Back to Journals » Journal of Inflammation Research » Volume 11
Regulatory T cells antagonize proinflammatory response of IL-17 during cutaneous tuberculosis
Authors Saini C , Kumar P, Tarique M, Sharma A, Ramesh V
Received 2 May 2018
Accepted for publication 17 July 2018
Published 28 September 2018 Volume 2018:11 Pages 377—388
DOI https://doi.org/10.2147/JIR.S172878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Chaman Saini,1 Praveen Kumar,1,2 Mohd Tarique,1 Alpana Sharma,1 Venkatesh Ramesh3
1Department of Biochemistry, All India Institute of Medical Sciences, New Delhi, India; 2Department of Microbiology, Government Medical College, Kota, India; 3Department of Dermatology, Safdarjung Hospital, New Delhi, India
Background: The clinical forms of cutaneous tuberculosis (CTB) consist of a spectrum that reflects the host’s immune response to Mycobacterium tuberculosis; it provides an ideal model to study the immunological dysregulation in humans. IL-17 plays an important role in initial immune response and is involved in both immune-mediated protection and pathology during M. tuberculosis infection. TGF-β producing regulatory T-cells (Tregs) are high in leprosy patients and responsible for immune suppression. However, in CTB, the involvement of Tregs and Th17 remains unevaluated.
Objective: To study the role of proinflammatory Th17 and Treg cells in the human CTB.
Methods: Blood and skin biopsies of CTB patients and healthy controls (HC) were included in the study. Flow cytometric analysis of IL-17, FOXP3, and TGF-β in blood was done followed by immunohistochemistry on paraffin-embedded skin sections. Expression of IFN-γ, TGF-β, and IL-17 was evaluated by quantitative real-time PCR.
Results: We found significant (P<0.0002) lower expression of proinflammatory IL-17 and IFN-γ (P<0.01) in CTB skins as compared to HC. However, the frequency of TGF-β producing Treg cells was found to be high in CTB patients (P<0.001) as compared to HC. A similar type of profile was observed by flow cytometric analysis. Treg cells produced suppressive cytokine TGF-β which showed a positive correlation with FOXP3 gene expression.
Conclusion: Our study found an increase in lineage-specific CD4+ Tregs in CTB as compared to the HC individuals. Such cells secrete TGF-β, a suppressive cytokine and may play a role in negatively regulating the T-cell immune responses in CTB. In addition, Tregs with TGF-β may downregulate Th17 cell responses leading to the antigen-specific anergy associated with CTB patients.
Keywords: FOXP3, IFN-γ, TGF-β, IL-17, regulatory T cells, cutaneous tuberculosis
Introduction
Cutaneous tuberculosis (CTB) is a paucibacillary infection caused by intracellular rod-shaped Mycobacterium tuberculosis complex, Mycobacterium bovis, and Bacillus Calmette-Guérin depending upon the individual immunity and other factors such as genetic and environmental ones.1–3 Skin manifestation shows 1 or more hypopigmented skin lesions and anesthetic patches. A typical CTB lesion is an epithelioid granuloma with central caseation necrosis.4,5 Since 5–6 decades, extensive studies have shown T-cell immunopathology of pulmonary and extrapulmonary TB.6 Among the extrapulmonary TB, CTB has been least studied. Because of good response to multidrug therapy, the skin lesions partially or even completely disappear within 6–8 weeks.
Cytokines are a key player for activation of cell-mediated immunity, while Th1 cells play a major role in the immune response to mycobacteria.7,8 Even though the production of IFN-γ is insufficient to control the bacillus, IFN- γ is one of the crucial components of the protective response against the pathogen.9 Ability to produce IFN-γ may differ in every individual. Some studies suggest that the levels of IFN-γ decrease in patients with active and advanced pulmonary disease. A distinct discovery was made in 1995 by Sakaguchi et al10 who showed that a subset of cells is responsible for maintaining homeostasis of organ-specific autoimmunity, and these cells were called as a Regulatory T (Treg) cell. Treg cells expressed CD25 and were later shown to also express FoxP3. The discovery of CD4+CD25+FOXP3+ cells as a suppressor of autoimmune responses and their presence in mice and man11,12 has revived the concept of a distinct lineage of T cells that negatively regulate the immune responses in order to maintain homeostasis and were designated as Tregs. These cells commonly produce TGF-β and are able to express TLRs and contributed to the immunopathology of mycobacterial diseases viz, leprosy and tuberculosis.13–16 On the other hand, Th17 cells have been recognized as an inflammatory subset of a distinct lineage of Th cells; associations between IL-17 and infectious disease have been known for many years. Earlier, our group has reported as nonpolarizing Th17 subset of T-helper cells in stable leprosy.17,18 Therefore, Th17 constitutes a potentially important factor at the onset of the infection, because they can influence the latency or progression of TB and leprosy.8 Study of T-cell pathology is a very important study for any infectious diseases in terms of appropriate treatment and therapeutic values. Among different populations of T-helper cells, Th1 and Th17 are the main effector populations which mediate protection and pathology during TB. Here, we made an attempt to identify the role of Tregs and Th17 cells, Treg-associated transcription factor FOXP3, and TGF-β in CTB patients. Furthermore, we also investigated IL-17 and IFN-γ expression levels in CTB patients in comparison to healthy individuals.
Materials and methods
Enrollment of patients and ethics
Ten newly diagnosed CTB patients with a differential diagnosis of lupus vulgaris (LV) and scrofuloderma were recruited from the Department of Dermatology and Venerology, Safdarjung Hospital, New Delhi, India. In addition, normal skin samples from 10 healthy controls (HC) (M: 04, F: 06, and age range: 18–38 years) undergoing cosmetic surgery for burn-free area were included as HC. Their chest X-rays were normal and they were negative for acid-fast bacilli. All these patients had normal chest X-rays except for 1 (Table 1).
Since there was no available data on this aspect, on a pilot basis we arbitrarily enrolled 10 patients in each group. The study protocol, informed consent forms, and all procedures were approved by the Institutional Ethical Committee of Safdarjung Hospital (No. 26–11-EC (25/31). Written informed consent was obtained from the patients prior to taking skin biopsies.
Diagnosis of CTB
CTB is essentially a paucibacillary type of mycobacterial infection. Diagnosis of CTB was made on the basis of the typical clinical picture, investigations (Mantoux test and histopathological finding of an epithelioid cell granuloma), and response to therapy within 2 months of starting antitubercular therapy (no organisms were seen on special stains on histopathology including Ziehl–Neelsen and periodic acid Schiff). In a recent meeting of experts on extrapulmonary tuberculosis, it was concluded that since culture and PCR-based tests have low sensitivity and since there is a lack of evidence from high-quality studies, they could not be recommended for confirmation of diagnosis, according to Index-TB guidelines on extra-pulmonary tuberculosis for India. Hence, response to therapy remains a reasonable way of diagnosing the patient as having any of the forms of CTB.19
Skin biopsies
Skin biopsies of the affected area were collected after obtaining the informed consent of patients and HC. In case of children, parent’s written and oral consent was obtained. Punch biopsies of 4–6 mm skin were taken under local anesthesia (1% lignocaine) from the edge of an active lesion. One-half of the biopsies were fixed in 10% buffered formalin for routine histopathological evaluation and immunohistochemical analysis and the other half were stored in RNA later (Ambion, Foster City, CA, USA), an RNA protection solution for RNA isolation and finally stored at –20°C for further processing.
Immunohistochemistry (IHC)
Serial sections of 5 µm thick formalin-fixed paraffin-embedded tissue were cut on Leica rotary microtome, adhered to poly-l-lysine (Sigma Aldrich, St. Louis, MO, USA)-coated slides, and deparaffinized in xylene and rehydrated with a sequence of isopropyl alcohol solutions. For antigen retrieval, sections were pretreated in EZ retriever microwave (Bio Genex, Fremont, CA, USA) with TE (50 mM Tris 2 mM EDTA, pH-9) buffer. The microwave conditions were 96°C for 8 minutes of 2 cycles followed by cooling to room temperature in the same buffer for 30–40 minutes. After cooling, endogenous peroxidase was quenched using 0.9% hydrogen peroxide in distilled water for 10 minutes followed by washing in PBS-T (Triton X-100 for nuclear markers), 2 changes of 5 minutes each. The nonspecific background was blocked using 3% BSA in PBS for 30 minutes followed by primary antibody incubation at the optimized dilutions overnight in a humidified chamber at 4°C. The antibodies used in this study were directed against human transcriptional factor of FOXP3 (Mouse monoclonal clone-236A/E7) and TGF-β (H-112). IHC was performed using enhancer HRP-polymer detection method (Bio Genex) according to the manufacturer’s instructions. The sections were counterstained with hematoxylin.
Assessment of staining
Sections were assessed under the microscope (Nikon, Tokyo, Japan) at 40× magnification, and photographs of 5 different fields were taken. Positively stained cells in the granuloma and cellular infiltration were determined by the presence of brown color due to DAB substrate. Negative cells lacked staining. Quantitative assessment of nuclear FOXP3 and TGF-β markers was done using Image Pro express 6.0 (Media Cybernetics, Rockville, MD, USA).
RNA extraction and cDNA synthesis
RNA isolation was done from individual skin tissue (20 mg), stored skin biopsies after thawing and crushing the tissue with liquid nitrogen in pestle and mortar, using Tri-reagent (Sigma Aldrich) according to the manufacturer’s instructions. The purity and concentration of RNA preparation were determined by Nanodrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). RNA was reverse transcribed to cDNA using Suprescript III First strand cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s recommendations using random hexamer primer sequences.
Real-time PCR (qPCR)
Quantitative gene expression of FOXP3, IL-17, and TGF-β was assessed by qPCR. Duplicate samples of cDNA from each subject was amplified with precoated primers (SA, Biosciences, Frederick, MD, USA, cat no PHAS-073), for FOXP3 (Hs.247700), IL-17 (Hs.41724), and TGF-b (Hs.645227) with 2 housekeeping genes b2M (Hs.534255) and HPRT1 (Hs.412707), using SYBER green chemistry. These were then subjected to qPCR (Bio-Rad CFX96, Bio-Rad, Hercules, CA, USA) for 2 hours. Threshold cycles values (Ct) were normalized and expressed as ΔCt: mean Ct of gene of interest-mean Ct of 2 housekeeping genes.17 Thermal profile was as follows: 95.0°C for 10:00 minutes for Stage 1, for Stage 2, 95.0°C for 0:15 (15 seconds) followed by 60.0°C for 1:00 minute (40 cycles).
Isolation and culture of peripheral blood mononuclear cells (PBMC)
Three milliliter venous blood was collected from each CTB patients and HC. PBMC were isolated using Ficoll (Sigma) density-gradient method. Cell yields ranged from 1.3 to 1.5×106/mL, and cell viability ranged from 95% to 98% as estimated by 0.2% trypan blue staining (Sigma Aldrich). After isolation of PBMC, 2×106 cells/mL were cultured for 48 hours in sterile flat bottom 24-well plates (Falcon, Somerset, NJ, USA) with and without purified protein derivative (PPD) (2.5 µg/mL) antigens and 5 µg/mL phytohemagglutinin (Sigma) as positive control (Figures S1 and S2) in Roswell Park Memorial Institute-1640 medium +10% fetal bovine serum (Sigma Aldrich) for 48 hours in 5% CO2 at 37°C in incubator. After the stimulation, cells were analyzed by flow cytometry.
Flow cytometric analysis
All reagents were obtained from BD Biosciences, San Diego, CA, USA and used as per manufacturer’s instructions. Eight hours prior to harvest, ex vivo cultured cells were incubated with BD Golgi Stop to block secretion of cytokine. For surface staining, 1.0×106 cells/100 µL in staining buffer were incubated with anti-human CD3 (Per-Cpcy-5.5 clone-UCHT), CD4 (APC-H7, clone:SK3), and CD25 (fluorescein isothiocyanate, clone:M-A251) with respective isotype controls. After the surface staining, cells were washed 2 times and permeabilized with permeabilizing/fixation solution for 30 minutes at 4°C. The cells were washed as above and resuspended in Perm/Wash buffer and incubated with anti-human IL-17A (Alexa Fluor-647, SCPL1362) at 4°C for 30 minutes in the dark followed by 2 washes as before, and resuspended in 500 µL. For FOXP3 staining, cells were incubated with 1xFOXP3 buffer A for 10 minutes at room temperature; cells were washed as above and permeabilized with buffer C for 30 minutes at room temperature. The cells were washed as before, resuspended in stain buffer, and incubated with anti-FOXP3 (APC, clone:259D/C7) and anti-TGF-β (PE, clone:TW4-9E7) at room temperature for 30 minutes in the dark followed by 2 washes as before and suspended in 500 µL of staining buffer. Stained cells were acquired by FACS Caliber (BD Biosciences) and analyzed by Flowing two software.
Statistical analysis
Data of cytokines are represented as mean ± standard deviation (SD) while gene expression data are represented as mean% ΔCt ± SD. P<0.05 was considered as statistically significant. Graph Pad Prism version 4 (GraphPad Software, Inc., San Diego, CA, USA) was used for 2-tailed Mann–Whitney test for significance and nonparametric Spearman’s rank correlation coefficient (r2).
Results
Clinical characteristics
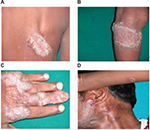
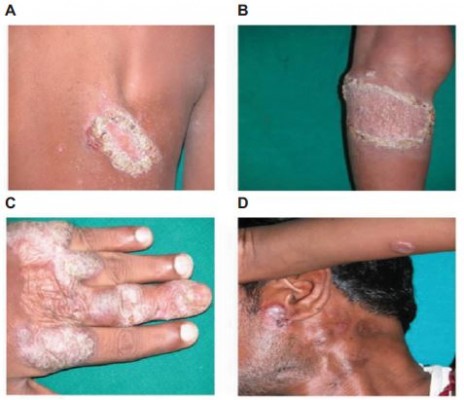
The 10 CTB patients were in the age group of 7–34 years with disease duration of 2–60 months. Patients had LV and keratotic plaques, scrofuloderma, and preauricular erythematous plaque on hand, back, and left knee (Figure 1).
Routine histopathology
Upon histopathological examination, 6 cases out of the 10 CTB patients were LV and 4 were SD (Table 1). All granulomas were ill-defined and small. Granulomas of LV predominantly had cellular infiltrate of few to increased lymphocytes, histiocytes, and multinucleated giant cells in some cases, and SD had predominantly epithelioid cells, few lymphocytes, plasma cells, and caseating necrosis. Both forms of CTB showed chronic inflammation.20
Th17 cells decrease in CTB patients
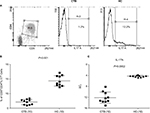
Flow cytometric analysis of data was done to identify the Th17 cell percentage in 48 hours PPD-stimulated PBMC cultures from CTB patients and HC. As expected (Figure 2A and B), the intracellular presence of IL-17A was associated with CD3+CD4+ cells in all the clinical groups. CTB patients showed significantly low (P<0.001) (mean% ± SD; 9.3±1.7) of CD3+-gated CD4+IL-17A+ cells as compared to nonreactive HC (mean% ± SD; 2.1±0.9).
Further validation of the above data was obtained with qPCR gene expression of IL-17A and IFN-γ in skin lesions of CTB and normal skin. Expression of IL-17A was significantly downregulated (P<0.0002, mean ± SD; 12.1±2.0) in CTB patients as compared to the normal healthy skin (IL-17; 4.2±0.3) (Figure 2C). Gene expression of IFN-γ showed significant downregulation (P<0.001, CTB; 7.7±1.1 and HC; 6.2±1.1) in CTB patients as compared to HC group.
Upregulation of FOXP3+ Treg cells and intracellular TGF-β cells in CTB patients
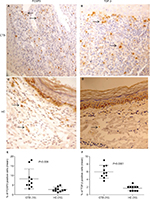
In situ: A total of 10 CTB and HC samples were studied for nuclear FOXP3 and cytoplasm TGF-β by IHC. The percentages of immune cell markers by quantitative image analysis are shown in Figure 3. Skin lesions of CTB patients and HC showed the presence of nuclear FOXP3+ staining using IHC (Figure 3A and C). However they were present in a limited pattern around as well as among the epithelioid cells of the tuberculoid granulomas; FOXP3+ cells were present among the foamy macrophages of the CTB granulomas in the dermis. The distribution of positive cells for all markers was not uniform, and 1,000 cells were enumerated to obtain a percentage of positive cells FOXP3+ cells. The percentage of FOXP3+ cells was significantly higher (P<0.008) in CTB (mean ± SD; 8.2±5.2) as compared to (2.6±1.1) normal skin. TGF-β-induced diffuse cytoplasmic pattern were also significantly higher in CTB skin (P<0.0001) (mean% ± SD; 5.9±1.6) as compared (1.7±0.6) to HC (Figure 3B and D).
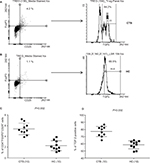
In vitro: The role of Treg during CTB was investigated using both flow cytometry (Figure 4A and B) and qPCR analysis (Figure 5A and B). Forty-eight hours-stimulated PBMC and skin lesions were investigated from both CTB patients and HC group. CTB patients showed a significant (P<0.002) increase in Treg cells (mean% ± SD; 5.3±1.0) as compared to the HC group (2.2±0.9) (Figure 4C). Of the interest, intracellular TGF-β+ Treg cells were also significantly higher (P<0.0001) in CTB (mean% ± SD; 77.14±7.0) patients as compared to HC (58.5±5.6) group (Figure 4D). In concordance with the above, using qPCR (Figure 5A and B) FOXP3 and TGF-β gene expressions were found to be significantly (P<0.005, P<0.001) higher in skin lesions of CTB patients as compared to normal skin.
Gene expression of FOXP3 and TGF-β showed positive correlation
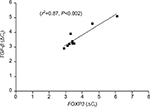
FOXP3 is a transcription factor of Treg cells and promotes TGF-β production in infectious diseases. As reported earlier, FOXP3 and TGF-β were found to be significantly upregulated in CTB patients (Figure 3A and B). In our study, a significant positive correlation of coexpression of FOXP3 and TGF-β was observed by qPCR (r2=0.87, P<0.002) in CTB patients’ skin (Figure 6). However, FOXP3 did not show any correlation with IL-17 gene expression.
Discussion
M. tuberculosis causes pulmonary and extrapulmonary tuberculosis. It is among the oldest microbe known to humanity, and it is a master manipulator of the immune system in terms of tolerance and pathogen persistence. Study of the immune mechanism during the pathogenesis of TB is still a matter of debate. The involvement of Th17 and Treg cells in pulmonary TB has been reported earlier.21 Th17 and Th1 subsets have emerged to build abundant layers of defense against various pathogens and diseases. However, concomitant with these effector responses, a network of Treg cells responses keep the effector cells of the cell-mediated immunity in homeostasis. The present investigations were undertaken on CTB patients with a view to understand the immune mechanisms underlying the unique clinical types in tuberculosis as well as to investigate the differences between circulating cells and local immune responses in the lesional skin. In general, expression of TGF-β, IL-17, and IFN-γ in skin lesion and circulating PBMCs may be related to the differences in numbers of cells in lesions or the stronger recall responses in circulating cells.
Diverse effector T-cell response controls such a balance, either containing the pathogen (paucibacillary disease) or rendering their survival via immune suppression leading to a multibacillary disease.22 Treg cells are found to be very high in CTB patients, thus suppressing the host immune system. Treg cells mediate suppression activity primarily through the secretion of the immunosuppressive cytokine TGF-β.14,23
TGF-β has been established as having an anti-inflammatory effect in nature and secreted by FOXP3+ Treg cells.24 This is the first study that investigated the role of TGF-β -producing Treg and Th17 cells in a CTB patient. In the present study, flow cytometric analysis revealed that the frequency of TGF-β-producing FOXP3+ Treg cells showed an increasing trend in CTB patients as compared to HC. These Treg cells produced significant amounts of TGF-β in CTB patients, indicating their suppressive in nature. Previously, it was reported that TGF-β plays an important role in CD4+CD25+ Treg expansion, and it was also shown to inhibit the polarization of Th17 and Th1 cells.25 Further, it was observed in flow cytometric analysis that the intracellular presence of IL-17A was associated with CD3+CD4+ cells in CTB and HC groups. CTB patients showed significantly low number of CD3+-gated CD4+IL-17A+ cells as compared to nonreactive healthy subjects.
Th17 cell-produced IL-17 played a protective role in the onset of infectious diseases and is pathogenic in autoimmune disorders.17,21,26–29 Moreover, it has been involved in tissue remodeling, and recruitment and survival of neutrophils in the early stages of infection leading to control of the bacterial load. A positive correlation between IL-17 and IFN-γ has also been reported.21,26,30–33 It has long been assumed that the central role of Th1 cells in the defense against TB is due to the ability of IFN-g to activate macrophages and stimulate phagocytosis, phagosome maturation, production of reactive nitrogen intermediates, and antigen presentation.22,34,35 Investigators have reported that IFN-γ mediated protection by inhibiting the harmful response of Th17 cells rather than by inhibiting M. tuberculosis duplication.36
In the present study, gene expression level of IFN-γ and IL-17A was estimated by qPCR in skin lesions of CTB and healthy control. Here we found that IL-17A and IFN-γ expression were significantly low in CTB patients. However, TGF-β was found to be significantly high in these patients as compared to HC group. Moreover, gene expression of IL-17 and IFN-γ were not showing any correlation. This may be unexplained kinetics of the production of cytokines. Results of the present study also demonstrated significant positive correlation and co-expression of FOXP3 and TGF-β by qPCR. To our surprise, IL-17 and IFN-γ did not show any correlations with FOXP3 and TGF-β gene expression. This may be because of Th17 and Treg cells are a distinct population of T helper cells.
To strengthen our study, we evaluated these findings by histopathological analysis followed by flow cytometric analysis to asses it at cellular levels; findings were further confirmed at the molecular level by qPCR. Expression of FOXP3, a transcription factor for Treg cells was also validated by real-time PCR. However, Quantification and analysis of IL-6 and IL-1β in all samples could have given a more insightful picture about this new aspect. Since Th17 and Treg cells have been established as opposite to each other. The differentiation of both cells depends on IL-6, IL-1β and TGF-β balance.37 The involvement of IL-6 and IL-1β might be possible in Th17 and Treg cells differentiation in CTB patients. Therefore further investigation of IL-6 and IL-1β could have given a more conclusive picture. We also understand the heterogeneity of our study population which consists of children and adult in terms of immune impairment in children. Collectively, this study point out a shift in our understanding of the immunological features that mediate and regulate the immune suppression and the disease progression in CTB patients with a new paradigm (TGF-β producing Tregs) that IL-17 may be protective in nature during CTB infection.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgment
CS is grateful to the Department of Science and Technology to National Postdoc Fellowship (PDF/2016/001997), New Delhi, India, for financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
Glassroth J. Clinical considerations in designing trials of vaccines for tuberculosis. Clin Infect Dis. 2000;30(Suppl 3):S229–S232. | ||
Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines. 2011;10(5):645–658. | ||
Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. | ||
Chakrabortty R, Rahman MA, Ferdousi KR, Paul BK. Cutaneous tuberculosis: an update. Mymensingh Med J. 2016;25(2):385–391. | ||
Dias MF, Bernardes Filho F, Quaresma MV, Nascimento LV, Nery JA, Azulay DR. Update on cutaneous tuberculosis. An Bras Dermatol. 2014;89(6):925–938. | ||
Lyadova IV, Panteleev AV. Th1 and Th17 cells in tuberculosis: protection, pathology, and biomarkers. Mediators Inflamm. 2015;2015:854507. | ||
Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280(5368):1432–1435. | ||
Jung YJ, LaCourse R, Ryan L, North RJ. Virulent but not avirulent Mycobacterium tuberculosis can evade the growth inhibitory action of a T helper 1-dependent, nitric oxide synthase 2-independent defense in mice. J Exp Med. 2002;196(7):991–998. | ||
Vilaplana C, Prats C, Marzo E, et al. To achieve an earlier IFN-γ response is not sufficient to control Mycobacterium tuberculosis infection in mice. PLoS One. 2014;9(6):e100830. | ||
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. | ||
Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16(11):1643–1656. | ||
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. | ||
Saini C, Nath I, Ramesh V. Role of Th17 and Foxp3 T cells mechanisms underlying the leprosy spectrum. In: LEPCON; March 28–30, 2014; PGIMR Chandigarh, India. | ||
Saini C, Ramesh V, Nath I. Increase in TGF-β secreting CD4+ CD25+ FOXP3+ T regulatory cells in anergic lepromatous leprosy patients. PLoS Negl Trop Dis. 2014;8(1):e2639. | ||
Kumar S, Naqvi RA, Ali R, Rani R, Khanna N, Rao DN. CD4+CD25+ T regs with acetylated FoxP3 are associated with immune suppression in human leprosy. Mol Immunol. 2013;56(4):513–520. | ||
Larson RP, Shafiani S, Urdahl KB. Foxp3(+) regulatory T cells in tuberculosis. Adv Exp Med Biol. 2013;783:165–180. | ||
Saini C, Ramesh V, Nath I. CD4+ Th17 cells discriminate clinical types and constitute a third subset of non Th1, non Th2 T cells in human leprosy. PLoS Negl Trop Dis. 2013;7(7):e2338. | ||
Saini C, Siddiqui A, Ramesh V, Nath I. Leprosy reactions show increased Th17 cell activity and reduced FOXP3+ Tregs with concomitant decrease in TGF-β and increase in IL-6. PLoS Negl Trop Dis. 2016;10(4):e0004592. | ||
Sharma SK, Ryan H, Khaparde S. Index TB Guidelines: guidelines on extra-pulmonary tuberculosis for India. Indian J Med Res. 2017;145(4):448–463. | ||
Santos JB, Figueiredo AR, Ferraz CE, de Oliveira MH, da Silva PG, de Medeiros VLS. Cutaneous tuberculosis: diagnosis, histopathology and treatment – part II. An Bras Dermatol. 2014;89(4):545–555. | ||
Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21(6):455–462. | ||
Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264(1):182–203. | ||
Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6(4):353–360. | ||
Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. | ||
Sadhu S, Khaitan BK, Joshi B, Sengupta U, Nautiyal AK, Mitra DK. Reciprocity between regulatory T cells and Th17 cells: relevance to polarized immunity in leprosy. PLoS Negl Trop Dis. 2016;10(1): e0004338. | ||
Umemura M, Yahagi A, Hamada S, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178(6):3786–3796. | ||
Gonzalez-Lombana C, Gimblet C, Bacellar O, et al. IL-17 mediates immunopathology in the absence of IL-10 following Leishmania major infection. PLoS Pathog. 2013;9(3):e1003243. | ||
Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126(2):177–185. | ||
Saini C, Nath I, Ramesh V. Role of TH17 cells in the leprosy spectrum. In: International Leprosy Congress; September 18–21, 2013; Brussels, Belgium. | ||
Hu S, He W, Du X, et al. IL-17 production of neutrophils enhances antibacteria ability but promotes arthritis development during Mycobacterium tuberculosis infection. EBioMedicine. 2017;23:88–99. | ||
Jurado JO, Pasquinelli V, Alvarez IB, et al. IL-17 and IFN-γ expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J Leukoc Biol. 2012;91(6):991–1002. | ||
Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol. 2009;159:109–119. | ||
Nath I, Curtis J, Sharma AK, Talwar GP. Circulating T-cell numbers and their mitogenic potential in leprosy – correlation with mycobacterial load. Clin Exp Immunol. 1977;29(3):393–400. | ||
Flynn JL, Chan J, Lin PL. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 2011;4(3):271–278. | ||
Ma J, Chen T, Mandelin J, et al. Regulation of macrophage activation. Cell Mol Life Sci. 2003;60(11):2334–2346. | ||
Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208(11):2251–2262. | ||
Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8(9):942–949. |
Supplementary materials
  | Figure S1 Flow cytometry dot plot are showing gating strategy and unstimulated and PHA control for Th17 cells. Abbreviation: PHA, phytohemagglutinin. |
  | Figure S2 Flow cytometry dot plot are showing gating strategy and unstimulated and PHA control for TGF-β producing Treg cells. Abbreviation: PHA, phytohemagglutinin. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.