Back to Journals » International Journal of General Medicine » Volume 14
Relationship Between Blood Glucose and Hemoglobin A1c Levels and Urinary Incontinence in Women
Authors Liu N, Xing L, Mao W, Chen S, Wu J, Xu B, Chen M
Received 15 June 2021
Accepted for publication 21 July 2021
Published 31 July 2021 Volume 2021:14 Pages 4105—4116
DOI https://doi.org/10.2147/IJGM.S324332
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Ning Liu,1 Li Xing,1 Weipu Mao,1– 3 Shuqiu Chen,1 Jianping Wu,1 Bin Xu,1 Ming Chen1– 3
1Department of Urology, Affiliated Zhongda Hospital of Southeast University, Nanjing, 210009, People’s Republic of China; 2Surgical Research Center, Institute of Urology, Southeast University Medical School, Nanjing, 210009, People’s Republic of China; 3Department of Urology, Nanjing Lishui District People’s Hospital, Zhongda Hospital Lishui Branches, Southeast University, Nanjing, 211200, People’s Republic of China
Correspondence: Ming Chen; Weipu Mao
Department of Urology, Affiliated Zhongda Hospital of Southeast University, No. 87 Dingjiaqiao, Hunan Road, Gulou District, Nanjing, 210009, People’s Republic of China
Tel/Fax +86-13913009977
; +86-18621571327
Email [email protected]; [email protected]
Objective: The purpose of this study was to evaluate the associations between blood glucose and hemoglobin A1c (HbA1c) levels with the degree of stress urinary incontinence (SUI) and urgency urinary incontinence (UUI) in women.
Methods: We conducted a cross-sectional study of female participants in the National Health and Nutrition Examination Survey (NHANES) database between 2007 and 2012. Univariate and multivariate logistic regressions were used to assess the relationship between blood glucose and HbA1c levels and the degree of SUI and UUI.
Results: A total of 3821 participants were enrolled in the study, of whom 2421 (63.4%) had no SUI, 1133 (29.7%) had monthly SUI, 267 (7.0%) had weekly SUI; 2883 (75.5%) had no UUI, 735 (19.2%) had monthly UUI, 203 (5.3%) had weekly UUI. The levels of blood glucose and HbA1c were positively correlated with SUI and UUI, and increased with increasing degree of UUI. Multivariate logistic regression showed that there was a positive association between blood HbA1c level and degree of SUI.
Conclusion: Our study found that blood glucose and HbA1c levels can be used as indicators of SUI and UUI severity in women.
Keywords: blood glucose, hemoglobin A1c, national health and nutrition examination survey, stress urinary incontinence, urgency urinary incontinence
Introduction
Urinary incontinence (UI) is one of the most common pelvic floor diseases affecting women’s health, and it is very common in women.1 The estimated prevalence of UI ranges from 10% to 40%, with the prevalence of about 10% in women aged 15–19 years, 30–40% in perimenopausal women, and more than 50% in women aged 70 years or older, with a steadily increasing prevalence.2,3 It was defined as a disease that “constitutes a social and health problem, and it can be objectively confirmed that there is involuntary urine outflow” by The International Continence Society (ICS).4 The prevalence of UI is high in the community and it increases with age. In particular, there is a greater proportion with prevalence of UI in elderly patients in nursing homes or patients with dementia or cognitive impairment.5,6 UI not only damages patients’ physical function but also has a serious negative impact on patients’ psychological state and quality of life.7,8
UI was divided into three types: stress urinary incontinence (SUI), urgency urinary incontinence (UUI) and mixed urinary incontinence (MUI). And also, MUI was accompanied by the symptoms of SUI and UUI. SUI, also known as active UI, is involuntary incontinence due to exertion, physical activity, sneezing or coughing in the absence of bladder contraction. UUI is involuntary incontinence, which can occur when strong, sudden voiding causes bladder contractions or spasms. And, MUI is an involuntary incontinence associated with urgency, physical effort, sneezing or coughing.9,10 Although UI is not life-threatening, its hidden troubles have seriously affected patients’ normal social activities, physical exercise and sexual life.11–13 Due to insufficient understanding, few affected women seek help. So, UI has gradually become a health burden bothering women and the elderly.14
The development of UI in women is affected by many factors. Current research shows that the possible risk factors include family history, age, delivery mode, smoking, obesity and coexisting medical conditions, such as diabetes.15–18 UI is common in older adults with diabetes, and they require home health care services.19 Some studies have shown that gestational diabetes increases the risk of UI in the 2 years after delivery.20 And there is also evidence that the incidence of UI in women with type 2 diabetes is 50% to 200% higher than in women with normal blood glucose levels.21 Little is known about the effect of diabetes control on the severity of UI in women. The purpose of this study was to determine whether the levels of blood glucose and hemoglobin A1c (HbA1c) were related to the severity of UI in women.
Methods
Patients Selection
The analysis was performed using the 2007–2012 National Health and Nutrition Examination Survey (NHANES) database (www.cdc.gov/nchs/nhanes/index.htm). NHANES is a nationally representative US database collected using a multi-stage hierarchical probabilistic clustering design to assess the health and nutritional status of noninstitutionalized civilians.
A total of 15,265 women participated in the study during the period 2007–2012, of which 6354 had complete survey data. The exclusion criteria were as follows: a) incomplete SUI/UUI survey (n = 7636); b) incomplete SUI/UUI degree (n = 9); c) unknown glucose (n = 444); d) unknown HbA1c (n = 84); e) unknown family poverty ratio (n = 592); f) unknown blood urea nitrogen/creatinine/uric acid (n = 108); g) unknown education levels (n = 38); h) comorbid neurological diseases (n = 23); i) obese participants (n = 2510).
As public data files were used, further ethical review by the Ethics Committee of the Affiliated Zhongda Hospital of Southeast University was not required for these analyses.
Assessments
Blood glucose and HbA1c levels were the main predictors of the measurements in this study. Blood glucose was measured using the hexokinase reference method and calibrated using a regression equation recommended by the National Cancer Research Center.22 HbA1c was calculated in whole blood samples using an automatic glycated hemoglobin analyzer and calibrated using an equal percentage equivalent method.23 We divided our blood glucose levels into three tertiles (<86 mg/dL, 86–97 mg/dL and >97 mg/dL). For HbA1c levels, we defined that less than 5.7 was normal, 5.7–6.5 was pre-diabetes and 6.5 or greater was diabetes mellitus.24 Other covariates included age (20–39 years, 40–59 years and 60+ years), race (Non-Hispanic white, Non-Hispanic black, Mexican American, and others), marital status (married, unmarried and others), education level (less than high school, high school or equivalent, college or above), participation in moderate or vigorous recreational activities (yes or no), blood urea nitrogen, creatinine, uric acid and estimated glomerular filtration rate (eGFR).25 Hypertension and diabetes were defined after being diagnosed by doctors.
UI was assessed for effective self-reporting problems used in previous studies.26 We define SUI as leaked or lost control of even a small amount of urine with an activity like coughing, lifting or exercise during the past 12 months. UUI is defined as leaked or lost control of even a small amount of urine with an urge or pressure to urinate and could not get to the toilet fast enough. In terms of clinical significance, we selected weekly or monthly episodes of UI as our primary outcome.
Statistical Analysis
All analyses were carried out using R version 3.5.3 (http://www.r-project.org/) and SPSS software (version 24.0). Mean ± standard deviation was used to describe the distribution of continuous variables, and proportion was used to calculate the distribution of classified variables. For categorical variables, P values were analyzed by chi-square tests. For continuous variables, the t-test for slope was used in generalized linear models. Risk factors for UI were assessed using a logistic regression model and calculated adjusted odds ratios (aOR) and 95% confidence intervals (CI). We constructed three logical regression models to evaluate the effect of blood glucose on the severity of UI in women. In the basic model, we adjusted the age, gender, marital status, education and family poverty ratio of the participants. Subsequently, taking into account the living conditions of the participants, we further adjusted diabetes, hypertension, and vigorous and moderate recreational activities in the core model. Finally, in the extended model, we adjusted blood urea nitrogen, creatinine, uric acid and eGFR. All statistical analyses with P-value ≤0.05 (two-sided) was considered statistically significant.
Results
General Characteristics of All Participants
According to the screening criteria in Figure 1, we included 3821 eligible participants for SUI and UUI in the NHANES database from 2007 to 2012, of whom 2421 (63.4%) had no SUI, 1133 (29.7%) had monthly SUI, 267 (7.0%) had weekly SUI; 2883 (75.5%) had no UUI, 735 (19.2%) had monthly UUI, 203 (5.3%) had weekly UUI. Tables 1 and 2 show the characteristics of female survey respondents in each category. The chi-square test in Table 1 shows significant differences in SUI severity for all variables (All p < 0.05), and Table 2 shows that the severity of UUI is significantly different for all variables except marital status (All p < 0.05). The proportion of ≥60 years of age increased with the degree of SUI (no SUI: 29.4% vs monthly SUI: 35.0% vs weekly SUI: 45.3%), and the same trend applied to the UUI group (no SUI: 25.5% vs monthly SUI: 48.6% vs weekly SUI: 67.0%). We also found that in participants with diabetic and pre-diabetic, the degree of SUI and UUI intensified as HbA1c and blood glucose levels increased.
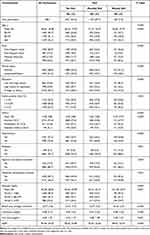 |
Table 1 Baseline Characteristics of All Participants According to SUI in Our Study |
 |  |  |
Table 2 Baseline Characteristics of All Participants According to UUI in Our Study |
 |
Figure 1 Schematic flow diagram of inclusion and exclusion criteria for our study cohort. |
Association Between Glucose, HbA1c and SUI or UUI
Subsequently, we focused on the levels of glucose and HbA1c in SUI and UUI participants. Figure 2 reveals that the levels of blood glucose (93.44±29.89, 94.12±25.17, 101.39±33.73) and HbA1c (5.50±0.86, 5.61±0.85, 5.72±0.93) increased gradually in the no SUI group, monthly SUI group and weekly SUI group, and this trend was also seen in the UUI (glucose: 92.52±26.76, 97.93±32.64, 104.53±39.26; HbA1c: 5.47±0.81, 5.74±0.97, 5.88±0.98), all of which were statistically significant. In addition, we found that the proportion of the group with blood glucose >97 mg/dL and HbA1c ≥6.5 increased with increasing degree of SUI and UUI (Figure 3).
 |
Figure 2 The values of glucose and HbA1c in SUI and UUI groups. (A) Glucose in SUI group; (B) HbA1c in SUI group; (C) Glucose in UUI group; (D) HbA1c in UUI group. **P<0.01; ****P<0.001. |
 |
Figure 3 The proportion of glucose and HbA1c in SUI and UUI groups. (A). Glucose in SUI group; (B) HbA1c in SUI group; (C) Glucose in UUI group; (D) HbA1c in UUI group. |
Risk Factors for Patients with SUI and UUI
Univariate regression analysis showed that blood glucose and HbA1c levels were positively associated with the degree of SUI and UUI, and that the degree of SUI and UUI increased with increasing blood glucose and HbA1c levels (Table 3). In addition, three multivariate logistic regression models were constructed to assess the association of blood glucose and HbA1c with the degree of SUI and UUI (Figure 4 and Table 3). The results showed that blood HbA1c was positively associated with the severity of SUI in both the base model (prediabetes vs normal: aOR 1.246, 95% CI=1.052–1.476, p=0.042; diabetes mellitus vs normal: aOR 1.376, 95% CI=1.012–1.870, p=0.011), core model (diabetes mellitus vs normal: aOR 1.379, 95% CI=1.048–1.994, p=0.013) and extended model (diabetes mellitus vs normal: aOR 1.320, 95% CI=1.034–1.914, p=0.019) (Table 3).
 |
Table 3 Relative Risk of Degree of SUI and UUI Were Calculated According to Glucose and HbA1c Levels a |
 |
Figure 4 Forest plots of the different glucose and HbA1c groups with the degree of SUI and UUI. (A) Glucose in SUI group; (B) HbA1c in SUI group; (C) Glucose in UUI group; (D) HbA1c in UUI group. |
Discussion
Our study revealed that blood glucose and HbA1c levels could be used as indicators of SUI and UUI severity in women. In the study of the NHANES 2007–2012 sample, nearly two out of five women reported weekly or monthly UI. As blood glucose and HbA1c levels increased, the more severe SUI and UUI became. Our study looked at the effect of blood glucose and HbA1c levels on SUI and UUI, and this study may provide guidance for the control of UI.
UI refers to the involuntary leakage of urine through the urethra, which is caused by excessive bladder pressure and/or excessively low urethral pressure. The older you are, the higher the incidence is, and it is more common in women than men.27 As UI is not a life-threatening disease, the prognosis is relatively good.28,29 Epidemiological studies have shown that one in three women has varying degrees of UI, more than half of whom are SUI. At present, there are many factors that induce female UI, such as age, overweight, family history, menopause, delivery and delivery times. Among which diabetes is the most important factor.30–32
With the increase in age, the bladder’s ability to store urine will decrease, and the bladder will contract more frequently, which will easily lead to UI. In Dr. Yuko Komesu’s study, women <65 years of age had a 7.49 (95% CI, −3.23 to −11.74) score reduction in the short-form symptom distress score of the overactive bladder questionnaire compared with women ≥65 years of age, regardless of treatment group. Young women are more likely to control UI, improve symptoms quickly, and have fewer urinary tract infections.33 In our study, it was also found that the severity of SUI and UUI increased with age in people over 60 years old (29.4% vs 35.0% vs 45.3%, 25.5% vs 48.6% vs 67.0%). Based on the findings from previous studies, the occurrence of UI in the marital status of elderly women (P = 0.001) had statistical significance.34 As found in our study, married people suffered weekly less from SUI or UUI than unmarried/others. This phenomenon may benefit from the careful care, financial support and psychological counseling of the partners in the marriage.
Previous studies have shown that the prevalence of UI in diabetic women is increasing.32,35,36 By analyzing data from 7270 women in the NHANES database, Wang et al37 found a 13% increased risk of UI for each one-unit increase in HbA1c, including a 34% increased risk of SUI, but no association with UUI or MUI. Lee et al38 investigated data from 6026 older women and found that HbA1c levels were not associated with the presence or absence of UI, but among women with UI, poor glycemic control (HbA1c ≥9%) was associated with more restriction of daily activities. Blood glucose and HbA1c levels were the focus of the study. However, in our study, we found a direct correlation between blood glucose and HbA1c and severity of UI, and increases in blood glucose and HbA1c levels may increase the severity of UI, both in SUI and UUI. This suggests that good glucose management should be considered as an effective tool for UI prevention. For women with UI, improved glycemic control is often advocated as a means to improve the symptoms and frequency of UI. In this regard, monitoring of blood glucose and HbA1c is more important. Poor glycemic control (glucose >97 mg/dL) and HbA1c (>6.5) control may exacerbate the symptoms and frequency of pre-existing UI. Since UI is a common condition in women, better glycemic control (glucose <86 mg/dL) and HbA1c (<5.7) control may reduce the occurrence of UI.
There are also shortcomings in our research. First, the data used in this study were cross-sectional and lacked prospective studies. Second, the presence or absence of UI was based on participants’ self-report and lacked objective measures. In addition, we were unable to obtain treatment-related information for UI participants.
In conclusion, increasing blood glucose and HbA1c levels are associated with SUI and UUI severity in women. This indicates that lifestyle intervention in this high-risk group is helpful to improve the severity of UI in the future.
Data Sharing Statement
The datasets are available in the NHANES repository and can be obtained from www.cdc.gov/nchs/nhanes/index.htm.
Ethical Approval
The present study was complied with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards (as revised in 2013). This study used previously collected deidentified data, which was deemed exempt from review by the Ethics Committee of the Affiliated Zhongda Hospital of Southeast University.
Acknowledgments
The authors are grateful for the invaluable support and useful discussions with other members of the urological department.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval for the version to be published, and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (81572517 to Bin Xu, 82070773 to Shuqiu Chen), the Scientific Research Foundation of Graduate School of Southeast University (YBPY2173 to Weipu Mao), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_0156), Jiangsu Provincial Key Research and Development Program (BE2019751 to Ming Chen), Innovative Team of Jiangsu Provincial (2017XKJQW07 to Ming Chen), and The National Key Research and Development Program of China (SQ2017YFSF090096 to Ming Chen).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Almousa S, Bandin van Loon A. The prevalence of urinary incontinence in nulliparous adolescent and middle-aged women and the associated risk factors: a systematic review. Maturitas. 2018;107:78–83. doi:10.1016/j.maturitas.2017.10.003
2. Troko J, Bach F, Toozs-Hobson P. Predicting urinary incontinence in women in later life: a systematic review. Maturitas. 2016;94:110–116. doi:10.1016/j.maturitas.2016.09.006
3. Lamerton TJ, Torquati L, Brown WJ. Overweight and obesity as major, modifiable risk factors for urinary incontinence in young to mid-aged women: a systematic review and meta-analysis. Obes Rev. 2018;19(12):1735–1745. doi:10.1111/obr.12756
4. Peate I. Urinary incontinence in women: treatment recommendations. Br J Nurs. 2019;28(22):1486–1488. doi:10.12968/bjon.2019.28.22.1486
5. Harris SS, Link CL, Tennstedt SL, Kusek JW, McKinlay JB. Care seeking and treatment for urinary incontinence in a diverse population. J Urol. 2007;177(2):680–684. doi:10.1016/j.juro.2006.09.045
6. Morrill M, Lukacz ES, Lawrence JM, Nager CW, Contreras R, Luber KM. Seeking healthcare for pelvic floor disorders: a population-based study. Am J Obstet Gynecol. 2007;197(1):86. doi:10.1016/j.ajog.2007.02.051
7. Lukacz ES, Santiago-Lastra Y, Albo ME, Brubaker L. Urinary Incontinence in women: a review. JAMA. 2017;318(16):1592–1604. doi:10.1001/jama.2017.12137
8. Bazi T, Takahashi S, Ismail S, et al. Prevention of pelvic floor disorders: international urogynecological association research and development committee opinion. Int Urogynecol J. 2016;27(12):1785–1795. doi:10.1007/s00192-016-2993-9
9. Kammerer-Doak D, Rizk DE, Sorinola O, Agur W, Ismail S, Bazi T. Mixed urinary incontinence: international urogynecological association research and development committee opinion. Int Urogynecol J. 2014;25(10):1303–1312. doi:10.1007/s00192-014-2485-8
10. Buckley BS, Lapitan MC. Epidemiology Committee of the Fourth International Consultation on Incontinence P. Prevalence of urinary incontinence in men, women, and children–current evidence: findings of the Fourth International Consultation on Incontinence. Urology. 2010;76(2):265–270.
11. Mota RL. Female urinary incontinence and sexuality. Int Braz J Urol. 2017;43(1):20–28. doi:10.1590/s1677-5538.ibju.2016.0102
12. Cayan S, Yaman O, Orhan I, et al. Prevalence of sexual dysfunction and urinary incontinence and associated risk factors in Turkish women. Eur J Obstet Gynecol Reprod Biol. 2016;203:303–308. doi:10.1016/j.ejogrb.2016.06.030
13. Melotti IGR, Juliato CRT, Tanaka M, Riccetto CLZ. Severe depression and anxiety in women with overactive bladder. Neurourol Urodyn. 2018;37(1):223–228. doi:10.1002/nau.23277
14. Soliman Y, Meyer R, Baum N. Falls in the elderly secondary to urinary symptoms. Rev Urol. 2016;18(1):28–32.
15. Ng KL, Ng KWR, Thu WPP, Kramer MS, Logan S, Yong EL. Risk factors and prevalence of urinary incontinence in mid-life Singaporean women: the integrated women’s health program. Int Urogynecol J. 2020;31(9):1829–1837. doi:10.1007/s00192-019-04132-3
16. Somoza Argibay I, Mendez Gallart R, Casal Beloy I, Garcia Gonzalez M. Urinary incontinence and lower urinary tract dysfunction prevalence in schoolchildren: risk factors. Cir Pediatr. 2019;32(3):145–149.
17. Shi W, Niu XY, Chen YY, et al. [A Study on the Risk Factors for Early Postpartum Urinary Incontinence in Chengdu]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2019;50(4):598–603. Chinese.
18. Lowenstein E, Jepsen R, Andersen LL, et al. Prevalence of urinary incontinence among women with diabetes in the Lolland-Falster Health Study, Denmark. Neurourol Urodyn. 2021;40(3):855–867. doi:10.1002/nau.24636
19. Northwood M, Markle-Reid M, Sherifali D, Fisher K, Ploeg J. Cross-sectional Study of Prevalence and Correlates of Urinary Incontinence in Older Home-Care Clients With Type 2 Diabetes in Ontario, Canada. Can J Diabetes. 2021;45(1):47–54 e4. doi:10.1016/j.jcjd.2020.05.005
20. Piculo F, Marini G, Vesentini G, et al. Pregnancy-specific urinary incontinence in women with gestational hyperglycaemia worsens the occurrence and severity of urinary incontinence and quality of life over the first year post partum. Eur J Obstet Gynecol Reprod Biol. 2020;252:336–343. doi:10.1016/j.ejogrb.2020.06.036
21. Brown JS, Vittinghoff E, Lin F, Nyberg LM, Kusek JW, Kanaya AM. Prevalence and risk factors for urinary incontinence in women with type 2 diabetes and impaired fasting glucose: findings from the National Health and Nutrition Examination Survey (NHANES) 2001–2002. Diabetes Care. 2006;29(6):1307–1312. doi:10.2337/dc05-2463
22. Wallace AS, Wang D, Shin JI, Selvin E. Screening and diagnosis of prediabetes and diabetes in US children and adolescents. Pediatrics. 2020;146(3). doi:10.1542/peds.2020-0265
23. Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160(8):517–525. doi:10.7326/M13-2411
24. Hannon TS. Promoting prevention, identification, and treatment of prediabetes and type 2 diabetes in youth. Pediatrics. 2020;146(3):e2020010272. doi:10.1542/peds.2020-010272
25. Mao W, Hu Q, Chen S, et al. Polyfluoroalkyl chemicals and the risk of kidney stones in US adults: a population-based study. Ecotoxicol Environ Saf. 2021;208:111497. doi:10.1016/j.ecoenv.2020.111497
26. Suskind AM, Cawthon PM, Nakagawa S, et al. Urinary Incontinence in older women: the role of body composition and muscle strength: from the health, aging, and body composition Study. J Am Geriatr Soc. 2017;65(1):42–50. doi:10.1111/jgs.14545
27. Forde JC, Chughtai B, Cea M, Stone BV, Te A, Bishop TF. Trends in ambulatory management of urinary incontinence in women in the United States. Female Pelvic Med Reconstr Surg. 2017;23(4):250–255. doi:10.1097/SPV.0000000000000365
28. Balk EM, Rofeberg VN, Adam GP, Kimmel HJ, Trikalinos TA, Jeppson PC. Pharmacologic and nonpharmacologic treatments for urinary incontinence in women: a systematic review and network meta-analysis of clinical outcomes. Ann Intern Med. 2019;170(7):465–479. doi:10.7326/M18-3227
29. Wang M. Acupuncture for stress urinary incontinence. JAMA. 2017;318(15):1500. doi:10.1001/jama.2017.13424
30. Blomquist JL, Munoz A, Carroll M, Handa VL. Association of delivery mode with pelvic floor disorders after childbirth. JAMA. 2018;320(23):2438–2447. doi:10.1001/jama.2018.18315
31. Abdalhk D, Riddell MC, Swayze S, Kuk JL. Association between metformin and physical activity with glucose control in adults with type 2 diabetes. Endocrinol Diabetes Metab. 2021;4(2):e00206. doi:10.1002/edm2.206
32. Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol. 2009;182(6 Suppl):S2–7. doi:10.1016/j.juro.2009.08.071
33. Komesu YM, Amundsen CL, Richter HE, et al. Refractory urgency urinary incontinence treatment in women: impact of age on outcomes and complications. Am J Obstet Gynecol. 2018;218(1):111e1- e9. doi:10.1016/j.ajog.2017.10.006
34. De Gagne JC, So A, Oh J, Park S, Palmer MH. Sociodemographic and health indicators of older women with urinary incontinence: 2010 national survey of residential care facilities. J Am Geriatr Soc. 2013;61(6):981–986. doi:10.1111/jgs.12258
35. Nazzal Z, Khatib B, Al-Quqa B, Abu-Taha L, Jaradat A. The prevalence and risk factors of urinary incontinence amongst Palestinian women with type 2 diabetes mellitus: a cross-sectional study. Arab J Urol. 2020;18(1):34–40. doi:10.1080/2090598X.2019.1699340
36. Broz J, Hronova M, Brunerova L. Metabolic syndrome and diabetes mellitus in women with and without stress urinary incontinence. Int Urogynecol J. 2019;30(5):847. doi:10.1007/s00192-019-03930-z
37. Wang R, Lefevre R, Hacker MR, Golen TH. Diabetes, glycemic control, and urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(5):293–297. doi:10.1097/SPV.0000000000000193
38. Lee SJ, Karter AJ, Thai JN, Van Den Eeden SK, Huang ES. Glycemic control and urinary incontinence in women with diabetes mellitus. J Womens Health. 2013;22(12):1049–1055. doi:10.1089/jwh.2012.4093
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
