic populations worldwide highlight the burden of fracture secondary to these conditions.5,6
The definition of metabolic syndrome (MetS) has evolved with time (Table 1), but it pivots on central obesity, hypertension (HPT), hyperglycaemia and dyslipidaemia. MetS alters the mechanical loading, hormonal and biochemical profile of the body, thereby producing complex effects on bone health. While increased mechanical loading and production of certain hormones are protective against osteoporosis, the proinflammatory and pro-oxidative body environment might be detrimental to bone health.7 Thus, the overall impact of MetS on bone health depends on the sum of these interactions. However, the relationship between MetS and bone health reported in epidemiological studies is heterogeneous. Although some studies reported a positive association between MetS and BMD, others reported a negative relationship.8,9 A potential sex difference in the relationship between MetS and bone is also observed.10–12
 |
Table 1 Definition of MetS |
This review aims to summarise the relationship between MetS and bone, primarily from epidemiological studies collecting BMD and bone fracture data. According to the World Health Organization, BMD measured by dual-energy X-ray absorptiometry is the standard in defining osteoporosis.13 Studies using alternative bone health assessment methods, such as quantitative ultrasonometry and peripheral quantitative computed tomography, were also discussed. The mechanistic basis of this relationship is discussed in the second part of this review. The review will help the readers to understand that influence of MetS on bone health, beyond its well-recognized impacts on cardiovascular diseases.
Relationship Between MetS and Bone Mineral Density
Positive Association Between MetS and Bone Mineral Density
A retrospective study by Park et al (n=399, women aged 59 ± 6.7 years) suggested a positive association between MetS on BMD. Age-adjusted femoral neck BMD was higher in post-menopausal Korean women with MetS compared to those without MetS, but their lumbar spine BMDs were similar.14 The difference in the strength of association between the two skeletal sites could be attributed by weight-bearing capacity, in which femoral neck endures more loading compared to lumbar spine.15
Multiple cross-sectional studies reported a positive relationship between MetS and BMD.16–19 The Third National Health and Nutrition Examination Survey (NHANES III) (n=8197, ≥20 years) reported a higher femoral neck BMD among subjects with MetS compared to subjects without MetS. Adjustment for BMI attenuated the difference in BMD between the two groups.17 However, this study only measured one skeletal site. The Rancho Bernardo Study (417 Caucasian men and 671 Caucasian women aged 38 to 97 years) reported that subjects with MetS had higher total hip BMD compared to non-MetS subjects. Men with MetS in the study also had a higher femoral neck BMD compared to men without MetS. However, BMI adjustment reversed the relationship between MetS and BMD.18 Additionally, a positive relationship between MetS and lumbar spine T-score was found among subjects from Saudi Arabia (1578 subjects, >35 years) with high BMI (BMI > 25 kg/m2 for 87.3% women and 79.4% men recruited).19
More conclusive evidence on the positive influence of MetS on BMD comes from a three-year longitudinal study by Kim et al (n=1128). In this study, Korean post-menopausal women with MetS experienced 31.1%, 26.7%, 21.7% and 12.0% less BMD loss at the lumbar spine, trochanter, total hip and femoral neck compared to non-MetS women, respectively. The rate of bone loss also decreased with an increasing number of MetS components.20 It should be noted the subjects were recruited from a health screening centre, who could be more health-conscious and have an upper socioeconomic background. Thus, they might not represent the general population.
In a meta-analysis by Xue et al (11 studies, n=13,122), lumbar spine and femoral neck BMD values unadjusted for BMI was higher in MetS subjects compared to non-MetS subjects. The association between MetS and femoral neck BMD was more prominent in the Caucasians than Asians. The unadjusted BMD was used because the adjustment could distort the relationship between BMD and MetS.8 Multiple studies suggested that the positive association between MetS and BMD was driven by mechanical loading reflected through BMI.17,18 This observation is evident when BMI-adjustment attenuates or reverses the association between MetS and BMD. However, a high BMI could reflect either large body size or high adiposity, whereas waist circumference is a better measure of adiposity.21 At its existing cut-offs, BMI is also an imprecise method to measure obesity in the elderly.22 In lieu of this opinion, a study among Korean post-menopausal women (n=3058) aged >50 years old classified the subjects into waist circumference (WC) obesity (>80 cm) and BMI obesity (>25 kg/m2). The study found that the prevalence of osteoporosis was lower in subjects classified as having BMI obesity with or without WC obesity.23 On the other hand, the prevalence of osteoporosis was higher among non-obese subjects and subjects with WC obesity only. Thus, BMI adjustment can delineate the effects of mechanical loading in the relationship between MetS and BMD.
Overall, the positive association between bone health and MetS may be mediated by body size reflected by BMD.
Negative Association Between MetS and Bone Mineral Density
Multiple studies among the East Asian populations demonstrated a negative relationship between MetS and BMD. A retrospective study by Chen et al reported that both MetS and the associated non-alcoholic fatty liver disease were positively associated with osteoporosis among Chinese post-menopausal women (n=938).24
The negative relationship was further consolidated in cross-sectional studies. Hwang and Choi reported that the lumbar spine BMD adjusted for age, weight and height decreased with increasing MetS components in Korean adult women (n=2548).25 Similarly, body weight-adjusted femoral neck BMD was lower in Korean men (n=1780, 55.7 ± 8.1 years) and post-menopausal women (n=1109, 57.1 ± 6.7 years) with MetS compared to subjects without MetS in the study by Kim et al.26 In a study by Jeon et al, pre- (n=1234) and post-menopausal Korean women (n=931) with MetS showed a lower lumbar spine BMD after multiple adjustments, including weight. The post-menopausal women with MetS also showed a lower femoral neck BMD.27 The authors postulate that the effects of MetS on BMD are more significant among post-menopausal women because they are not protected by oestrogen.
The studies in this section considered only women. Since apparent differences in body composition and hormonal profile exist between men and women, it would be interesting to explore if sex influences the relationship between MetS and BMD.
Sex-Specific Relationship MetS and Bone Mineral Density
Several sex-specific trends on the association between MetS and BMD have been observed. Two retrospective studies among Taiwanese population showed protective effects of MetS on skeletal health of men but not in women >50 years. In these studies, men with MetS had a higher BMD at the lumbar spine and the hip28 and were less likely to have osteoporosis compared to non-MetS men.29 The relationship between MetS and BMD was negative in women was negative28 or negligible.29 Multiple adjustments for confounders attenuated the relationship between MetS and bone health in both sexes.29 Thus, the overall effects of MetS on bone are weak and inconsistent in both studies.28,29
Two studies on the Caucasian populations showed that MetS protects bone health in women but not in men.10,12 In the Camargo Cohort Study (n=1508, >50 years), women with MetS showed higher age-adjusted BMD at the femoral neck, lumbar spine and total hip compared to women without MetS. Again, adjustment for BMI attenuated most differences, except for the total hip.12 The presence of MetS did not influence BMD of men in the same study.12 In the Berlin Aging Study II (n=1402, 68±4 years), the relationship between MetS and lumbar spine and total hip BMD was positive among women, but not significant among men.10
A series of studies in Korean population showed that MetS was a negative predictor of BMD in men but not in women. The Korean National Health and Nutrition Examination Survey (KNHANES, n=3207) reported that BMD of the femoral neck, total hip and lumbar spine between subjects with MetS (defined by Joint Interim Statement)30 and those without MetS was similar after multiple adjustments, including BMI. However, men with MetS had higher odds of suffering from suboptimal bone health (T-score < −1).31 Re-analysis using National Cholesterol Education Program Adult Treatment Panel III definition32 showed that adjusted BMD of the lumbar spine and femoral neck was consistently lower in young (<45 years) and older men with MetS compared to subjects without MetS (n=2989). However, pre- and post-menopausal women with MetS showed similar BMD values.11 Using the criteria of American Heart Association/National Heart, Lung, and Blood Institute,33 Korean men (n=6659, aged 43.62±0.3 years) with MetS showed lower total hip and femoral neck BMD compared to non-MetS counterparts after multiple adjustments, including BMI. Again, the difference was not observed in pre- (n=4547, aged 35.15±0.19 years) and post-menopausal women (n=3279, aged 63.17±0.26 years).34
A meta-analysis by Zhou et al (9 studies, n=18,380) showed that subjects without MetS had a higher lumbar spine and femoral neck BMD. The negative effects of MetS were more prominent in men compared to women.9
Overall, there is an apparent sex difference in the relationship between MetS and bone health. When sex difference is studied, men seem to be more susceptible to the negative effects of MetS on bone. Some researchers postulate that at any given BMI, men had higher visceral fat compared to women, so they are more susceptible to the adverse effects of MetS.34
Relationship Between MetS and Quantitative Ultrasound
Quantitative ultrasound is another method of assessing individual bone health.35 Two of the basic quantitative ultrasound indices (QUIs) are speed of sound, which reflects the distance travelled by the ultrasound across time (m/s), and broadband ultrasound attenuation, which refers to the slope between ultrasound signal attenuation due to absorption by cortical bone or scattering by trabecular bone in relationship with its frequency (dB/MHz).36 Other composite QUIs can be derived from these two basic indices, but they differ between devices. In general, higher values of QUIs indicate better bone health.35 QUI has been shown to correlate with bone mass and microarchitecture,37,38 and it can predict fracture risk.39 QUIs also showed a high diagnostic accuracy of osteoporosis with modified cut-offs.40,41
In the PREMED study (124 men aged 55 −80 years and 127 women, aged 60–80 years), BUA and QUI were higher in subjects with MetS compared to those without after adjustment for sex. BUA was also higher in subjects with diabetes compared to normal subjects.42 In the study of Cvijetic et al (n=211 drawn from voter registry, aged 77.9±4.5 years), QUI was significantly lower in men with MetS but higher in women with MetS compared to their non-MetS counterparts. In the multivariate regression model, adjustment for BMI weakened the relationship between MetS and QUI in women but not in men. Further adjustment for glucose level diminished the relationship between MetS and QUI in men.43
In the Camargo Cohort Study, women with MetS (n=788, aged 63±9 years) showed higher age-adjusted QUI compared to those without MetS but the BMI-adjusted QUI values were similar between the two groups. MetS was not associated with QUI in all models for men (n=421 aged 65±9 years old) in the study.44 Similarly, Chin et al showed reported that MetS was not significantly associated with the calcaneal speed of sound among 303 Malaysian men of Chinese and Malay ethnicity aged >40 years old.45
Overall, the effects of MetS on QUI may be similar to BMD, which might be mediated by mechanical loading reflected by BMI.
Relationship Between MetS and Bone Geometry
Evidence on the association between MetS and bone geometry in human is somewhat limited. The Rotterdam Study showed that MetS was associated with lower bone width and cortical bucking ratio, indicative of bone instability, in women (n=2040, aged 72.38 ±6.81 years) but not in men (n=1510, aged 72.04 ±6.51 years).46
Table 2 summarises the relationship between MetS and bone health, including BMD, QUI and bone geometry.
 |  |  |  |  |  |
Table 2 Summary of the Relationship Between MetS and Bone Health |
Relationship Between MetS and Bone Fracture
Another indicator of osteoporosis is fragility fracture since most patients are not aware of their bone health status until a fracture occurs. Each component of MetS exerts distinct influence on fracture risk. For instance, increased mechanical loading and estradiol synthesis due to obesity could preserve bone mass and prevent fracture.47,48 Type 2 diabetes is associated with increased BMD but higher fracture risk.49,50 Variation in lipid profile is also associated with fracture risk.51
Several studies reported reduced fracture risk among MetS subjects compared to non-MetS subjects.52–54 In the Tromsø Study (n=26,991, 25–98 years), subjects with MetS showed decreased non-vertebral fracture (NVF) risk compared to non-MetS subjects. Particularly, NVF risk decreased in men with increased blood pressure and in women with increased BMI.52
A sex-specific trend in the relationship between MetS and fracture risk was also observed. In a cross-sectional study by Yu et al (n=897 men and 1792 women; ≥40 years), MetS was associated with increased risk of any fracture in women (22.8% in MetS vs 16.3% in non-MetS women) and the inverse happened in men.55 In the MINOS study, men with MetS showed a reduced incidence of vertebral fractures (VF) and peripheral fractures compared to men without MetS.56 The Rotterdam study found a reduced risk of any fracture and NVF among men with an increasing number of MetS components, but a lack of significant relationship among women.46 A Chinese study (2814 males and 4675 females, ≥40 years) also showed that women with MetS experienced an increased risk of any fractures compared to non-MetS women.57 Other studies among the Chinese population also obtained similar findings.58,59
In contrast to the studies mentioned earlier, the Camargo cohort study found no significant difference in the prevalence of VF and NVF between MetS and non-MetS subjects of either sex.44 Other smaller studies, such as PREMED also discovered a lack of association between MetS and fracture risk.42
Overall, the majority of the studies found a significant association between MetS and fracture risk except for a few smaller studies. Women with MetS might have elevated fracture risk, whereas MetS might protect men from fracture. Could these sex discrepancies be attributed to the fact that women with higher fat mass than men suffer from the negative effects of MetS on bone, while men benefit from increased mechanical loading? This question worth further investigation.
Table 3 summarises the relationship between MetS and fracture risk.
 |
Table 3 The Relationship Between MetS and Fracture Risk |
Mechanistic Basis of the Relationship Between MetS and Bone
The effects of MetS on BMD and fracture risk are underlaid by altered cellular and mechanical properties of the skeletal system. Each MetS components exert a distinct effect on bone, and the sum determines the overall effects of MetS on BMD and fracture risk.
The Effects of MetS on Osteoblastogenesis and Osteoclastogenesis
Bone remodelling is a process of bone repair consisting of bone formation by osteoblasts and bone resorption by osteoclasts. Various endogenous and exogenous factors influence the differentiation of osteoblasts and osteoclasts from their corresponding progenitors of mesenchymal and haematopoietic origins. Hormones, nutrients, drugs and mechanical loading are some of the factors influencing bone remodelling. The early event of osteoblastogenesis requires the presence of Wnt and bone morphogenetic protein-2 (BMP-2) to direct the commitment of mesenchymal stem cells (MSCs) into pre-osteoblastic fate along with induction of other osteoblast-specific transcription factors, such as Runt-related transcription factor 2 (Runx-2) and Osterix (OSX) to promote osteoblastogenesis.60 Alkaline phosphatase (ALP) is highly expressed in committed pre-osteoblasts, thus represent an early marker of osteoblast phenotype. Subsequently, active and mature osteoblasts secrete other bone matrix proteins including collagen I (COL1), osteocalcin (OCN), osteopontin (OPN), osteonectin and bone sialoprotein (BSP) associated with mineralization and calcification.61 Osteoblasts regulate the differentiation of osteoclasts through receptor activator of nuclear factor kappa-Β (RANK) ligand (RANKL) and osteoprotegerin (OPG). RANKL binds to the RANK receptor on osteoclast progenitors and stimulates their differentiation, whereas OPG is a decoy receptor of RANKL which prevents its binding to RANK.62,63 The binding of RANKL to RANK recruits tumour necrosis factor receptor-associated factor 6 (TRAF6) and activates nuclear factor-kappa B (NFκB) and Fos proto-oncogene (c-Fos). The latter interacts with Jun proto-oncogene (c-Jun) and forms activator protein 1 (AP-1) complex, which cooperates with NF-κB to trigger nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) for osteoclast differentiation and function.61 Bone formation and bone resorption markers are the by-products of osteoblast and osteoclast activities, respectively. They serve as an assessment tool complementary to BMD, which measure bone remodelling rate and ultimately predict fracture risk.64
Omental adipose tissue-derived MSCs isolated from MetS subjects expressed lower ALP and osteonectin.65 The Camargo Cohort Study reported lower propeptide of type 1 collagen (P1NP) and C-terminal telopeptide of type I collagen (CTX) in individuals with MetS as compared to healthy controls in either sex.12 A population-based study of health in Pomerania also found that higher bone turnover markers (OCN, P1NP and CTX) were associated with lower odds for MetS or T2DM among 2671 adult men and women.66 The MINOS study demonstrated lower OCN, CTX and higher deoxypyridinoline (DPD) in 762 older men with MetS.56 The association between MetS and OPG was inconsistent whereby some studies reported no correlation,67,68 while some studies indicated positive associations.69,70 In short, the accumulating evidence from human studies demonstrated heterogeneous outcomes on the association between MetS and bone markers, thus the net effect of MetS on bone needs to be resolved.
The Effects of MetS on Osteocyte-Driven Bone Remodelling
Osteocytes, the terminally differentiated osteoblasts entombed by the organic matrix, are the mechanosensor of the skeletal system playing a significant role in coordinating the bone remodelling process.71 Osteocytes also secrete regulators of bone remodelling similar to osteoblasts (OPG and RANKL), Wnt pathway [sclerostin (SOST) and Dickkopf-related protein 1 (DKK1)] and phosphate homeostasis [fibroblast growth factor-23 (FGF-23), phosphate-regulating neutral endopeptidase (Phex) and dentin matrix protein (DMP1)].72 The interplay between MetS and Wnt signalling has been proposed based on several considerations: (a) Increased level of β-catenin upon activation of the canonical Wnt pathway decreased peroxisome proliferator-activated receptor-gamma (PPAR-γ) and CCAAT-enhancer-binding protein alpha (C/EBPα) transcription factors essential for adipocyte gene expressions, (b) Glycogen synthase kinase 3β (GSK3β) has an important role in regulating blood glucose whereby its sustained activation-induced apoptosis of islet β cells, reduction of insulin secretion and inhibition of glycogen synthase, and (c) low-density lipoprotein receptor-related protein 6 (LRP6) forms a complex with low-density lipoprotein receptor (LDLR) and clathrin to facilitate low-density lipoprotein (LDL) clearance.73–75 The activation of Wnt/β-catenin results in the expression of direct downstream Wnt target genes, Runx-2, thus promoting osteogenesis.76 Wnt antagonists (eg SOST and DKK1) bind to LRP5/6 and antagonize the Wnt signalling, favouring bone resorption and bone loss.77 Recent report delineated the levels of SOST and DKK-1 were significantly increased in a MetS-induced bone loss animal model, suggesting the involvement of osteocyte-mediated Wnt inhibition during MetS.78
On the other hand, a previous study reported an inverse relationship between MetS and the level of phosphate. Patients with a higher number of MetS components had a lower circulating phosphate level.79 FGF-23 acts as a regulator for phosphate homeostasis by increasing phosphate excretion and reducing phosphate reabsorption.80 FGF-23 also inhibits 1α-hydroxylase, an enzyme essential for the conversion of inactive 25-hydroxyvitamin D [25(OH)D] to 1.25-dihydroxy vitamin D [1,25(OH)2D], suggesting its pivotal role in bone metabolism.81 In MetS animals induced by a high-carbohydrate high-fat diet, the level of FGF-23 in bone was increased, subsequently contributing to bone loss.78 In an epidemiological study, serum FGF-23 was higher in elderly with MetS and was associated with increased TG, BMI, waist circumference, trunk and body fat, as well as reduced HDL-c.82 Patients with impaired glucose tolerance also showed higher circulating FGF-23 level compared to normal control.83 Based on this evidence, the reduction of phosphate level in MetS might be explained by the increase in FGF-23 that suppressed phosphate reabsorption and enhanced phosphate excretion. The synergistic action of FGF-23 on phosphate and vitamin D homeostasis postulated the possible association between MetS and bone loss.
However, the link between MetS on bone through the osteocyte-driven bone remodelling in humans is yet to be investigated extensively. Thus, the findings obtained from in vivo study await further validation from human studies.
The Effects of Individual MetS Components on Bone
Each component of MetS affects bone metabolism. The role of adiposity on bone health is complex. Adiposity increases body mass and exerts mechanical loading on the bone, thereby stimulating bone accrual.84 Aromatase enzymes in the abdominal subcutaneous and visceral adipose tissue synthesize oestrogens which are protective of bone health.85,86 On the other hand, adipose tissue is also a major source of proinflammatory enzymes, such as interleukin (IL)-1 (IL-1), IL-6 and tumour necrosis factor-alpha (TNF-α).87 In particular, visceral fat was shown to be a significant source of IL-1β, IL-6 and IL-15 in obese men.88 These cytokines encourage the formation of osteoclasts and bone resorption activities while decreasing the formation of osteoblasts and bone formation.89 Adipose tissue also sequesters lipid-soluble hormones, such as testosterone and vitamin D essential in maintaining bone health, making them unavailable to bone tissue.90,91 A study in the Chinese population demonstrated that visceral fat area, an index of visceral adiposity, correlated negatively with serum 25-hydroxyvitamin D3 level.92 Apart from that, visceral adipose tissue also secretes adipokines, such as leptin and adiponectin.93 Leptin regulates energy metabolism by controlling satiety.93 Leptin has both anabolic and catabolic effects on the skeleton. Leptin exerts anabolic effects on osteoblasts, and it inhibits osteoclast formation by acting through RANKL/OPG pathway. At the same time, leptin exerts catabolic actions on the bone by acting on the sympathetic nervous system and increasing catecholamine and neuropeptide Y (NPY) secretion.94 Overall, a higher leptin level is associated with higher BMD and bone mineral content according to a meta-analysis, especially post-menopausal women.95 Adiponectin improves the insulin sensitivity of liver and muscle,93 but its level is inversely associated with BMD in humans,96 although preclinical studies reveal an anabolic effect, through inhibition of SOST and modulation of RANKL/OPG pathway.97
Type 2 diabetes mellitus (T2DM) is associated with increased BMD and increased fracture risk.49,98 Individuals with MetS suffer from impaired glucose tolerance and are predisposed to T2DM.99 Chronic hyperglycaemia will trigger the formation of advanced glycation end products (AGEs) when carbonyl group of a reducing sugar reacts with the amino group of macromolecules. The resultant Amadori products are unstable and will undergo subsequent reactions to form AGEs.100 Intracellular AGEs induce apoptosis of osteoblasts through endoplasmic reticulum stress.101 AGEs possess a biphasic effect on osteoclasts, whereby early exposure to AGEs inhibit osteoclasts differentiation and bone resorption, whereas late-stage exposure increases osteoclast fusion, podosome number and bone resorption.102 High glucose and AGEs increase SOST level production by osteocytes, thereby inhibiting bone formation. RANKL production by osteocytes is suppressed by AGEs, thereby inhibiting bone resorption.103 In combination, the effects caused low bone turnover and affect bone material properties. Human studies showed that skin AGEs were negatively correlated with bone material strength index regardless of diabetic status.104,105 In the Baltimore Longitudinal Study of Aging, both impaired glucose tolerance and diabetes status were negatively associated with hip geometry parameters and hip bending strength in women. This highlights a progressive bone impairment which starts even in prediabetic state.106 Besides, diabetes is also associated with bone marrow adiposity. Since adipocyte and osteoblast share the same mesenchymal progenitor, increased adipocyte differentiation decreases osteoblast differentiation, thereby limiting the osteoblast available for bone turnover.107 Insulin resistance is linked to high circulating insulin level, which exerts anabolic effects on the skeleton through direct and indirect actions on osteoblasts.108 All these effects precipitate altered bone geometry, such as increased intracortical porosity but increased trabecular bone density due to ineffective load distribution, as well as reduced bone strength observed in T2DM patients.109 Impaired glucose tolerance, when progresses to T2DM, has other indirect effects on fracture risk. Patients with diabetes have a higher risk of sarcopenia,110 which could increase their risk of falls and fractures.111
HPT due to high circulating level of sodium ion prevents the reabsorption of calcium, thereby increasing calcium excretion. Subsequently, parathyroid hormone (PTH) production will increase and lead to bone resorption.112 Mutations in thiazide‐sensitive sodium-chloride co‐transporter (NCCT) responsible in sodium resorption at the distal convoluted tubule have also been postulated as a genetic link between HPT and bone health. Inactivating mutation of this transporter, as in the case of Gitelman’s syndrome, leads to excessive sodium excretion but high BMD phenotype. Heterozygote of mutated NCCT may also confer protection to the bone but the prevalence of this genotype is not clear.113
A diet rich in fat will increase the free fatty acid (FA) in the blood. Oxidized FA can activate PPAR-γ,114 which subsequently induces the differentiation of adipocytes in the bone marrow and suppresses differentiation of osteoblasts.115 Oxidized LDL impairs osteoblast differentiation and mineralization process.116 Accumulation of oxidized LDL can induce apoptosis of osteoblasts by lysosomal membrane damage.117 Oxidized LDL can increase the production of RANKL of osteoblasts without corresponding changes in OPG level and stimulate the differentiation of osteoclasts.118 On the other hand, oxidized LDL can directly suppress the formation of osteoclasts and the fusion of lysosomes to the ruffled border, thereby affecting their bone resorption activity, via scavenger receptor-A.119,120 Mevalonate pathway responsible for the synthesis of cholesterol and isoprenoids is the drug target for dyslipidaemia treatment. Isoprenoids generated from the mevalonate pathway are involved in the prenylation of GTPases, such as Rho, Ras and Rac. Suppression of GTPase prenylation though mevalonate is known to suppress osteoclast differentiation, function and survival.121,122
HDL can prevent oxidized LDL-induced apoptosis of osteoblast by preserving lysosome integrity.123 Paraoxonase 1 associated with HDL has been shown to prevent oxidation of LDL and lipid peroxidation products,124 thereby contributing to the protection of HDL on the bone. Apart from cholesterol transport, HDL also suppresses proinflammatory cytokine synthesis from macrophages,125 which would otherwise promote the formation of osteoclasts over osteoblasts, leading to bone loss.
The Role of Inflammation and Oxidative Stress in Linking MetS and Bone
Chronic low-grade inflammation and induction of oxidative stress are the hallmark features in the pathogenesis of MetS. Proinflammatory cytokines and free radical species are known to modulate the bone remodelling process, favouring bone loss.126–128 As aforementioned, visceral adipose tissue has a major role in for the activation of the inflammatory mechanism via the release of cytokines, adipokines and chemokines. In osteoblast, the mechanistic pathways involved under inflammatory condition include the activation of NF-κB, suppressor of mothers against decapentaplegic (SMAD) ubiquitylation regulatory factor (SMURF) 1 and SMURF2, as well as inhibition of mitogen-activated protein kinase (MAPK) activities. These signalling events inhibit osteoblast-specific gene transcription.129 Additionally, the interaction of M-CSF, RANKL, TNF-α, IL-6 and monocyte chemoattractant protein-1 (MCP-1) with their respective receptors signals through the activation of NF-κB, MAPK and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathways, leading to the massive upregulation of downstream signalling in osteoclasts129,130 and cytokine production.131
The increased production of reactive oxygen species (ROS) during MetS is triggered by the excessive macronutrient intake such as fat and carbohydrate in the oxidative phosphorylation process. Hyperglycaemia can activate a metabolic pathway involving diacylglycerol/protein kinase C/NADPH-oxidase, which leads to the generation of free oxygen radicals.132 The depletion of antioxidant capacity in the body to counterbalance the synthesis of ROS results in oxidative stress. Inflammation is also a contributor to oxidative stress and vice versa. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxidase, myeloperoxidase, lipoxygenase and cyclooxygenase are catalytic enzymes for the generation of ROS that sensitive to inflammatory conditions.133 Inversely, oxidative stress induces an inflammatory response through the activation of NF-κB transcription factor.134 Excessive oxidative stress promotes osteoclast differentiation, osteoblast and osteocyte apoptosis but suppresses osteoclast apoptosis and osteoblast differentiation, thereby affecting bone homeostasis.135,136
A meta-analysis showed that MetS is associated with increased proinflammatory cytokines (IL-6 and TNF-α), adipokines (leptin) and prooxidants (oxidized LDL and uric acid), as well as decreased anti-inflammatory cytokine (IL-10) and antioxidant (paraoxonase 1).137 Overview from a cross-sectional survey consisting of 10,475 participants revealed that the level of inflammatory mediator [C-reactive protein (CRP)] was inversely correlated with total BMD in both men and women.138 Another study also demonstrated higher levels of cytokines [interferon alpha 2 (IFNα2), interferon-gamma (IFN-γ), interleukin-12p70 (IL-12p70) and interleukin-33 (IL-33)] and chemokines (MCP-1) in osteoporotic post-menopausal women.139 Treating rheumatoid arthritis and ankylosing patients with anti-TNF-α therapy for a year were found to slow down generalized bone loss with decreased DKK-1, cathepsin K (CTSK) and increased P1NP.140 Taken together, the available evidence reiterated that inflammation and oxidative stress are simultaneously found in MetS and bone loss.
The mechanistic basis of the relationship between MetS, osteoblastogenesis and osteoclastogenesis is summarized in Figure 1.
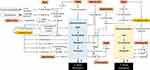 |
Figure 1 The molecular action of MetS and its components on osteoblastogenesis and osteoclastogenesis. |
Effects of MetS Treatment on Bone
Management of MetS using pharmacological agents targeting individual components are commonly implemented. The improvements of glycaemic status, lipid profile and blood pressure of the patients will lead to reduced inflammation and oxidative status of the patients and benefit their bone health. These drugs also exert other pleiotropic effects directly on the skeleton. For example, statins are known to suppress the mevalonate pathway by inhibiting 3-hydroxy-3-methyl-glutaryl-CoA reductase, thereby reducing prenylation of GTPases, which favours bone formation. The different classes of anti-diabetic agents possess distinct actions on bone remodelling. Biguanides, insulin, sulfonylureas, glucagon-like peptide-1 and dipeptidyl peptidase-4 inhibitors promote osteoblast differentiation, while sodium-glucose co-transporter 2 inhibitors and thiazolidinedione enhance bone loss.141 Anti-diabetic agents are often associated with hypoglycaemia, which contributes to increased fracture risk.142 A meta-analysis shows that sulfonylurea increased fracture risk by 14%, which was a rate similar to thiazolidinedione, lower than insulin but higher than metformin.143 The use of thiazide diuretics and beta-blockers are associated with a small benefit in fracture risk reduction.144 Therefore, an understanding of the baseline bone health status of the patients may help to minimize skeletal adverse effects of the drugs.
Weight reduction through diet modification and physical exercise is recommended to patients with MetS and abdominal obesity as per the recommendation of the American Heart Association/National Heart, Lung, and Blood Institute.33 Since mechanical loading is important in maintaining bone health, excess weight reduction might be harmful to the bone. A meta-analysis of randomized controlled trials showed that hip BMD was significantly reduced by weight loss after 4 months, and spine BMD reduction was significant after 13 months. In comparison, weight loss by exercise did not induce BMD reduction.145 Other researchers suggest that the changes in BMD due to weight loss are exaggerated due to variation in the surrounding soft tissue, and the new BMD should be ratioed to the new bodyweight.146 The effects of high impact exercise could offset the loss of mechanical loading by exercise.147 Resistant training could also enhance lean mass and reduce fat mass, which could be beneficial to bone health.147
Bariatric surgery is recommended for morbidly obese patients. A meta-analysis showed that bariatric surgery might be associated with increased PTH, bone turnover and reduced circulating calcium level and BMD.148 Another meta-analysis showed that bariatric surgery increased the risk of total and non-vertebral fractures, especially of the upper arms.149 Comparing two forms of bariatric surgery, circulating PTH is higher and 25(OH)D is lower in patients undergoing gastric bypass compared to sleeve gastrectomy.150 Deficiency of other nutrients may occur, such as protein, folate, vitamin B6, B12 and trace elements.151 Therefore, ensuring sufficient intake of calcium, vitamin D, protein and other micronutrients, as well as exercise, are important steps to counteract the adverse effects of bariatric surgery to bone.151
Conclusion
The relationship between MetS and BMD is complex. After adjusting for the effects of mechanical loading exerted by BMI, the association seems to be negligible or negative. In addition, the relationship may be mediated by sex. The proinflammatory, pro-oxidative and pro-calciuric body environment may contribute to the negative effects of MetS on bone. Improving the metabolic profile of the patients though medications could potentially alleviate the negative effects on BMD. However, excessive weight loss due to MetS management could be detrimental to the bone, and exercises can balance it. In particular, the combination of calorie restriction and exercise can promote a reduction in fat mass while retaining lean and bone mass. Thus, proper management of MetS can benefit not only the cardiovascular system but also the skeletal system.
Acknowledgments
The researchers thank Universiti Kebangsaan Malaysia for providing Fundamental Research Grant (FF-2018-405).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Edwards MH, Dennison EM, Aihie Sayer A, Fielding R, Cooper C. Osteoporosis and sarcopenia in older age. Bone. 2015;80:126–130. doi:10.1016/j.bone.2015.04.016
2. Aspray TJ, Hill TR. Osteoporosis and the ageing skeleton. In: Harris JR, Korolchuk VI, editors. Biochemistry and Cell Biology of Ageing: Part II Clinical Science. Singapore: Springer Singapore; 2019:453–476.
3. Wang H, Ba Y, Xing Q, Du J-L. Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open. 2019;9(1):e024067. doi:10.1136/bmjopen-2018-024067
4. Johansson H, Kanis JA, Odén A, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223–233. doi:10.1002/jbmr.2017
5. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
6. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. doi:10.1038/s41574-019-0176-8
7. Wong SK, Chin K-Y, Suhaimi F, Ahmad F, Ima-Nirwana S. The relationship between metabolic syndrome and osteoporosis: a review. Nutrients. 2016;8(6):347. doi:10.3390/nu8060347
8. Xue P, Gao P, Li Y. The association between metabolic syndrome and bone mineral density: a meta-analysis. Endocrine. 2012;42(3):546–554. doi:10.1007/s12020-012-9684-1
9. Zhou J, Zhang Q, Yuan X, et al. Association between metabolic syndrome and osteoporosis: a meta-analysis. Bone. 2013;57(1):30–35. doi:10.1016/j.bone.2013.07.013
10. Eckstein N, Buchmann N, Demuth I, et al. Association between metabolic syndrome and bone mineral density–data from the Berlin Aging Study II (BASE-II). Gerontology. 2016;62(3):337–344. doi:10.1159/000434678
11. Kim T, Park S, Pak YS, Lee S, Lee EH. Association between metabolic syndrome and bone mineral density in Korea: the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV), 2008. J Bone Miner Metab. 2013;31(6):652–662. doi:10.1007/s00774-013-0459-4
12. Hernández JL, Olmos JM, Pariente E, et al. Metabolic syndrome and bone metabolism: the Camargo Cohort study. Menopause. 2010;17(5):955–961. doi:10.1097/gme.0b013e3181e39a15
13. World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a World Health Organization Study Group. Geneva: World Health Organization; 1994.
14. Park KK, Kim SJ, Moon ES. Association between bone mineral density and metabolic syndrome in postmenopausal Korean women. Gynecol Obstet Invest. 2010;69(3):145–152. doi:10.1159/000264665
15. Grimston SK, Willows ND, Hanley DA. Mechanical loading regime and its relationship to bone mineral density in children. Med Sci Sports Exerc. 1993;25(11):1203–1210. doi:10.1249/00005768-199311000-00002
16. Chin K, Chan C, Subramaniam S, et al. Positive association between metabolic syndrome and bone mineral density among Malaysians. Int J Med Sci. 2020;17(16):2585–2593. doi:10.7150/ijms.49030
17. Kinjo M, Setoguchi S, Solomon DH. Bone mineral density in adults with the metabolic syndrome: analysis in a population-based U.S. sample. J Clin Endocrinol Metab. 2007;92(11):4161–4164. doi:10.1210/jc.2007-0757
18. von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int. 2007;18(10):1337–1344. doi:10.1007/s00198-007-0385-1
19. Wani K, Yakout SM, Ansari MGA, et al. Metabolic syndrome in Arab adults with low bone mineral density. Nutrients. 2019;11(6):1405. doi:10.3390/nu11061405
20. Kim BJ, Ahn SH, Bae SJ, et al. Association between metabolic syndrome and bone loss at various skeletal sites in postmenopausal women: a 3-year retrospective longitudinal study. Osteoporos Int. 2013;24(8):2243–2252. doi:10.1007/s00198-013-2292-y
21. Chan DC, Watts GF, Barrett PH, Burke V. Waist circumference, waist-to-hip ratio and body mass index as predictors of adipose tissue compartments in men. QJM. 2003;96(6):441–447. doi:10.1093/qjmed/hcg069
22. Pasco JA, Holloway KL, Dobbins AG, Kotowicz MA, Williams LJ, Brennan SL. Body mass index and measures of body fat for defining obesity and underweight: a cross-sectional, population-based study. BMC Obes. 2014;1(1):9. doi:10.1186/2052-9538-1-9
23. Kim HY, Kim Y. Associations of obesity with osteoporosis and metabolic syndrome in Korean postmenopausal women: a cross-sectional study using national survey data. Arch Osteoporos. 2019;14(1):64. doi:10.1007/s11657-019-0615-0
24. Chen DZ, Xu QM, Wu XX, et al. The combined effect of nonalcoholic fatty liver disease and metabolic syndrome on osteoporosis in postmenopausal females in Eastern China. Int J Endocrinol. 2018;2018:2314769. doi:10.1155/2018/2314769
25. Hwang DK, Choi HJ. The relationship between low bone mass and metabolic syndrome in Korean women. Osteoporos Int. 2010;21(3):425–431. doi:10.1007/s00198-009-0990-2
26. Kim KC, Shin DH, Lee SY, Im JA, Lee DC. Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med J. 2010;51(6):857–863. doi:10.3349/ymj.2010.51.6.857
27. Jeon YK, Lee JG, Kim SS, et al. Association between bone mineral density and metabolic syndrome in pre- and postmenopausal women. Endocr J. 2011;58(2):87–93. doi:10.1507/endocrj.K10E-297
28. Loke SS, Chang HW, Li WC. Association between metabolic syndrome and bone mineral density in a Taiwanese elderly population. J Bone Miner Metab. 2018;36(2):200–208.
29. Lin HH, Huang CY, Hwang LC. Association between metabolic syndrome and osteoporosis in Taiwanese middle-aged and elderly participants. Arch Osteoporos. 2018;13(1):48. doi:10.1007/s11657-018-0467-z
30. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645.
31. Kim H, Oh HJ, Choi H, Choi WH, Lim SK, Kim JG. The association between bone mineral density and metabolic syndrome: a Korean population-based study. J Bone Miner Metab. 2013;31(5):571–578. doi:10.1007/s00774-013-0446-9
32. National Cholesterol Education Program (NCEP) Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. doi:10.1161/circ.106.25.3143
33. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi:10.1161/CIRCULATIONAHA.105.169404
34. Kim YH, Cho KH, Choi YS, et al. Low bone mineral density is associated with metabolic syndrome in South Korean men but not in women: the 2008–2010 Korean National Health and Nutrition Examination Survey. Arch Osteoporos. 2013;8:142. doi:10.1007/s11657-013-0142-3
35. Chin KY, Ima-Nirwana S. Calcaneal quantitative ultrasound as a determinant of bone health status: what properties of bone does it reflect? Int J Med Sci. 2013;10(12):1778–1783. doi:10.7150/ijms.6765
36. Oo WM, Naganathan V, Bo MT, Hunter DJ. Clinical utilities of quantitative ultrasound in osteoporosis associated with inflammatory rheumatic diseases. Quant Imaging Med Surg. 2018;8(1):100–113. doi:10.21037/qims.2018.02.02
37. Qin Y-X, Lin W, Mittra E, et al. Prediction of trabecular bone qualitative properties using scanning quantitative ultrasound. Acta Astronaut. 2013;92(1):79–88. doi:10.1016/j.actaastro.2012.08.032
38. Langton CM, Langton DK. Comparison of bone mineral density and quantitative ultrasound of the calcaneus: site-matched correlation and discrimination of axial BMD status. Br J Radiol. 2000;73(865):31–35. doi:10.1259/bjr.73.865.10721317
39. Moayyeri A, Adams JE, Adler RA, et al. Quantitative ultrasound of the heel and fracture risk assessment: an updated meta-analysis. Osteoporos Int. 2012;23(1):143–153. doi:10.1007/s00198-011-1817-5
40. Nayak S, Olkin I, Liu H, et al. Meta-analysis: accuracy of quantitative ultrasound for identifying patients with osteoporosis. Ann Intern Med. 2006;144(11):832–841. doi:10.7326/0003-4819-144-11-200606060-00009
41. Subramaniam S, Chan CY, Soelaiman IN, et al. The performance of a calcaneal quantitative ultrasound device, CM-200, in stratifying osteoporosis risk among Malaysian population aged 40 years and above. Diagnostics. 2020;10(4):178. doi:10.3390/diagnostics10040178
42. Bulló M, Garcia-Aloy M, Basora J, Covas MI, Salas-Salvado J. Bone quantitative ultrasound measurements in relation to the metabolic syndrome and type 2 diabetes mellitus in a cohort of elderly subjects at high risk of cardiovascular disease from the PREDIMED study. J Nutr Health Aging. 2011;15(10):939–944. doi:10.1007/s12603-011-0046-0
43. Cvijetic S, Pavlovic M, Pasalic D, Dodig S. Ultrasound bone measurement in an older population with metabolic syndrome. Aging Clin Exp Res. 2011;23(1):29–34. doi:10.1007/BF03324950
44. Hernández JL, Olmos JM, de Juan J, et al. Heel quantitative ultrasound parameters in subjects with the metabolic syndrome: the Camargo Cohort Study. Maturitas. 2011;69(2):162–167. doi:10.1016/j.maturitas.2011.02.017
45. Chin KY, Ima-Nirwana S, Mohamed IN, et al. The association between bone health indicated by calcaneal quantitative ultrasound and metabolic syndrome in Malaysian men. J Diabetes Metab Disord. 2015;14:9. doi:10.1186/s40200-015-0136-3
46. Muka T, Trajanoska K, Kiefte-de Jong JC, et al. The association between metabolic syndrome, bone mineral density, hip bone geometry and fracture risk: the Rotterdam study. PLoS One. 2015;10(6):e0129116. doi:10.1371/journal.pone.0129116
47. Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(3):S116–124. doi:10.1067/mjd.2001.117432
48. Migliaccio S, Greco EA, Fornari R, Donini LM, Lenzi A. Is obesity in women protective against osteoporosis? Diabetes Metab Syndr Obes. 2011;4:273–282. doi:10.2147/DMSO.S11920
49. Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–332. doi:10.1007/s10654-012-9674-x
50. Schneider AL, Williams EK, Brancati FL, Blecker S, Coresh J, Selvin E. Diabetes and risk of fracture-related hospitalization: the atherosclerosis risk in communities study. Diabetes Care. 2013;36(5):1153–1158. doi:10.2337/dc12-1168
51. Wang Y, Dai J, Zhong W, Hu C, Lu S, Chai Y. Association between serum cholesterol level and osteoporotic fractures. Front Endocrinol (Lausanne). 2018;9:30. doi:10.3389/fendo.2018.00030
52. Ahmed LA, Schirmer H, Berntsen GK, Fønnebø V, Joakimsen RM. Features of the metabolic syndrome and the risk of non-vertebral fractures: the Tromsø study. Osteoporos Int. 2006;17(3):426–432. doi:10.1007/s00198-005-0003-z
53. El Maghraoui A, Rezqi A, El Mrahi S, Sadni S, Ghozlani I, Mounach A. Osteoporosis, vertebral fractures and metabolic syndrome in postmenopausal women. BMC Endocr Disord. 2014;14:93. doi:10.1186/1472-6823-14-93
54. Lee SH, Baek S, Ahn SH, et al. Association between metabolic syndrome and incident fractures in Korean men: a 3-year follow-up observational study using national health insurance claims data. J Clin Endocrinol Metab. 2014;99(5):1615–1622. doi:10.1210/jc.2013-3608
55. Yu CY, Chen FP, Chen LW, Kuo SF, Chien RN. Association between metabolic syndrome and bone fracture risk: a community-based study using a fracture risk assessment tool. Medicine (Baltimore). 2017;96(50):e9180. doi:10.1097/MD.0000000000009180
56. Szulc P, Varennes A, Delmas PD, Goudable J, Chapurlat R. Men with metabolic syndrome have lower bone mineral density but lower fracture risk–the MINOS study. J Bone Miner Res. 2010;25(6):1446–1454. doi:10.1002/jbmr.13
57. Sun M, Cao M, Fu Q, et al. Association of calcaneal quantitative ultrasound parameters with metabolic syndrome in middle-aged and elderly Chinese: a large population-based cross-sectional study. BMC Endocr Disord. 2014;14:14. doi:10.1186/1472-6823-14-14
58. Qin L, Yang Z, Zhang W, et al. Metabolic syndrome and osteoporotic fracture: a population-based study in China. BMC Endocr Disord. 2016;16(1):27. doi:10.1186/s12902-016-0106-x
59. Wang D, Liu N, Gao Y, Li P, Tian M. Association between metabolic syndrome and osteoporotic fracture in middle-aged and elderly Chinese peoples. Cell Biochem Biophys. 2014;70(2):1297–1303. doi:10.1007/s12013-014-0054-x
60. Marcellini S, Henriquez JP, Bertin A. Control of osteogenesis by the canonical Wnt and BMP pathways in vivo: cooperation and antagonism between the canonical Wnt and BMP pathways as cells differentiate from osteochondroprogenitors to osteoblasts and osteocytes. Bioessays. 2012;34(11):953–962. doi:10.1002/bies.201200061
61. Rucci N. Molecular biology of bone remodelling. Clin Cases Miner Bone Metab. 2008;5(1):49–56.
62. Siddiqui JA, Partridge NC. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology (Bethesda, Md). 2016;31(3):233–245.
63. Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen. 2020;40:2.
64. Shetty S, Kapoor N, Bondu JD, Thomas N, Paul TV. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocrinol Metab. 2016;20(6):846–852. doi:10.4103/2230-8210.192914
65. Oliva-Olivera W, Leiva Gea A, Lhamyani S, et al. Differences in the osteogenic differentiation capacity of omental adipose-derived stem cells in obese patients with and without metabolic syndrome. Endocrinology. 2015;156(12):4492–4501. doi:10.1210/en.2015-1413
66. Lerchbaum E, Schwetz V, Nauck M, Völzke H, Wallaschofski H, Hannemann A. Lower bone turnover markers in metabolic syndrome and diabetes: the population-based Study of Health in Pomerania. Nutr Metab Cardiovasc Dis. 2015;25(5):458–463. doi:10.1016/j.numecd.2015.02.002
67. Gannagé-Yared MH, Fares F, Semaan M, Khalife S, Jambart S. Circulating osteoprotegerin is correlated with lipid profile, insulin sensitivity, adiponectin and sex steroids in an ageing male population. Clin Endocrinol (Oxf). 2006;64(6):652–658. doi:10.1111/j.1365-2265.2006.02522.x
68. Nabipour I, Kalantarhormozi M, Larijani B, Assadi M, Sanjdideh Z. Osteoprotegerin in relation to type 2 diabetes mellitus and the metabolic syndrome in postmenopausal women. Metabolism. 2010;59(5):742–747. doi:10.1016/j.metabol.2009.09.019
69. Pérez de Ciriza C, Moreno M, Restituto P, et al. Circulating osteoprotegerin is increased in the metabolic syndrome and associates with subclinical atherosclerosis and coronary arterial calcification. Clin Biochem. 2014;47(18):272–278. doi:10.1016/j.clinbiochem.2014.09.004
70. Bernardi S, Fabris B, Thomas M, et al. Osteoprotegerin increases in metabolic syndrome and promotes adipose tissue proinflammatory changes. Mol Cell Endocrinol. 2014;394(1–2):13–20. doi:10.1016/j.mce.2014.06.004
71. Bonewald LF. The role of the osteocyte in bone and nonbone disease. Endocrinol Metab Clin North Am. 2017;46(1):1–18. doi:10.1016/j.ecl.2016.09.003
72. Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94(1):25–34. doi:10.1007/s00223-013-9774-y
73. Ali A, Ali A, Ahmad W, et al. Deciphering the role of WNT signaling in metabolic syndrome-linked Alzheimer’s disease. Mol Neurobiol. 2020;57(1):302–314.
74. Abou Ziki MD, Mani A. The interplay of canonical and noncanonical Wnt signaling in metabolic syndrome. Nutr Res. 2019;70:18–25. doi:10.1016/j.nutres.2018.06.009
75. Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107(2):519–527. doi:10.1111/j.1432-1033.1980.tb06059.x
76. Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–33140. doi:10.1074/jbc.M500608200
77. Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. doi:10.1016/j.bone.2016.10.007
78. Wong SK, Chin KY, Ima-Nirwana S. The effects of tocotrienol on bone peptides in a rat model of osteoporosis induced by metabolic syndrome: the possible communication between bone cells. Int J Environ Res Public Health. 2019;16(18):3313. doi:10.3390/ijerph16183313
79. Stoian M, Stoica V. The role of disturbances of phosphate metabolism in metabolic syndrome. Maedica. 2014;9(3):255–260.
80. Jüppner H. Phosphate and FGF-23. Kidney Int Suppl. 2011;79(121):S24–S27. doi:10.1038/ki.2011.27
81. Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293(5):F1577–1583. doi:10.1152/ajprenal.00463.2006
82. Mirza MAI, Alsiö J, Hammarstedt A, et al. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler Thromb Vasc Biol. 2011;31(1):219–227. doi:10.1161/ATVBAHA.110.214619
83. Gateva A, Assyov Y, Tsakova A, Kamenov ZJH. metabolisme mrH-uSHe. Prediabetes is characterized by higher FGF23 levels and higher prevalence of vitamin D deficiency compared to normal glucose tolerance subjects. Horm Metab Res. 2019;51(2):106–111. doi:10.1055/a-0813-3164
84. Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. doi:10.1186/1749-799X-6-30
85. Purohit A, Reed MJ. Regulation of estrogen synthesis in postmenopausal women. Steroids. 2002;67(12):979–983. doi:10.1016/S0039-128X(02)00046-6
86. Hetemäki N, Savolainen-Peltonen H, Tikkanen MJ, et al. Estrogen metabolism in abdominal subcutaneous and visceral adipose tissue in postmenopausal women. J Clin Endocrinol Metab. 2017;102(12):4588–4595. doi:10.1210/jc.2017-01474
87. Nishimura S, Manabe I, Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med. 2009;8(41):55–60.
88. Jonas MI, Kurylowicz A, Bartoszewicz Z, et al. Interleukins 6 and 15 levels are higher in subcutaneous adipose tissue, but obesity is associated with their increased content in visceral fat depots. Int J Mol Sci. 2015;16(10):25817–25830. doi:10.3390/ijms161025817
89. Agidigbi TS, Kim C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int J Mol Sci. 2019;20(14):3576. doi:10.3390/ijms20143576
90. Migliaccio S, Di Nisio A, Mele C, et al. Obesity and hypovitaminosis D: causality or casualty? Int J Obes Suppl. 2019;9(1):20–31. doi:10.1038/s41367-019-0010-8
91. Di Nisio A, Sabovic I, De Toni L, et al. Testosterone is sequestered in dysfunctional adipose tissue, modifying androgen-responsive genes. Int J Obes (Lond). 2020;44(7):1617–1625. doi:10.1038/s41366-020-0568-9
92. Zhang M, Li P, Zhu Y, et al. Higher visceral fat area increases the risk of vitamin D insufficiency and deficiency in Chinese adults. Nutr Metab (Lond). 2015;12:50. doi:10.1186/s12986-015-0046-x
93. Khan M, Joseph F. Adipose tissue and adipokines: the association with and application of adipokines in obesity. Scientifica. 2014;2014:328592. doi:10.1155/2014/328592
94. Reid IR, Baldock PA, Cornish J. Effects of leptin on the skeleton. Endocr Rev. 2018;39(6):938–959. doi:10.1210/er.2017-00226
95. Liu K, Liu P, Liu R, Wu X, Cai M. Relationship between serum leptin levels and bone mineral density: a systematic review and meta-analysis. Clin Chim Acta. 2015;444:260–263. doi:10.1016/j.cca.2015.02.040
96. Biver E, Salliot C, Combescure C, et al. Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(9):2703–2713. doi:10.1210/jc.2011-0047
97. Pal China S, Sanyal S, Chattopadhyay N. Adiponectin signaling and its role in bone metabolism. Cytokine. 2018;112:116–131. doi:10.1016/j.cyto.2018.06.012
98. Jia P, Bao L, Chen H, et al. Risk of low-energy fracture in type 2 diabetes patients: a meta-analysis of observational studies. Osteoporos Int. 2017;28(11):3113–3121.
99. Dragsbæk K, Neergaard JS, Laursen JM, et al. Metabolic syndrome and subsequent risk of type 2 diabetes and cardiovascular disease in elderly women: challenging the current definition. Medicine. 2016;95(36):e4806–e4806. doi:10.1097/MD.0000000000004806
100. Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194–222. doi:10.3390/biom5010194
101. Suzuki R, Fujiwara Y, Saito M, et al. Intracellular accumulation of advanced glycation end products induces osteoblast apoptosis via endoplasmic reticulum stress. J Bone Miner Res. 2020. doi:10.1002/jbmr.4053
102. Li Z, Li C, Zhou Y, et al. Advanced glycation end products biphasically modulate bone resorption in osteoclast-like cells. Am J Physiol Endocrinol Metab. 2016;310(5):E355–366. doi:10.1152/ajpendo.00309.2015
103. Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun. 2015;461(2):193–199. doi:10.1016/j.bbrc.2015.02.091
104. Samakkarnthai P, Sfeir JG, Atkinson EJ, et al. SUN-LB68 advanced glycation endproducts are associated with worse bone material strength in older adults with and without type 2 diabetes. J Endocrine Soc. 2020;4(Supplement_1). doi:10.1210/jendso/bvaa046.2045.
105. Samakkarnthai P, Sfeir JG, Atkinson EJ, et al. Determinants of bone material strength and cortical porosity in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2020;105(10). doi:10.1210/clinem/dgaa388.
106. Moseley KF, Chia CW, Simonsick EM, Egan JM, Ferrucci L, Sellmeyer DE. Sex-specific differences in progressive glucose intolerance and hip geometry: the Baltimore longitudinal study of aging. Osteoporos Int. 2015;26(5):1555–1562. doi:10.1007/s00198-015-3027-z
107. Kim TY, Schafer AL. Diabetes and Bone Marrow Adiposity. Curr Osteoporos Rep. 2016;14(6):337–344.
108. Zhang N, Jiang H, Bai Y, et al. The molecular mechanism study of insulin on proliferation and differentiation of osteoblasts under high glucose conditions. Cell Biochem Funct. 2019;37(5):385–394. doi:10.1002/cbf.3415
109. Burghardt AJ, Issever AS, Schwartz AV, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(11):5045–5055. doi:10.1210/jc.2010-0226
110. Trierweiler H, Kisielewicz G, Hoffmann Jonasson T, Rasmussen Petterle R, Aguiar Moreira C, Zeghbi Cochenski Borba V. Sarcopenia: a chronic complication of type 2 diabetes mellitus. Diabetol Metab Syndr. 2018;10(1):25. doi:10.1186/s13098-018-0326-5
111. Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10(3):485–500.
112. Caudarella R, Vescini F, Rizzoli E, Francucci CM. Salt intake, hypertension, and osteoporosis. J Endocrinol Invest. 2009;32(4Suppl):15–20.
113. Cruz DN. The renal tubular Na-Cl co-transporter (NCCT): a potential genetic link between blood pressure and bone density? Nephrol Dial Transplant. 2001;16(4):691–694. doi:10.1093/ndt/16.4.691-a
114. Itoh T, Fairall L, Amin K, et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol. 2008;15(9):924–931. doi:10.1038/nsmb.1474
115. Zhuang H, Zhang X, Zhu C, et al. Molecular mechanisms of PPAR-γ governing MSC osteogenic and adipogenic differentiation. Curr Stem Cell Res Ther. 2016;11(3):255–264. doi:10.2174/1574888X10666150531173309
116. Mazière C, Savitsky V, Galmiche A, Gomila C, Massy Z, Mazière JC. Oxidized low density lipoprotein inhibits phosphate signaling and phosphate-induced mineralization in osteoblasts. Involvement of oxidative stress. Biochim Biophys Acta. 2010;1802(11):1013–1019. doi:10.1016/j.bbadis.2010.07.010
117. Brodeur MR, Brissette L, Falstrault L, Ouellet P, Moreau R. Influence of oxidized low-density lipoproteins (LDL) on the viability of osteoblastic cells. Free Radic Biol Med. 2008;44(4):506–517. doi:10.1016/j.freeradbiomed.2007.08.030
118. Mazière C, Salle V, Gomila C, Mazière JC. Oxidized low density lipoprotein enhanced RANKL expression in human osteoblast-like cells. Involvement of ERK, NFkappaB and NFAT. Biochim Biophys Acta. 2013;1832(10):1756–1764. doi:10.1016/j.bbadis.2013.05.033
119. Dawodu D, Patecki M, Dumler I, Haller H, Kiyan Y. oxLDL inhibits differentiation of mesenchymal stem cells into osteoblasts via the CD36 mediated suppression of Wnt signaling pathway. Mol Biol Rep. 2019;46(3):3487–3496. doi:10.1007/s11033-019-04735-5
120. Dawodu D, Patecki M, Hegermann J, Dumler I, Haller H, Kiyan Y. oxLDL inhibits differentiation and functional activity of osteoclasts via scavenger receptor-A mediated autophagy and cathepsin K secretion. Sci Rep. 2018;8(1):11604. doi:10.1038/s41598-018-29963-w
121. Weivoda MM, Oursler MJ. The roles of small GTPases in osteoclast biology. Curr Res. 2014;3:1000161.
122. Wan Hasan WN, Chin KY, Jolly JJ, Abd Ghafar N, Soelaiman IN. Identifying potential therapeutics for osteoporosis by exploiting the relationship between mevalonate pathway and bone metabolism. Endocr Metab Immune Disord Drug Targets. 2018;18(5):450–457. doi:10.2174/1871530318666180423122409
123. Brodeur MR, Brissette L, Falstrault L, Moreau R. HDL3 reduces the association and modulates the metabolism of oxidized LDL by osteoblastic cells: a protection against cell death. J Cell Biochem. 2008;105(6):1374–1385. doi:10.1002/jcb.21938
124. Eren E, Ellidag HY, Aydin O, Yılmaz N. HDL-associated paraoxonase 1 as a bridge between postmenopausal osteoporosis and cardiovascular disease. Chonnam Med J. 2014;50(3):75–81. doi:10.4068/cmj.2014.50.3.75
125. Inoue M, Niki M, Ozeki Y, et al. High-density lipoprotein suppresses tumor necrosis factor alpha production by mycobacteria-infected human macrophages. Sci Rep. 2018;8(1):6736. doi:10.1038/s41598-018-24233-1
126. Singh A, Mehdi AA, Srivastava RN, Verma NS. Immunoregulation of bone remodelling. Int J Crit Illn Inj Sci. 2012;2(2):75–81. doi:10.4103/2229-5151.97271
127. Wang X, Chen B, Sun J, et al. Iron-induced oxidative stress stimulates osteoclast differentiation via NF-κB signaling pathway in mouse model. Metabolism. 2018;83:167–176. doi:10.1016/j.metabol.2018.01.005
128. Zhao B. TNF and bone remodeling. Curr Osteoporos Rep. 2017;15(3):126–134. doi:10.1007/s11914-017-0358-z
129. Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11(3):234–250.
130. Boyce BF, Xiu Y, Li J, Xing L, Yao Z. NF-κB-mediated regulation of osteoclastogenesis. Endocrinol Metab. 2015;30(1):35–44. doi:10.3803/EnM.2015.30.1.35
131. Krum SA, Chang J, Miranda-Carboni G, Wang C-Y. Novel functions for NFκB: inhibition of bone formation. Nat Rev Rheumatol. 2010;6(10):607–611. doi:10.1038/nrrheum.2010.133
132. Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9(2):119. doi:10.1038/s41419-017-0135-z
133. Vona R, Gambardella L, Cittadini C, Straface E, Pietraforte D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid Med Cell Longev. 2019;2019:8267234. doi:10.1155/2019/8267234
134. Biswas SK. Does the Interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;2016:5698931. doi:10.1155/2016/5698931
135. Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT. Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab. 2017;14(2):209–216. doi:10.11138/ccmbm/2017.14.1.209
136. Zhang B, Xie Q-Y, Quan Y, Pan X-M, Liao D-F. Reactive oxygen species induce cell death via Akt signaling in rat osteoblast-like cell line ROS 17/2.8. Toxicol Ind Health. 2015;31(12):1236–1242. doi:10.1177/0748233713491801
137. Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginian population. Int J Med Sci. 2016;13(1):25–38. doi:10.7150/ijms.13800
138. de Pablo P, Cooper MS, Buckley CD. Association between bone mineral density and C-reactive protein in a large population-based sample. Arthritis Rheum. 2012;64(8):2624–2631. doi:10.1002/art.34474
139. Ilesanmi-Oyelere BL, Schollum L, Kuhn-Sherlock B, et al. Inflammatory markers and bone health in postmenopausal women: a cross-sectional overview. Immun Ageing. 2019;16:15. doi:10.1186/s12979-019-0155-x
140. Gulyás K, Horváth Á, Végh E, et al. Effects of 1-year anti-TNF-α therapies on bone mineral density and bone biomarkers in rheumatoid arthritis and ankylosing spondylitis. Clin Rheumatol. 2020;39(1):167–175. doi:10.1007/s10067-019-04771-3
141. Adil M, Khan RA, Kalam A, et al. Effect of anti-diabetic drugs on bone metabolism: evidence from preclinical and clinical studies. Pharmacol Rep. 2017;69(6):1328–1340. doi:10.1016/j.pharep.2017.05.008
142. Hung YC, Lin CC, Chen HJ, et al. Severe hypoglycemia and hip fracture in patients with type 2 diabetes: a nationwide population-based cohort study. Osteoporos Int. 2017;28(7):2053–2060. doi:10.1007/s00198-017-4021-4
143. Zhang Z, Cao Y, Tao Y, et al. Sulfonylurea and fracture risk in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Res Clin Pract. 2020;159:107990. doi:10.1016/j.diabres.2019.107990
144. Wiens M, Etminan M, Gill SS, Takkouche B. Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. J Intern Med. 2006;260(4):350–362. doi:10.1111/j.1365-2796.2006.01695.x
145. Soltani S, Hunter GR, Kazemi A, Shab-Bidar S. The effects of weight loss approaches on bone mineral density in adults: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2016;27(9):2655–2671.
146. Seimon RV, Wild-Taylor AL, Keating SE, et al. Effect of weight loss via severe vs moderate energy restriction on lean mass and body composition among postmenopausal women with obesity: the TEMPO diet randomized clinical trial. JAMA Netw Open. 2019;2(10):e1913733–e1913733. doi:10.1001/jamanetworkopen.2019.13733
147. Hunter GR, Plaisance EP, Fisher G. Weight loss and bone mineral density. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):358–362. doi:10.1097/MED.0000000000000087
148. Liu C, Wu D, Zhang JF, et al. Changes in bone metabolism in morbidly obese patients after bariatric surgery: a meta-analysis. Obes Surg. 2016;26(1):91–97. doi:10.1007/s11695-015-1724-5
149. Zhang Q, Chen Y, Li J, et al. A meta-analysis of the effects of bariatric surgery on fracture risk. Obes Rev. 2018;19(5):728–736. doi:10.1111/obr.12665
150. Tian Z, Fan XT, Li SZ, Zhai T, Dong J. Changes in bone metabolism after sleeve gastrectomy versus gastric bypass: a meta-analysis. Obes Surg. 2020;30(1):77–86. doi:10.1007/s11695-019-04119-5
151. Ben-Porat T, Elazary R, Sherf-Dagan S, et al. Bone health following bariatric surgery: implications for management strategies to attenuate bone loss. Adv Nutr. 2018;9(2):114–127.
152. Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Med. 1998;15(7):539–553. doi:10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
153. Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. doi:10.1001/jama.285.19.2486
154. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi:10.1016/S0140-6736(05)67402-8
155. Kim HY, Choe JW, Kim HK, et al. Negative association between metabolic syndrome and bone mineral density in Koreans, especially in men. Calcif Tissue Int. 2010;86(5):350–358. doi:10.1007/s00223-010-9347-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

