Back to Journals » Cancer Management and Research » Volume 12
Regulatory T Cells in Cancer Immunotherapy: Basic Research Outcomes and Clinical Directions
Authors Zeng G, Jin L, Ying Q, Chen H , Thembinkosi MC, Yang C, Zhao J, Ji H, Lin S, Peng R, Zhang M, Sun D
Received 3 June 2020
Accepted for publication 8 September 2020
Published 21 October 2020 Volume 2020:12 Pages 10411—10421
DOI https://doi.org/10.2147/CMAR.S265828
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Guoming Zeng,1,2,* Libo Jin,3,4,* Qinsi Ying,5 Haojie Chen,3 Murinda Charmaine Thembinkosi,3 Chunguang Yang,6 Jinlong Zhao,6 Hao Ji,3 Sue Lin,3 Renyi Peng,3 Maolan Zhang,1,3 Da Sun3
1Chongqing University of Science and Technology, Chongqing 401331, People’s Republic of China; 2China Metallurgical Construction Engineering Group Co., Ltd., Chongqing 400044, People’s Republic of China; 3Institute of Life Sciences, Wenzhou University, Wenzhou, Zhejiang 325035, People’s Republic of China; 4Biomedical Collaborative Innovation Center of Zhejiang Province & Engineering Laboratory of Zhejiang Province for Pharmaceutical Development of Growth Factors, Biomedical Collaborative Innovation Center of Wenzhou, Wenzhou, Zhejiang 325035, People’s Republic of China; 5Wenzhou Institute, University of Chinese Academy of Sciences, Wenzhou, Zhejiang 325035, People’s Republic of China; 6Institute of Metal Research, Chinese Academy of Sciences, Shenyang, Liaoning 110016, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Da Sun
Institute of Life Sciences, Wenzhou University, Higher Educational Park, Wenzhou City 325035, Zhejiang Province, People’s Republic of China
Email [email protected]
Maolan Zhang
Chongqing University of Science and Technology, No. 19, Xijiao Branch Road, Jiulongpo District, Chongqing 400050, People’s Republic of China
Email [email protected]
Abstract: Cancer immunotherapy is a promising approach that has recently gained its importance in treating cancer. Despite various approaches of immunotherapies being used to target cancer cells, they are either not effective against all types of cancer or for all patients. Although efforts are being made to improve the cancer immunotherapy in all possible ways, one important hindrance that lowers the immune response to kill cancer cells is the infiltration of Regulatory T (Treg) cells into the tumor cells, favoring tumor progression, on one hand, and inhibiting the activation of T cells to respond to cancer cells, on the other hand. Therefore, new anti-cancer drugs and vaccines fail to show promising results against cancer. This is due to the infiltration of Treg cells into the tumor region and suppression of anti-cancer activity. Thus, regardless of various types of immunotherapies being practiced, understanding the mechanisms of how Treg cells favor tumor progression and inhibition of anti-cancer activity is worthwhile. Therefore, the review highlights the importance of Tregs cells and how depletion of Treg cells can pave the way to an effective immunotherapy by activating the immune responses against cancer.
Keywords: cancer immunotherapy, Treg cells, FoxP3, targeting Treg cells, anti-cancer
Introduction
Cancer is the most important and leading cause of mortality worldwide. Although various conventional treatments to modern therapeutics are emerging, the mortality rates are still rising. Our immune system is well developed in such a way it protects us from various infectious and degenerative diseases. It also helps in prevention of other viral diseases and cancer. Activation of the immune system thus acts as a great tool to combat cancer. Recently, cancer immunotherapy has become an exciting, interesting, and beneficial approach in treating cancer.1 It has shown a significant improvement in the last few years and “Cancer immunotherapy” was named as the “Breakthrough of the Year” by Science in 2013.2 Diverse mechanisms of activation of our own immune system using cancer immunotherapies are being tested in a rapid manner. They have some of the most common and effective approaches of immunotherapy, such as immune checkpoint inhibitors, chimeric antigen receptor T-cell transfer therapy, and non-specific method of immune stimulation.3 Although immunotherapies are underway to treat many types of cancer, they are not as effective as conventional therapies such as chemotherapy, radiation therapy, and surgery. Not all patients or all types of cancers respond to immunotherapy. This is because of the cancer cells becoming resistant to immune cells, thereby evading immunity. The cancer cells can downregulate the major histocompatibility complexes, suppress the co-stimulatory signals, secretion of immunosuppressive agents and pro-inflammatory agents by cancer cells and many more, making an ambience conducive for cancer progression.4,5 Researchers are working to identify and understand the mechanism of cancer immunotherapy as a novel target to combat cancer with limited side-effects to the possible extent. One such advancement of effective cancer immunotherapy is the depletion of Regulatory T (Treg) cells. A large number of studies have reported that this is due to the infiltration of Treg cells into the tumor sites suppressing the activity of T cells to act against cancer cells, thereby leading to poor prognosis and cure.6 Thus, understanding the mechanisms of Treg cells in promoting cancer progression and the ways of depleting Treg cells to prevent tumor progression has become an ideal targeted therapeutic mechanism in the field of cancer therapeutics, favoring anti-tumor immune response on one side, and preventing adverse effects of autoimmunity on the other side. This forms the basis of this review that outlines the role of Treg cells in causing cancer and how its depletion plays a vital role in cancer immunotherapy by preventing cancer progression and metastasis.
Immunosuppressive Property of Treg Cells and Cancer
Treg cells play a vital role in the maintenance of self-tolerance, immune homeostasis, preventing autoimmunity and inflammatory diseases.7 The suppressive concept of Treg cells depends on several complex mechanisms and varies according to its role in normal and disease conditions. Earlier evidence shows that Treg cells are a part of CD4+CD25+ T-Cell population.8 Since CD25 is a marker of effector T cells, researchers were skeptical about the immuno-suppressive property of T cells. Later a distinct difference between CD25+ effector T cells and Treg cells were demonstrated.9 Although the complex mechanism is yet to be fully understood, research in the early 2000s revealed that Treg cells work through primarily Forkhead Box transcription factor, FoxP3.10–12 It has been demonstrated that deletion of FoxP3 in the T cells of newborn mice led to improper development of Treg cells and increases autoimmunity and vice versa.13,14
Scientists strongly believe that epigenetic mechanisms also play an important role in activating Treg cells.15 Evidential reports on demethylation of conventional T cells are supposed to be an important cause for Treg cells activation.16–18 The Treg cells are not only restricted to these functions but also exert an important role in tumor immunity.19,20 Although their immunosuppressive property helps to prevent autoimmunity and graft-vs-host rejection during transplantation therapies,21 this similar property poses a serious threat through its diverse novel mechanisms mediating immunosuppression and cancer progression.22
An important aspect of the action of Treg cells is via transforming growth factor (TGF) beta pathway. Treg cells are known to produce TGF beta, which can promote differentiation of naive CD4+ T cells into Treg cells via FOXP3 expression.23 Further, TGF beta is also known to dampen effector T cells and antigen presenting cells (APCs).24 In general, Tregs can suppress anti-tumor immune response through TGF beta. Malignant cells escape this pathway, which leads to immunosuppressive tumor microenvironment (TME), subsequently resulting in cancer progression.25,26 Similarly, Treg cells via secretion of interleukin-10 (IL10) play a significant role in regulating immune responses. It is apparent that, under malignant conditions, the Treg cells secreting IL10 within malignant inflammatory TME impair the T Helper Type 1 cell-mediated anti-cancer activity.27 In general, Granzyme B assists natural killer cells and cytotoxic T cells to kill cancer cells.28 However, under malignant conditions, alternatively, Treg cells utilizes perforin-granzyme B-mediated pathway to suppress the anti-tumor activity.29
Another important aspect of Treg cells causing cancer progression is by promoting angiogenesis.30 The infiltration of Treg at TME activates angiogenic markers such as vascular endothelial growth factor, thereby promoting tumor angiogenesis via several direct and indirect mechanisms. For instance, the Treg cells promote tumor angiogenesis by inhibiting tumor-reactive T cells.31 Yet another mechanism by which the Treg cells promote tumor tolerance and angiogenesis is via CCL28-mediated hypoxic environment.32 The role of Treg cells in cancer is summarized in Figure 1.
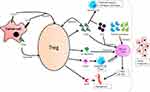 |
Figure 1 Role of Treg cells in cancer progression. |
Due to the activity of these Treg cells as an anti-tumor substance through various mechanisms, anti-cancer drugs fail to activate the endogenous immune cells against cancer. Thus, an immunosuppressive TME is developed, leading to cancer progression.
The role of Tregs in cancer prognosis is an interesting and debated topic. A meta-analysis in this regards associated FOXP3+ Tregs with: A) decreased overall survival in cervical, renal, melanoma, hepatocellular, gastric, and breast cancer cases, B) increased overall survival in colorectal, head and neck, and esophageal cancer cases, and C) no effect on overall survival in pancreatic and ovarian cancer.6 However, there is a considerable variation in the results amongst individual studies; few studies claim decreased overall survival in a particular cancer while few others claim no effects or even better overall survival in the same cancer. This difference is attributable to the molecular heterogeneity of the cancers; for example FOXP3+ Treg cells are of prognostic significance in A) estrogen receptor-positive breast cancer, but not in estrogen receptor-negative breast cancer,33 and B) mismatch repair-proficient type colorectal cancer but not in mismatch repair-deficient type.34 Further, in the light of effector T cells expressing FOXP3, using multiple markers to characterize Tregs cells is shown to yield more consistent prognostic results.35 Overall, Treg cells are potent regulators of tumor immunity and contribute to the prognosis of the cancer.
Targeting Treg Cells in Cancer Immunotherapy
It is apparent that infiltration of Treg cells into tumor microenvironment is a hurdle in treating cancer cells. Infiltration of Treg cells is indirectly proportional to anti-tumor activity and the survival of cancer patients. Thus, it is of paramount importance to identify an appropriate target of Treg cells, thereby increasing anti-cancer activity and the survival of cancer patients. Mechanisms by which Treg cells are targeted are not restricted but limited to depletion of Treg cells, suppression of Treg cells functions, disruption of Treg cells infiltrating to TME, and so on.36
The immunosuppressive TME is a major challenge in developing successful anti-cancer therapies, due to the inhibition of immune response against cancer. This property is due to the infiltration of immunosuppressive cells such as Treg cells, tumor associated macrophages, and so on. The Treg cells were earlier identified by the CD4+CD25+ cells. Later, FoxP3 was identified as the key transcriptional factor of Treg cells and was found to be responsible for development and function of Treg cells. The successful inhibition of cancer progression depends on depletion of CD25+ and FoxP3+ Treg cells. Along with immunosuppressive cells, the TME is also found to possess some key immune checkpoints such as programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), the blockade of which using anti-PD-1 and anti-CTLA4 is also shown to result in depletion of Treg cells.37,38 Treg cells therapeutic targets and the outcomes are summarized in Figure 2.
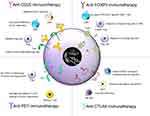 |
Figure 2 Therapeutic targets of Treg cells and its outcome. |
Thus, this article focuses on inhibition of these four important molecules as a target for depletion of Treg cells for their successful anti-cancer activity and patients survival in clinical settings.
CD25+ Targeted Depletion of Treg Cells
Before the identification of FoxP3-mediated Treg cell activity, anti-CD25 antibodies are being used to target specific interference with Treg cells. With the inhibition of CD25+ mediated Treg cell interface, CD4+ T cells mediate tumor rejection leading to prevention of cancer.39 Similarly, removal of CD25+ cells in a mice model increased the infiltration of CD8+ T cells, resulting in tumor regression. Clinical trials exploring the combination of various vaccines and drugs involving the inhibition of Treg cells via CD25 as a target showed varying impacts on the number of circulating Treg cells and vaccine-induced immunity. A study by Rech et al40 showed that daclizumab-mediated CD25 blockade could significantly deplete Treg cells in HLA-A2+ metastatic breast cancer patients, and demonstrated robust immune response to the experimental peptide vaccine at the peak of daclizumab-mediated Treg depletion. In contrast, depletion of CD25+ Treg cells in metastatic melanoma patients failed to demonstrate an antitumor immune response and augmentation of efficacy of cancer vaccination.41,42 This limited activity of anti-CD25 antibodies has been attributed to their ability to deplete Treg cells in the periphery and not those infiltrating the tumor.43 The study demonstrated that upregulation of inhibitory FC gamma receptor (FcγR) at the tumor site can effectively inhibit tumor-infiltrating Treg cells and consequently reject tumors. Associated clinical trials are further warranted. However, since CD25+ cells are also an important parameter for effector T cells, selective depletion of tumor infiltrating Treg cells without affecting effector T cells are of serious concern to prevent autoimmunity.44 Further in-depth investigations are warranted to ensure the prevention of autoimmunity.
FoxP3 Targeted Depletion of Treg Cells
Yet another approach of depleting Treg cells is by depletion of FoxP3+ Treg cells. Although a number of studies have been confined to the fact that working towards depletion of FoxP3-mediated Treg cells acts as a potential target in treating cancer as well as development of cancer vaccine,45–47 its clinical translation is not easy, owing to its intracellular location.48 In 2008, Morse et al49 demonstrated the ability of denileukin diftitox, an immunotoxin, in depleting the FoxP3+ Treg cells both in vitro and in patients with carcinoembryonic antigen (CEA)-expressing malignancies, after multiple doses. Compared to the control group, the treatment group demonstrated an earlier induction CD4+ and CD8+ T cells response to CEA; however, the difference failed to reach statistical significance. Conversely, in HLA-A2+ metastatic melanoma patients, denileukin diftitox failed to effectively deplete peripheral Foxp3 Treg cells with no improvement in clinical activity.50 Sugiyama et al51 noted that most of the tumor infiltrating terminally differentiated FoxP3+ Treg cells express CCR4 antibody. Further in the study, on administering anti-CCR4 antibody (Mogamulizumab) in NY-ESO-1-expressing adult T cell leukemia-lymphoma patients, a significant reduction in respective FoxP3+ Treg cells, with a consequent increase in NY-ESO-1 specific CD8+ T cell response, was observed. Similarly, another anti-CCR4 antibody (KW-0761) also demonstrated a significant decrease in peripheral FoxP3+ Treg cells while eliciting immune response in lung and esophageal cancer patients.44 Furthermore, the mogamulizumab is also shown to deplete circulating Treg cells in peripheral T cell lymphoma and cutaneous T cell lymphoma patients.52
Thus, it is very important to understand the role of FoxP3 in effector T cells and Treg cells, thereby discriminating the differences in the presence of this population leading to tumor progression or prevention. Similarly, it is postulated that the FoxP3-mediated anti-tumor activity may not be effective against all types of cancer, and results are inconclusive yet, in many types of cancers, paving the way for further research to explore its potential role in other types of cancers.
Suppression of Treg Function via Checkpoint Inhibitors
Immune checkpoints are a cascade of pathways, most crucial for healthy cells, allowing them to prevent from autoimmunity and damage. However, these immune checkpoint proteins might be dysregulated by cancer cells and become resistant to immune cells. Thus, immune checkpoint blockades have become a fascinating area of cancer immunotherapy. Out of several on-going clinical trials, checkpoint inhibitors have successfully entered various phases of clinical trials and many more checkpoint inhibitors are underway.53 Among the various immune checkpoint inhibitors, the most evidential results in clinical cancer immunotherapy are CTLA-4 and PD-1. Although, on one hand, these immune checkpoint inhibitors are gaining consensus in cancer immunotherapy for several types of cancer, on the other hand, its application and efficacy over a wide range of cancer types is uncertain and the clinical significance and responsiveness of patients towards immune checkpoint blockades are warranted in multiple types of cancers.
CTLA-4 Inhibitor
CTLA-4 expressed on the surface of activated T cells and Treg cells was the first clinically targeted immune checkpoint molecule. CTLA-4 activates infiltration of Treg cells via down-regulation of costimulatory molecules (CD80/86 expression) on APCs, leading to cancer progression and prevention of immune response. Hence, several studies have tried suppressing CTLA-4 expression to prevent Treg cells infiltration, further assessing its clinical outcome. Romano et al54 showed for the first time that ipilimumab, an IgG1 anti-CTLA4 antibody, was able to induce CD16+ monocyte-mediated lysis of Treg cells, in an antibody-dependent cell-mediated cytotoxicity (ADCC) fashion, in metastatic melanoma patients. However, a recent immunohistochemical and mass cytometry analysis of the CD4+, CD8+, and FoxP3 in stage-matched melanoma, prostate cancer, and bladder cancer samples demonstrated that both ipilimumab and tremelimumab (an IgG2 anti CTLA-4 antibody) did not deplete the FoxP3+ Treg cells within the TME.55 A few other studies have also reported this increase in the absolute number of circulating FOXP3+ Treg cells in response to anti-CTLA4 antibody.56–58 Kavanagh et al57 demonstrated that an anti-CTLA4 antibody may exert its effects in metastatic prostate cancer patients through activation of effector T cells but not by depleting FoxP3+ Treg cells. Although an increase in circulating levels of FOXP3+ Treg cells is observed, it is vital to decrease the number of tumor infiltrating Treg cells to elucidate anti-tumor effects of the anti-CTLA4 immunotherapy. While there are mixed results in achieving this with an anti-CTLA4 antibody alone,59,60 a more promising approach is to further augment it with activation of FC-receptor expressing macrophages, that results in selective depletion of Treg cells in TME.61,62
Although the mechanism of CTLA-4 knockout-mediated cancer immunotherapy in clinical settings is known, with its improvement in patient’s survival, further research on its mechanism of anti-tumor activity and autoimmunity is of paramount importance, especially with respect to resolving the Treg cells controversy, so as to further improve the therapeutic strategies in cancer treatments.
Programmed Cell Death Protein 1 (PD-1 Inhibitors)
PD-1, a transmembrane immunoinhibitory protein of the CD28 Ig superfamily, plays a crucial role in tumor immune escape. PD-1 is expressed on activated T and B cells, natural killer T cells, activated monocytes, tumor-infiltrating lymphocytes, and some subsets of dendritic cells. High levels of PD-1 are observed in tumor infiltrating T cells of various cancer patients. Pembrolizumab and Nivolumab are FDA-approved humanized monoclonal antibodies that block PD-1/PD-L1 interaction, prevent the Treg cells activity, and promote anti-tumor activity. Very few clinical studies have looked into the effect of anti-PD-1 therapy on Treg cells in cancer patients. In 2017, Ribas et al63 reported no change in the percentage of circulating Treg cells in pembrolizumab-treated metastatic melanoma patients, while a recent preclinical study on osteosarcoma animal model noted a decrease in the tumor-infiltrating Treg cells in response to anti-PD-1 therapy.64 However, in resected high-risk melanoma patients treated with nivolumab, a paradoxical increase in the proportion of circulating Treg cells with a reduction in the Treg-suppressive capacity was observed.65 Analysis on how anti-PD-1 therapy may induce hyper-progressive disease in advanced gastric cancer patients showed a significant increase in circulating and intra-tumoral levels of PD-1+ Treg cells, thus inhibiting antitumor immunity in such patients.66 In this regards, studies point at the anti-PD-1-mediated activation of TGF-beta/Smad3 pathway, which can promote Treg cells induction and immunosuppression.67,68 Consequently, blocking TGF beta along with PD-1 blockade is a viable option to suppress tumor-infiltrating Treg cells and promote infiltration of effector T cells, resulting in cytotoxic destruction of tumor.69 Furthermore, combining a PD-1 inhibitor with CTLA-4 inhibitor has demonstrated superior efficacy against melanoma and non-small cell lung carcinoma.70 Such therapy has been shown to effectively deplete Treg cells while expanding CD8+ effector T cells within TME, compared to monotherapy with either of the inhibitors.71,72 Furthermore, PD-1 inhibitor combined with FC-optimized CD25 inhibitor effectively repressed established tumors in the preclinical models of sarcoma, colon cancer, and melanoma, as compared to monotherapy.43
The clinical response with reference to PD-1 blockade has drastically envisaged the clinical cancer immunotherapy into the next phase of research and clinical trial.73 Progress towards identification of prospective biomarkers and clinical response of PD-1 blockage with respect to Treg cells are underway, thereby paving the way for early prognosis and treatment strategies.
Conclusions
Cancer immunotherapy is an emerging field of cancer therapeutics (Table 1). Immunotherapies work in two different ways, either by stimulating effector mechanisms or by neutralizing suppressive mechanisms. An important challenge in cancer immunotherapy is to neutralize the immunosuppressive mechanisms, so as to strengthen our immune system to act against cancer cells. An immunosuppressive tumor microenvironment poses a serious threat due to inhibition of immune responses against cancer. The rationale for the same is due to the infiltration of immunosuppressive cells such as Treg cells and immunosuppressive checkpoint molecules such as PD-1 and CTLA-4 into the TME, leading to cancer progression, poor prognosis and cure. Evidence from various research articles demonstrates the mechanisms of Treg cells infiltration and importance of depletion of Treg cells as a key parameter in altering the TME, thereby activating more T cells and helping cancer immunotherapy work better. The scientists believe that working towards clinical cancer immunotherapy coupled with depletion of various Treg cells pathways to prevent tumor progression favors anti-tumor immune response and prevents adverse effects of auto-immunity, thereby making cancer immunotherapy more promising, and improves the survival of patients. Thus, it is evident that depletion of Treg cells is directly proportional to the anti-tumor activity and inversely proportional to tumor progression.
 |
Table 1 Clinical Application of the Cancer Immunotherapy Targeting Tregs |
Depletion of immunosuppressive Treg cells is carried out by targeting either markers of Treg cells such as CD25+ or the nuclear transcription factor, FoxP3. Besides, immune checkpoints such as CTLA-4 and PD-1 are also an important target of depletion of Treg cells. The effects of various efficient immunotherapeutic agents are under progress and few of the promising molecules have entered different phases of clinical trials. Some of these promising immunotherapy drugs towards targeting the expression markers of Treg cells (CD25 or FoxP3) or targeting immune checkpoints (CTLA-4 or PD-1/PDL1) along with their outcome are highlighted. With the development of various cancer immunotherapeutic drugs that have emerged so far, including the most evident and successful inhibitors mentioned in this review, it is understood that deletion of Treg cells via these compounds act as a promising therapeutic tool in treating various types of cancers. However, either these molecules are not effective against all types of cancer or show few contradictory results, as shown in this review, warranting further in-depth investigations on the outcome of anti-tumor activity, the survival of patients, and long-term benefits. With the existing advancement in clinical trials on cancer immunotherapy and with further advancements in the near future, it is postulated that such a therapeutic regime would improve the overall survival of patients in metastatic cancer conditions and help to combat cancer. However, Treg cells immunotherapy has several limitations. Depleting Treg cells will upset the natural immune balance that may trigger severe allergic responses, autoimmune disorders, and may breach feto-maternal tolerance in pregnant patients. Further, the target markers such as CD25 and FoxP3 are also expressed on effector T cells, and thus may get suppressed with targeted antibodies resulting in unwanted consequences. Thus, te mechanisms of depleting Treg cells and preventing autoimmunity in most of the cancer immunotherapeutic drugs still remain elusive and need to be further investigated to understand their mechanisms in prevention of cancer and autoimmunity, thereby bringing Treg cells-mediated cancer immunotherapy as a cutting-edge in the field of clinical settings.
Acknowledgments
This work was supported by the Natural Science Foundation of Zhejiang Province (Grant No.Q19E010021, LQ20C020003 and LQ20C200015), National Natural Science Foundation of China (Grant No.51901160, 51808086), Shenzhen Science and Technology Research Funding (JCYJ20160608153641020), and Special project of basic research and frontier exploration in Chongqing (Grant No. cstc2018jcyjAX0078).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Klener P, Otahal P, Lateckova L, Klener P. Immunotherapy approaches in cancer treatment. Curr Pharm Biotechnol. 2015;16(9):771–781. doi:10.2174/1389201016666150619114554
2. Couzin-Frankel J. Breakthrough of the year 2013. Cancer Immunother Sci. 2013;342(6165):1432–1433. doi:10.1126/science.342.6165.1432
3. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–3337. doi:10.1172/JCI83871
4. Rodriguez PC, Zea AH, Ochoa AC. Mechanisms of tumor evasion from the immune response. Cancer Chemother Biol Response Modif. 2003;21:351–364.
5. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–1284. doi:10.1101/gad.314617.118
6. Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi:10.1038/srep15179
7. Piccirillo CA. Regulatory T cells in health and disease. Cytokine. 2008;43(3):395–401. doi:10.1016/j.cyto.2008.07.469
8. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164.
9. Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11(1):7–13. doi:10.1038/ni.1818
10. Li Z, Li D, Tsun A, Li B. FOXP3+ regulatory T cells and their functional regulation. Cell Mol Immunol. 2015;12(5):558–565. doi:10.1038/cmi.2015.10
11. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi:10.1038/ni904
12. Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–342. doi:10.1038/ni909
13. Liu Y, Zheng P. FoxP3: a life beyond regulatory T cells. Int J Clin Exp Pathol. 2009;2(3):205–210.
14. Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi:10.1038/83784
15. Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114(18):3727–3735.
16. Polansky JK, Kretschmer K, Freyer J, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38(6):1654–1663. doi:10.1002/eji.200838105
17. Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204(7):1543–1551. doi:10.1084/jem.20070109
18. Polansky JK, Schreiber L, Thelemann C, et al. Methylation matters: binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J Mol Med (Berl). 2010;88(10):1029–1040. doi:10.1007/s00109-010-0642-1
19. Paluskievicz CM, Cao X, Abdi R, Zheng P, Liu Y, Bromberg JS. T regulatory cells and priming the suppressive tumor microenvironment. Front Immunol. 2019;10:2453.
20. Magnuson AM, Kiner E, Ergun A, et al. Identification and validation of a tumor-infiltrating Treg transcriptional signature conserved across species and tumor types. Proc Natl Acad Sci. 2018;115(45):E10672–E10681. doi:10.1073/pnas.1810580115
21. Romano M, Tung SL, Smyth LA, Lombardi G. Treg therapy in transplantation: a general overview. Transpl Int. 2017;30(8):745–753. doi:10.1111/tri.12909
22. Whiteside TL. Clinical Impact of Regulatory T cells (Treg) in Cancer and HIV. Cancer Microenviron. 2015;8(3):201–207. doi:10.1007/s12307-014-0159-1
23. Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9(2):194–202.
24. Sanjabi S, Oh SA, Li MO. Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017;9(6).
25. Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50(4):924–940. doi:10.1016/j.immuni.2019.03.024
26. Chen J, Gingold JA, Su X. Immunomodulatory TGF-beta signaling in hepatocellular carcinoma. Trends Mol Med. 2019;25(11):1010–1023. doi:10.1016/j.molmed.2019.06.007
27. Dennis KL, Blatner NR, Gounari F, Khazaie K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr Opin Oncol. 2013;25(6):637–645. doi:10.1097/CCO.0000000000000006
28. Cullen SP, Brunet M, Martin SJ. Granzymes in cancer and immunity. Cell Death Differ. 2010;17(4):616–623.
29. Cao X, Cai SF, Fehniger TA, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–646. doi:10.1016/j.immuni.2007.08.014
30. Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72(9):2162–2171. doi:10.1158/0008-5472.CAN-11-3687
31. Casares N, Arribillaga L, Sarobe P, et al. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171(11):5931–5939. doi:10.4049/jimmunol.171.11.5931
32. Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475(7355):226–230. doi:10.1038/nature10169
33. Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–5380. doi:10.1200/JCO.2006.05.9584
34. Frey DM, Droeser RA, Viehl CT, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126(11):2635–2643.
35. deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18(11):3022–3029. doi:10.1158/1078-0432.CCR-11-3216
36. Tanaka A, Sakaguchi S. Targeting Treg cells in cancer immunotherapy. Eur J Immunol. 2019;49(8):1140–1146.
37. Walker LS. Treg and CTLA-4: two intertwining pathways to immune tolerance. J Autoimmun. 2013;45:49–57. doi:10.1016/j.jaut.2013.06.006
38. Wu X, Gu Z, Chen Y, et al. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J. 2019;17:661–674.
39. Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32(11):3267–3275. doi:10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1
40. Rech AJ, Mick R, Martin S, et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4(134):134ra162. doi:10.1126/scitranslmed.3003330
41. Jacobs JF, Punt CJ, Lesterhuis WJ, et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a Phase I/II study in metastatic melanoma patients. Clin Cancer Res. 2010;16(20):5067–5078. doi:10.1158/1078-0432.CCR-10-1757
42. Powell DJ, Felipe-Silva A, Merino MJ, et al. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol. 2007;179(7):4919–4928. doi:10.4049/jimmunol.179.7.4919
43. Arce Vargas F, Furness AJS, Solomon I, et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46(4):577–586. doi:10.1016/j.immuni.2017.03.013
44. Kurose K, Ohue Y, Wada H, et al. Phase Ia study of FoxP3+ CD4 Treg depletion by infusion of a humanized anti-CCR4 antibody, KW-0761, in cancer patients. Clin Cancer Res. 2015;21(19):4327–4336. doi:10.1158/1078-0432.CCR-15-0357
45. Teng MW, Swann JB, von Scheidt B, et al. Multiple antitumor mechanisms downstream of prophylactic regulatory T-cell depletion. Cancer Res. 2010;70(7):2665–2674. doi:10.1158/0008-5472.CAN-09-1574
46. Pastille E, Bardini K, Fleissner D, et al. Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res. 2014;74(16):4258–4269. doi:10.1158/0008-5472.CAN-13-3065
47. Sato K, Sato N, Xu B, et al. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci Transl Med. 2016;8(352):352ra110. doi:10.1126/scitranslmed.aaf6843
48. Dao T, Mun SS, Scott AC, et al. Depleting T regulatory cells by targeting intracellular Foxp3 with a TCR mimic antibody. Oncoimmunology. 2019;8(7):1570778. doi:10.1080/2162402X.2019.1570778
49. Morse MA, Hobeika AC, Osada T, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112(3):610–618. doi:10.1182/blood-2008-01-135319
50. Luke JJ, Zha Y, Matijevich K, Gajewski TF. Single dose denileukin diftitox does not enhance vaccine-induced T cell responses or effectively deplete Tregs in advanced melanoma: immune monitoring and clinical results of a randomized Phase II trial. J Immunother Cancer. 2016;4:35. doi:10.1186/s40425-016-0140-2
51. Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110(44):17945–17950. doi:10.1073/pnas.1316796110
52. Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32(11):1157–1163. doi:10.1200/JCO.2013.52.0924
53. Chen Y, Qiu J, Chen B, et al. Long non-coding RNA NEAT1 plays an important role in sepsis-induced acute kidney injury by targeting miR-204 and modulating the NF-kappaB pathway. Int Immunopharmacol. 2018;59:252–260. doi:10.1016/j.intimp.2018.03.023
54. Romano E, Kusio-Kobialka M, Foukas PG, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112(19):6140–6145. doi:10.1073/pnas.1417320112
55. Sharma A, Subudhi SK, Blando J, et al. Anti-CTLA-4 immunotherapy does not deplete FOXP3(+) regulatory T cells (Tregs) in human cancers. Clin Cancer Res. 2019;25(4):1233–1238. doi:10.1158/1078-0432.CCR-18-0762
56. Calabro L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, Phase 2 trial. Lancet Oncol. 2013;14(11):1104–1111. doi:10.1016/S1470-2045(13)70381-4
57. Kavanagh B, O’Brien S, Lee D, et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112(4):1175–1183. doi:10.1182/blood-2007-11-125435
58. Calabro L, Morra A, Fonsatti E, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med. 2015;3(4):301–309. doi:10.1016/S2213-2600(15)00092-2
59. Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105(39):14987–14992. doi:10.1073/pnas.0806075105
60. Ribas A, Comin-Anduix B, Economou JS, et al. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res. 2009;15(1):390–399. doi:10.1158/1078-0432.CCR-08-0783
61. Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi:10.1084/jem.20130579
62. Du X, Tang F, Liu M, et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res. 2018;28(4):416–432. doi:10.1038/s41422-018-0011-0
63. Ribas A, Shin DS, Zaretsky J, et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res. 2016;4(3):194–203. doi:10.1158/2326-6066.CIR-15-0210
64. Yoshida K, Okamoto M, Sasaki J, et al. Anti-PD-1 antibody decreases tumour-infiltrating regulatory T cells. BMC Cancer. 2020;20(1):25. doi:10.1186/s12885-019-6499-y
65. Woods DM, Ramakrishnan R, Laino AS, et al. Decreased suppression and increased phosphorylated STAT3 in regulatory T cells are associated with benefit from adjuvant PD-1 blockade in resected metastatic melanoma. Clin Cancer Res. 2018;24(24):6236–6247. doi:10.1158/1078-0432.CCR-18-1100
66. Kamada T, Togashi Y, Tay C, et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A. 2019;116(20):9999–10008. doi:10.1073/pnas.1822001116
67. Dodagatta-Marri E, Meyer DS, Reeves MQ, et al. α-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by α-TGFβ antibody to promote durable rejection and immunity in squamous cell carcinomas. J Immunother Cancer. 2019;7(1):62.
68. Donkor MK, Sarkar A, Savage PA, et al. T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-beta1 cytokine. Immunity. 2011;35(1):123–134. doi:10.1016/j.immuni.2011.04.019
69. Loffek S. Transforming of the tumor microenvironment: implications for TGF-beta inhibition in the context of immune-checkpoint therapy. J Oncol. 2018;2018:9732939. doi:10.1155/2018/9732939
70. Chae YK, Arya A, Iams W, et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J Immunother Cancer. 2018;6(1):39. doi:10.1186/s40425-018-0349-3
71. Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–4280. doi:10.1073/pnas.0915174107
72. Wei SC, Anang N-A-AS, Sharma R, et al. Combination anti–CTLA-4 plus anti–PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci. 2019;116(45):22699–22709. doi:10.1073/pnas.1821218116
73. Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv324. doi:10.1126/scitranslmed.aad7118
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
