Back to Journals » Cancer Management and Research » Volume 12
Refractory Metastatic Colorectal Cancer: Current Challenges and Future Prospects
Authors Lam M , Lum C , Latham S , Tipping Smith S , Prenen H , Segelov E
Received 13 May 2020
Accepted for publication 26 June 2020
Published 15 July 2020 Volume 2020:12 Pages 5819—5830
DOI https://doi.org/10.2147/CMAR.S213236
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Marissa Lam,1 Caroline Lum,1 Sarah Latham,1 Sam Tipping Smith,1 Hans Prenen,2 Eva Segelov1,3
1Department of Medical Oncology, Monash Medical Center, Clayton, Australia; 2Department of Oncology, University Hospital Antwerp, Edegem, Belgium; 3Faculty of Medicine, Monash University, Clayton, Australia
Correspondence: Eva Segelov Level 7 MHTP, Monash Health 246 Clayton Road, Clayton VIC 3168, Australia
Tel +613 85722392
Fax +613 8572 2446
Email [email protected]
Abstract: Despite advances, patients with metastatic colorectal cancer (mCRC) still have poor long-term survival. Identification of molecular subtypes is important to guide therapy through standard treatment pathways and holds promise for the development of new treatments. Following standard first- and second-line chemotherapy plus targeted agents, many patients retain a reasonable performance status, and thus are seeking further effective treatment to extend life and maintain symptom control. The challenge lies in selecting the most appropriate therapy in the third- and fourth-line settings, from a range of options including the relatively new oral agents TAS-102 and regorafenib, or rechallenge with previous chemotherapy or anti-epidermal growth factor receptor (anti-EGFR) monoclonal antibodies (mAB). Beyond this, therapy consists of trials involving novel agents and new combinations of treatments with theoretical synergy and/or non-overlapping toxicity. There is a great focus on enhancing immunogenicity in mCRC, to reflect the impressive results of immunotherapy drugs in the small cohort with mismatch repair deficient (dMMR) mCRC. Rare molecular subtypes of mCRC are increasingly being identified, including Her2-positive disease, NTRK fusions and others. Clinical trials exploring the efficacy of immunomodulatory and precision agents are plentiful and will hopefully yield clinically meaningful results that can be rapidly translated into routine care.
Keywords: molecular targets, genomic profiling, immunotherapy, precision medicine
Introduction
Colorectal cancer (CRC) is a major global health issue, being the third most commonly diagnosed malignancy with an estimated global incidence of over 1.8 million in 2018, predicted to increase to 2.2 million in 2030.1,2 CRC is the second commonest cause of global cancer mortality with 0.5 million deaths in 2018, predicted to increase to 1.1 million by 2030.1,2 Twenty percent of patients have metastatic colorectal cancer (mCRC) at presentation, whilst up to 50% of the patients who present with early-stage disease relapse later, despite curative-intent surgery, (neo)adjuvant chemotherapy and/or radiotherapy.
Over the last decade, clinical outcomes in mCRC have improved significantly, largely due to the identification of molecular subtypes. The median overall survival (OS) now approximates 30 months in clinical trial populations and two years in the general population.3 Molecular profiling of tumors is now routinely performed, to identify the approximately 40% that are RAS wild type (WT) that are susceptible to anti-epidermal growth factor receptor (anti-EGFR) monoclonal antibody (mAB) therapy;4 tumors with deficiencies in mismatch repair genes (dMMR) which are highly responsive to immune checkpoint inhibitors; targeted antibodies (AB) for tumors with Her2 amplification or mutation; combination therapy for tumors with BRAF mutations; and other rarer subtypes.
After two lines of chemotherapy, 44–50% of the patients may retain a good performance status and be suitable to receive further therapy to improve quantity and quality of life.5 Understanding the mechanisms that drive treatment resistance is essential in guiding the development of new therapies in this refractory stage. This manuscript provides an overview of currently available agents, and emerging options after failure of standard treatment.
Initial Therapy of Metastatic Colorectal Cancer
For more than 50 years, fluoropyrimidine therapy with 5-fluorouracil (5-FU) administered as an infusional agent or an oral form, capecitabine, has been the cornerstone of treatment for mCRC. Standard combinations include oxaliplatin, irinotecan or both (regimens such as “FOLFOX”, “FOLFIRI”, and “FOLFOXIRI”) plus either the anti-vascular endothelial growth factor (anti-VEGF) mAB bevacizumab, or one of the anti-EGFR mABs in patients with no tumor mutations in RAS genes, ie wild type (WT). Alternative anti-VEGF mABs that can be used in the second-line setting include ramucirumab or aflibercept, with efficacy demonstrated in the “RAISE” and “VELOUR” phase 3 trials, respectively, when used with “FOLFIRI”.6,7 The treatment pathway usually includes a de-escalation or maintenance phase. In this article, patients whose disease has progressed beyond these therapies are defined as refractory.
Although there is Level 1 evidence for third- and fourth-line treatment, not all are globally available. Options include Trifluridine/Tipiracil (TAS-102); regorafenib; rechallenge with oxaliplatin; or single-agent anti-EGFR mAB in RAS WT disease. Older regimens such as Mitomycin C plus 5-FU are rarely prescribed due to low efficacy.8
Treatment of Chemorefractory mCRC
Trifluridine/Tipiracil (TAS-102)
TAS-102 is an orally administered combination of trifluridine, a cytotoxic nucleic acid analogue, and tipiracil, a thymidine phosphorylase inhibitor that prevents enzymatic breakdown of the active compound.9 TAS-102 became a standard of care option based on the multicenter randomized phase 3 “RECOURSE” trial (n=800) of TAS-102 compared to placebo for mCRC patients who had received all prior chemotherapy plus anti-VEGF therapy and/or anti-EGFR mAB for RAS WT mCRC.9 The primary endpoint was met, with median OS 7.1 versus (v) 5.3 months (m) [hazard ratio (HR) 0.68; 95% confidence interval (CI) 0.58–0.81; p<0.001], and small improvement in median progression-free survival (PFS) [2.0 v 1.7 m; HR 0.48; p<0.001].7 Of note, 17–20% of the patients had received regorafenib.9 Grade 3 or higher adverse effects (AEs) were reported in 69% of the patients; neutropenia was the most frequent although only 4% experienced febrile neutropenia.9 Interestingly, a post-hoc association between TAS-102-induced neutropenia and efficacy has been demonstrated, suggesting that dose incrementing to neutropenia may be of value.10,11
Due to concern regarding ethnic variation in pharmacogenomics, the “TERRA” trial was undertaken in a similar Asian population, but with no requirement for previous anti-VEGF or anti-EGFR therapy. There was similar improvement in median OS [7.8 v 7.1 m; HR 0.79; 95% CI; p=0.035] and PFS [2 vs 1.8 m; HR 0.43; p<0.001].12
In an effort to improve efficacy and harness potential synergy, TAS-102 is being trialed in a number of combinations. TAS-102 plus bevacizumab for refractory mCRC is supported by pre-clinical and early trial evidence; a phase 1/2 single-arm study (“C-TASK FORCE”) reported a PFS rate of 42.9% at 16 weeks, with median PFS 3.7 m and median OS 11.4 m in the primary analysis.13 A subsequent phase 2 study (n=93) reached the primary endpoint of improved median PFS for the combination compared to TAS-102 alone [4.6 v 2.6 m; HR 0.45; 95% CI 0.29–0.72; p=0.0015]; median OS was also improved [9.4 v 6.7 m; HR 0.55; 95% CI 0.32–0.94; p=0.028].14 Ramucirumab, another anti-VEGF mAB, is being combined with TAS-102 in the “REMETY” phase 1 study which reported a disease control rate (DCR) at 8 weeks of 58.3%, with PFS and OS data awaited.15 A phase 2b study using the combination is ongoing.16
Oxaliplatin plus TAS-102 is being investigated in a phase 2 trial, consequent to a phase 1 study demonstrating a DCR of 67% at 8 weeks and no dose-limiting toxicities.17 Despite supportive preclinical data, a phase 1/2 trial of TAS-102 plus panitumumab in 56 patients with RAS WT mCRC (with no prior anti-EGFR or regorafenib) reported a 33.3% PFS rate at 6 m, below the prespecified threshold for activity.18 With regard to immunotherapy, a phase 2 study of TAS-102 plus nivolumab in patients with proficient MMR (pMMR) refractory mCRC was disappointing, with no observed responses and median PFS of 2.8 m.19
Regorafenib
Regorafenib is an oral inhibitor of multiple oncogenic kinases, including VEGF receptors.20 The United States Food and Drug Administration (FDA) granted approval for regorafenib in 2012 for use in patients with refractory mCRC based on results of the phase 3 “CORRECT” trial (n=760) comparing regorafenib to placebo. The primary end point was met, with improved median OS [6.4 v 5.0 m; HR 0.77; 95% CI 0.64–0.94; p=0.0052] and small improvement in PFS [1.9 v 1.7 m, HR 0.49; p<0.0001].21 Similar to TAS-102, the small PFS gain would not be clinically meaningful in the absence of the OS benefit. The DCR was significant [41% v 15%, p<0.0001], although ORR was 1%.21
In the Asian population, the “CONCUR” study was similar but did not require prior anti-VEGF or anti-EGFR therapy. This demonstrated an improved median OS [8.8 v 6.3 m; HR 0.55; 95% CI 0.4–0.77, p=0.00016] and median PFS [3.2 v 1.7 m; HR 0.31; p<0.0001].22
The rate of adverse effects with regorafenib at the trial dose of 160mg daily for 21 days of a 28-day cycle was concerningly high. Over 50% of the patients had Grade 3 or higher toxicity; most commonly palmar-plantar erythrodysesthesia (PPE), hypertension, fatigue and diarrhea; dose modification was required in over 70% of the patients.21,22
To address this, the “ReDOS” phase 2 trial investigated an alternative dosing schedule, by commencing at 80mg daily and titrating up by 40mg per week to 160mg. On this schedule, more patients initiated cycle 3 of treatment compared to standard dosing.23 However, progression occurred prior to cycle 3 in 47% of the patients on the modified schedule arm v 37% with standard dosing, raising concerns about the utility of this strategy, although OS and PFS were not statistically significantly different.23 The dose escalation strategy is still used in clinical practice, particularly for patients with more indolent disease. This overall poor tolerability has limited regorafenib use in the real-world mCRC setting.
Rechallenge Strategies
Given the limited options, re-introducing oxaliplatin is an attractive strategy. In selected patients with previous oxaliplatin response (defined in this article as a PFS interval of at least 6 months), the “RE-OPEN”, “RE-OX” and a similar Korean trial demonstrated disease control rates of 39–68%, with OS ranging from 14.5 to 18.5 m.24–26 Grade 1–2 oxaliplatin-induced neuropathy (OIN) occurred in 53% of the patients in “RE-OPEN”, and grade 2–3 in 14.5% of the patients in the Korean study.
Patients who ceased oxaliplatin because of OIN without concurrent disease progression present a challenging clinical scenario.27 A retrospective analysis of 106 patients demonstrated feasibility with close toxicity monitoring, although one-third of patients developed worsening neuropathy.28 The clinical decision must balance the impact of worsening OIN on quality of life for patients already with a short prognosis.
Rechallenge with the anti-EGFR mABs cetuximab or panitumumab despite prior progression is based on data demonstrating the dynamic nature of clonal populations with EGFR resistance mutations which are now well defined, measured by circulating tumor deoxyribonucleic acid (ctDNA).29 Ct-DNA-detected KRAS mutant clones were shown to rise during anti-EGFR therapy as a selection pressure phenomenon, with a decay half-life of 4.4 m after drug cessation, leading to the strategy of interval dosing.30,31 A retrospective review demonstrated non-statistically significant trends towards improved median PFS and overall response rate (ORR) as the time intervals between anti-EGFR cycles increased [ORR 32% for >2 half-lives v 20% <1 half-life].31
Monitoring of RAS-resistant mutations using ct-DNA was taken forward in the “CRICKET” study.32 This examined rechallenge with cetuximab plus irinotecan in the third-line setting in patients with previous response to cetuximab-containing therapy. An ORR of 21% [95% CI 10–40%] and DCR of 54% [95% CI 36–70%] were observed.32 Patients with RAS WT ctDNA had a longer PFS than those with ctDNA-detected RAS mutations [median PFS 4.0 v 1.9 m; HR 0.44; 95% CI 0.18–0.98; p=0.03].32
A 2019 systematic review supported anti-EGFR mAB rechallenge after evaluating 26 studies of retreatment.33 An ongoing prospective trial utilizing ctDNA monitoring will provide definitive proof.34
Choosing Between Currently Available Therapy
TAS-102 and regorafenib have not been compared head-to-head but appear to have similar efficacy in two meta-analyses, with regorafenib having higher toxicity of any grade.35,36 The clinical decision regarding choice and order of use should be based on matching the side effect profile with each patient’s comorbidities and pace of disease (Table 1).
 |
Table 1 Currently Available Therapies for Refractory mCRC |
Emerging Therapies
Anti-VEGF Strategies
Two novel oral agents are in clinical trials. Fruquitinib, a selective inhibitor of VEGF receptors 1, 2 and 3, is being investigated in the “FRESCO” phase 3 trial in a Chinese refractory mCRC population naive to anti-VEGF therapy.37,38 This is based on phase 2 data where fruquintinib improved median OS to 9.3 m, v 6.6 m with placebo [HR 0.65; 95% CI 0.51–0.83; p<0.001].38 The “FRESCO-2” trial is being conducted in a similar population but allows previous treatment with anti-VEGF mAB, TAS-102 or regorafenib.39
Famitinib is an oral tyrosine kinase inhibitor (TKI) with activity that includes inhibition of c-kit, VEGF receptors 2 and 3, platelet-derived growth factor receptor, and FMS-like tyrosine kinases.40 This agent was compared to placebo in a phase 2 trial in refractory mCRC. The primary end point was met, with improved median PFS 2.8 v 1.5 m [HR 0.60; 95% CI 0.41–0.86; p=0.004] with OS data awaited.41 The most common Grade 3–4 AEs included PPE, thrombocytopenia and neutropenia.41
Targeting Her2
Based on success in breast and gastric cancer, agents have been used with the hope of similar responses in patients with mCRC containing Her2 aberrations. Her2 amplification is found in around 5% of patients with KRAS/BRAF WT mCRC, and around 1% with RAS mutant tumors.42 Amplifications are notably more prevalent in Chinese populations; around 14% of patients with KRAS/BRAF WT mCRC and 4.4% with RAS mutant disease.43 An analysis of patients from the “FOCUS” and “PICCOLO” studies (n=1342) in mCRC demonstrated that Her2 amplification confers resistance to anti-EGFR treatment.42,44
Her2 activating mutations, which are also rare in breast and gastric cancer, are found in around 2% of mCRC; these will not be identified by immunohistochemistry.45
The combination of the anti-Her2 mABs trastuzumab and pertuzumab was investigated in the phase 2 “TRIUMPH” study of 19 patients with Her2-amplified, RAS WT refractory mCRC.46 Her2 amplification was confirmed on tumor tissue and/or ctDNA, with analysis of ORR reported by detection method. Both had similar ORR, around 33–35%.46 Combined median PFS was 4 months. Interestingly, the patients who had disease progression had baseline ct-DNA-detected KRAS, BRAF, PIK3CA or Her2 activating mutations.46
In the phase 2 “MOUNTAINEER” trial, 22 patients with RAS WT, Her2 amplified refractory mCRC received trastuzumab plus tucatinib, an oral TKI that inhibits the Her2 receptor.47 The ORR was 55%, median PFS was 6.2 m [95% CI 3.5-NE] with median duration of response not reached at a median 10.6 m of follow up; median OS was 17.3 m [95% CI 12.3-NE].47
Trastuzumab plus the oral dual-target TKI, lapatinib, appears to be an efficacious combination in mCRC based on the phase 2 “HERACLES” trial.48 In the heavily pretreated cohort, the median PFS was 21 weeks [95% CI 16–32] and median OS 46 weeks [95% CI 33–68 weeks].49 The primary endpoint was met with ORR 30.3% [95% CI 17–47%] and DCR 70% [95% CI 52–82%].50 The combination was chosen due to efficacy not seen with either single agent in preclinical models. Although this regimen is now standard for refractory disease, these drugs are not universally funded by health schemes, and the cost and efficiency of screening many patients must be considered. However, trials examining utility in earlier lines of therapy are in progress. Her2 activating mutations also confer susceptibility to trastuzumab and lapatinib in xenograft models.51
The phase 2 trial “HERACLES-B” (n=30) investigated pertuzumab and trastuzumab-emtansine (TDM1) in a similar population.52 However, with an ORR of 10% [95% CI 0–28%], the trial did not meet its primary endpoint.52 Notably, the DCR was 80% [24 of 30; stable disease (SD) in 70%] and median PFS was 4.8 m [95% CI 3.6–5.8].52
Single-agent TDM1 is being investigated in a phase 2 trial after progression on trastuzumab and lapatinib.53 A novel Her2-targeted AB-drug conjugate, trastuzumab deruxtecan, is being investigated in a similar population.54 A phase 2 study (n=35) is investigating neratinib plus trastuzumab v neratinib plus cetuximab.55
In the “MyPathway” study, an ongoing phase 2a multi-basket trial for Her2-amplified cancers, 57 patients with refractory CRC received trastuzumab and pertuzumab.56 In an updated report, the objective response rate was 32% [95% CI 20–45%].54 The follow-on randomized phase 2 study “CETIRI” is comparing trastuzumab plus pertuzumab to cetuximab and irinotecan (in the second or later line).57
Neurotrophin Tropomyosin Receptor Kinase (NTRK) Fusions
NTRK fusions are reported at a prevalence of 0.2–2.4% in CRC, but 4% with dMMR present.58 Current drugs exploiting this target are the first-generation tropomyosin kinase (TRK) inhibitors larotrectinib and entrectinib; and the next-generation agents selitrectinib (LOXO-195) and repotrectinib.
Larotrectinib and entrectinib both inhibit TRK A, B and C; entrectinib is a multikinase inhibitor (MKI) with activity also against ALK and ROS1.59 They demonstrated ORR of 75–79% and 57% respectively in a number of solid tumor basket studies, where 5% of the patients had mCRC.60–63 Larotrectinib was well tolerated, with 93% of AEs being Grade 1, and no treatment-related Grade 3–4 events.60 Entrectinib was associated with neurotoxic AEs including cognitive disorder, cerebellar ataxia, paresthesia and peripheral sensory neuropathy; the most common Grade 3 or higher AEs were weight gain and anemia.63 Both drugs were granted accelerated FDA approval within the last two years for TRK fusion-positive cancers. Although the number of mCRC patients in these studies was small, it would appear larotrectinib is preferred due to a better toxicity profile, particularly lack of neurotoxic AEs.
Resistance to TRK inhibitors has been shown via development of TRK mutations, amongst other mechanisms.64 Selitrectinib was specifically designed to overcome resistance mutations. Data from phase 1 and expanded access programs in patients with previous TRK inhibitor therapy have shown tolerability and an ORR of 34%; ORR was 45% in patients who developed mutations whilst on a TRK inhibitor.64 Common AEs included dizziness, nausea and vomiting, anemia, abdominal pain and fatigue.64 A second drug, repotrectinib, has activity in patients with resistance to first-generation TRK inhibitors due to acquired TRK, ALK or ROS1 mutations.65,66
Targeting BRAF Mutations
BRAF mutations occur in approximately 6–9% of mCRC, with 95% comprising a point mutation in the V600E allele.6,67,68 These tumors have aggressive biology and an unusual pattern of metastases (lung, brain, bone and peritoneum, with less liver involvement). Their poor prognosis (median OS around 12 m) means that only a third of patients are suitable for second-line therapy.67 About 12% of BRAF-mutant patients are concurrently dMMR, almost always due to a sporadic intra-tumoral mutation; they have a similarly poor prognosis.67,69
BRAF-mutant tumors respond poorly to standard therapy. The triplet regimen “FOLFOXIRI” plus or minus bevacizumab appears more active and is often the regimen of choice in the first-line setting.70,71 In the second line, BRAF inhibitor monotherapy failed to demonstrate activity, unlike melanoma. A proposed mechanism is EGFR-mediated rapid reactivation of ERK, after initial phospho-ERK inhibition.72 Anti-EGFR mABs were subsequently trialed also as monotherapy; however, two meta-analyses found no benefit.73,74 This resistance mechanism was overcome by combining BRAF and EGFR inhibitor therapy in preclinical studies.72 The combination has been taken forward in a number of trials (Table 2).
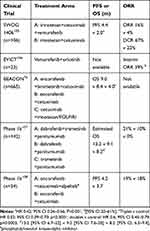 |
Table 2 Clinical Trials of Agents Targeting BRAF V600E Mutations in Advanced CRC |
Current NCCN guidelines suggest encorafenib plus anti-EGFR with or without binimetinib, or dabrafenib plus trametinib plus anti-EGFR as second-line therapy in patients with BRAF V600E mutations.75 The phase 3 “BEACON” trial suggested an OS benefit for triplet over doublet therapy [HR 0.79; 95% CI 0.59–1.06] but was not powered for this comparison.76 Notably, 62% of the patients receiving triplet therapy had Grade 3 diarrhea, compared to 33% with the doublet, making a clinical argument for the latter for many patients.76
Immune Checkpoint Inhibitors
Immune checkpoint inhibition (ICI) with anti-programmed death (PD-1 or PD-ligand-1) mAB therapy has proven highly effective in many cancers, but in mCRC patients only benefits those with microsatellite instability-high (MSI-H) or the correlative dMMR tumors.77 In patients with refractory mCRC, interim results from two phase 2 studies (“KEYNOTE-016” using pembrolizumab and “Checkmate-142” using nivolumab) demonstrated response rates of 40% and 31% respectively in patients with dMMR mCRC, but no response in patients with pMMR tumors.77,78 PD-1 expression did not appear to predict ICI response.77 A current phase 3 study is evaluating dual checkpoint blockade combining ipilimumab, a cytotoxic T-lymphocyte-associated protein 4 (CLTA-4) inhibitor, with nivolumab.79 ICI is currently approved by the FDA for use in the second line or later for dMMR mCRC, although recent data show dramatic benefit in the neoadjuvant and first-line setting.80
Concurrent BRAF or RAS mutations in dMMR mCRC are associated with a slightly lower response rate to single-agent anti-PD-1 compared to the WT (25–27% v 41%); however, the DCR was not statistically different.78 With dual checkpoint inhibition, ORR and DCR were similar whether BRAF was mutant or WT, in the presence of dMMR.81
Improving Tumor Immunogenicity in pMMR CRC
Using Known Agents
Combining ICI with an agent that causes tumor cell apoptosis and antigen presentation to induce an antitumor immune response, such as chemotherapy, radiation, or targeted therapy, seems the most promising next step in making ICI effective in pMMR mCRC. Standard cytotoxic chemotherapy has been shown to increase antigen presentation and the ratio of cytotoxic to regulatory T-cells, with resulting increased PD-1 expression.82 In mouse CRC models, both 5-FU and oxaliplatin increased PDL-1 expression, and when followed by an anti-PD1 agent improved OS.82,83 Irinotecan and anti-PD1 demonstrated an additive effect on tumor regression in mice.84 Multiple clinical trials, mainly in the refractory setting, are now examining combinations with ICI (Table 3). The risk of increased toxicity and impact on quality of life is paramount to document.
 |
Table 3 Trials Using Combination Therapy with ICI |
Using Novel Agents
A phase 1b trial of the anti-CD20 mAB obinutuzumab followed by cibisatamab (a mAB with bispecificity for tumor CarcinoEmbryonic Antigen (CEA) and T-cell CD3) aims to promote cytotoxic immune cell recruitment, and is being combined with atezolizumab.85 The innate immune system is another targetable pathway, with autologous infusions of universal donor natural killer cells being tested with a cytokine support agent, ALT803.86 Another trial is using an injectable vaccine to promote secretion of donor granulocyte macrophage colony-stimulating factor (GM-CSF).87 Indoleamine 2,3 dehydrogenase (IDO) inhibitors are being combined with anti-OX40 AB (promoting cytotoxic T-cells) and a bifunctional anti-PD-L1/TGFβ fusion mAB in a phase 1 trial.88
Antitumor vaccines aim to stimulate presentation of tumor-associated antigens by antigen-presenting cells (APCs) to cytotoxic and memory T-cells. A phase 1b basket trial using the personalised tumor vaccine RO7198457 in combination with atezolizumab is currently underway, as well as an Australian phase 1 trial using the TetMYB vaccine together with tiselizumab, an anti-PD1 agent.89,90 An anti-Her2 tumor vaccine is being examined in a phase 1 trial for Her2-amplified mCRC.91
Novel Targeted Therapy and Drug Delivery Vehicles
mABs against CEA, a membrane-anchored glycoprotein, such as labetuzumab, aim to target payloads to tissue CEA which is overexpressed in over 90% of CRC.92 Such payloads include targeted photodynamic therapy, radio-guided surgery, and radioimmunotherapy for peritoneal metastases.93–95 A phase 1/2 trial using the payload govitecan (a liposomal irinotecan metabolite) demonstrated tumor response in 38% of the 72 patients enrolled, with SD in 48%.96 Diarrhea and cytopenias were the major toxicities (7–16% of patients) and phase 2 of the trial is ongoing.96
Another focus is loco-regional cytotoxic delivery, using nanomedicines. Alginate microcapsules prevented the degradation of anti-CD44 agents in the gastrointestinal tract, with accumulation of the micelles in CD44-positive colorectal tumors.97 Targeted micelles may provide improved drug delivery to poorly vascularized tumors.
Engineered viruses with inherent tropism for cancer cells are also being investigated as a targeting method. In mouse mCRC models, TG6002 with 5-fluorocytosine and intraperitoneal vaccines have improved survival.98,99 Direct intra-tumoral injection of talimogene laherparepvec (TVEC), an oncolytic herpes virus with activity in melanoma, is currently in phase 1b/2 trials.100
CAR-T Cell Therapy
Individualized chimeric antigen receptor T-cells (CAR-T cells) have been developed with success mainly in hematological malignancy, with focus turning to solid tumors. Intraperitoneal delivery of anti-CEA CAR-T cells induced distal tumor response and prevented peritoneal reseeding in murine models.101 A phase 1 study using hepatic arterial injection of the same CAR-T cells met safety targets, with tumor necrosis seen in 4 of 6 patients.102
Supportive Care
Early integration of palliative care is shown to reduce in-hospital deaths and end-of-life healthcare costs and prolong overall survival in mCRC patients.103,104 Maximal symptom control to maintain good quality of life requires excellent holistic care. Patients with mCRC have particular, complex end-of-life issues, including nutrition, stomal complications, recurrent ascites and bowel obstructions due to peritoneal disease.
Conclusion
Increasing numbers of patients with mCRC maintain good performance status even with disease progression, and seek active anti-cancer treatment in the third- and fourth-line setting and beyond. There have been relatively few advances in chemotherapy and only a handful of new agents entering standard practice. Benefit from immunotherapy is currently restricted to the small number of patients with tumors harboring deficient mismatch repair genes. Multiple new agents are in clinical trials at various stages, with combinations of therapy aimed at overcoming innate and acquired resistance. Toxicity and costs to both the patient and healthcare systems are important factors to weigh against modest gains in treatment efficacy. Biomarkers to guide patient selection for refractory therapies are eagerly sought. It is hoped that the next decade brings significant advances in this area of great need.
Disclosure
Prof. Dr Eva Segelov reports personal fees from Merck, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683. doi:10.1136/gutjnl-2015-310912
3. Pericay C, Gallego J, Montes AF, et al. Real world data in colorectal cancer: a retrospective analysis of overall survival in metastatic colorectal cancer patients between 2011–2015 treated in Spain, preliminary results (RWD-ACROSS study). Ann Oncol. 2018;29:v78. doi:10.1093/annonc/mdy151.276
4. Chiu JW, Krzyzanowska MK, Serra S, et al. Molecular profiling of patients with advanced colorectal cancer: Princess Margaret Cancer Centre experience. Clin Colorectal Cancer. 2018;17(1):73–79. doi:10.1016/j.clcc.2017.10.010
5. Ducreux M, Malka D, Mendiboure J, et al. Sequential versus combination chemotherapy for the treatment of advanced colorectal cancer (FFCD 2000–05): an open-label, randomised, phase 3 trial. Lancet Oncol. 2011;12(11):1032–1044. doi:10.1016/S1470-2045(11)70199-1
6. Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. doi:10.1016/S1470-2045(15)70127-0
7. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–3506. doi:10.1200/JCO.2012.42.8201
8. Ferrarotto R, Machado K, Mak MP, et al. A multicenter, multinational analysis of mitomycin C in refractory metastatic colorectal cancer. Eur J Cancer. 2012;48(6):820–826. doi:10.1016/j.ejca.2012.01.008
9. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi:10.1056/NEJMoa1414325
10. Hamauchi S, Yamazaki K, Masuishi T, et al. Neutropenia as a predictive factor in metastatic colorectal cancer treated with TAS-102. Clin Colorectal Cancer. 2017;16(1):51–57. doi:10.1016/j.clcc.2016.07.005
11. Makihara K, Fukui R, Uchiyama H, Shigeoka Y, Toyokawa A. Decreased percentage of neutrophil is a predict factor for the efficacy of trifluridine and tipiracil hydrochloride for pretreated metastatic colorectal cancer. J Gastrointest Oncol. 2019;10(5):878–885. doi:10.21037/jgo.2019.04.04
12. Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase iii trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol. 2018;36(4):350–358. doi:10.1200/JCO.2017.74.3245
13. Kuboki Y, Nishina T, Shinozaki E, et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol. 2017;18(9):1172–1181. doi:10.1016/S1470-2045(17)30425-4
14. Pfeiffer P, Yilmaz M, Möller S, et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21(3):412–420. doi:10.1016/S1470-2045(19)30827-7
15. Moehler M, Stein A, Trojan J, et al. Regorafenib with TAS-102 (REGOTAS) in metastatic colorectal cancer patients who progressed after at least two standard therapies: efficacy and safety results of a multicenter Phase I study (REMETY). J Clin Oncol. 2020;38:158. doi:10.1200/JCO.2020.38.4_suppl.158
16. Kasper S, Zur Hausen G, Stein A, et al. A phase IIb study of ramucirumab in combination with TAS102 versus TAS102 monotherapy in metastatic, chemotherapy refractory colorectal cancer patients: the RAMTAS trial of the German AIO (KRK-0316). J Clin Oncol. 2019;37(15_suppl):TPS3617–TPS3617. doi:10.1200/JCO.2019.37.15_suppl.TPS3617
17. Cecchini M, Kortmansky J, Fischbach N, et al. A phase I study of TAS-102 in combination with oxaliplatin (TAS-OX) for refractory metastatic colorectal cancer (mCRC). J Clin Oncol. 2019;37:630. doi:10.1200/JCO.2019.37.4_suppl.630
18. Kuboki Y, Yoshino T, Kato T, et al. APOLLON: a phase I/II study of panitumumab combined with TAS-102 in patients (pts) with RAS wild-type (wt) metastatic colorectal cancer (mCRC). J Clin Oncol. 2018;36(15_suppl):3523. doi:10.1200/JCO.2018.36.15_suppl.3523
19. Patel M, Falchook G, Hamada K, et al. Results of a phase II study evaluating trifluridine/tipiracil plus nivolumab in patients with heavily pretreated microsatellite-stable (MSS) metastatic colorectal cancer (mCRC). J Clin Oncol. 2019;37:48. doi:10.1200/JCO.2019.37.8_suppl.48
20. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255. doi:10.1002/ijc.25864
21. Grothey A, Cutsem EV, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi:10.1016/S0140-6736(12)61900-X
22. Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–629. doi:10.1016/S1470-2045(15)70156-7
23. Bekaii-Saab TS, Ou F-S, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(8):1070–1082. doi:10.1016/S1470-2045(19)30272-4
24. Suenaga M, Mizunuma N, Matsusaka S, et al. Phase II study of reintroduction of oxaliplatin for advanced colorectal cancer in patients previously treated with oxaliplatin and irinotecan: RE-OPEN study. Drug Des Devel Ther. 2015;9:3099–3108. doi:10.2147/DDDT.S85567
25. Costa T, Nuñez J, Felismino T, Boente L, Mello C. REOX: evaluation of the efficacy of retreatment with an oxaliplatin-containing regimen in metastatic colorectal cancer: a retrospective single-center study. Clin Colorectal Cancer. 2017;16(4):316–323. doi:10.1016/j.clcc.2017.03.002
26. Yang Q, Huang Y, Jiang Z, et al. Rechallenge of oxaliplatin-containing regimens in the third- or later-line therapy for patients with heavily treated metastatic colorectal cancer. Onco Targets Ther. 2018;11:2467–2473. doi:10.2147/OTT.S154220
27. Kokotis P, Schmelz M, Kostouros E, Karandreas N, Dimopoulos M-A. Oxaliplatin-induced neuropathy: a long-term clinical and neurophysiologic follow-up study. Clin Colorectal Cancer. 2016;15(3):e133–e140. doi:10.1016/j.clcc.2016.02.009
28. Besora S, Santos C, Izquierdo C, Martinez-Villacampa MM, Bruna J, Velasco R. Rechallenge with oxaliplatin and peripheral neuropathy in colorectal cancer patients. J Cancer Res Clin Oncol. 2018;144(9):1793–1801. doi:10.1007/s00432-018-2691-8
29. Tonini G, Imperatori M, Vincenzi B, Frezza AM, Santini D. Rechallenge therapy and treatment holiday: different strategies in management of metastatic colorectal cancer. J Exp Clin Cancer Res. 2013;32:92. doi:10.1186/1756-9966-32-92
30. Siravegna G, Mussolin B, Buscarino M, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21(7):795–801. doi:10.1038/nm.3870
31. Parseghian CM, Loree JM, Morris VK, et al. Anti-EGFR-resistant clones decay exponentially after progression: implications for anti-EGFR re-challenge. Ann Oncol. 2019;30(2):243–249. doi:10.1093/annonc/mdy509
32. Cremolini C, Rossini D, Dell’Aquila E, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2018;5:343–350.
33. Mauri G, Pizzutilo EG, Amatu A, et al. Retreatment with anti-EGFR monoclonal antibodies in metastatic colorectal cancer: systematic review of different strategies. Cancer Treat Rev. 2019;73:41–53. doi:10.1016/j.ctrv.2018.12.006
34. Fondazione del Piemonte. Rechallenge with panitumumab driven by RAS dynamic of resistance. Available from: https://ClinicalTrials.gov/show/NCT03227926. NLM identifier: NCT03227926.
35. Abrahao A, Ko Y-J, Berry S, Chan K. A comparison of regorafenib and TAS-102 for metastatic colorectal cancer: a systematic review and network meta-analysis. Clin Colorectal Cancer. 2017;17:113–120.
36. Casadei Gardini A, Gelsomino F, Spallanzani A, Tamburini E, Scartozzi M, Cascinu S. Is there an optimal choice in refractory colorectal cancer? A network meta-analysis. Ann Oncol. 2019;30:iv14. doi:10.1093/annonc/mdz155.051
37. Cao J, Zhang J, Peng W, et al. A phase I study of safety and pharmacokinetics of fruquintinib, a novel selective inhibitor of vascular endothelial growth factor receptor-1,-2, and −3 tyrosine kinases in Chinese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;78:259–269. doi:10.1007/s00280-016-3069-8
38. Li J, Qin S, Xu RH, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. 2018;319(24):2486–2496. doi:10.1001/jama.2018.7855
39. Hutchison. A study of efficacy and safety of fruquintinib (HMPL-013) in patients with metastatic colorectal cancer (FRESCO-2). Available from: https://ClinicalTrials.gov/show/NCT04322539. NLM identifier: NCT04322539. Updated August.
40. Zhou A, Zhang W, Chang C, et al. Phase I study of the safety, pharmacokinetics and antitumor activity of famitinib. Cancer Chemother Pharmacol. 2013;72(5):1043–1053. doi:10.1007/s00280-013-2282-y
41. Xu R-H, Shen L, Wang K-M, et al. Famitinib versus placebo in the treatment of refractory metastatic colorectal cancer: a multicenter, randomized, double-blinded, placebo-controlled, phase II clinical trial. Chin J Cancer. 2017;36(1):97. doi:10.1186/s40880-017-0263-y
42. Richman SD, Southward K, Chambers P, et al. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol. 2016;238(4):562–570. doi:10.1002/path.4679
43. Dong Q, Shi B, Zhou M, et al. Growth suppression of colorectal cancer expressing S492R EGFR by monoclonal antibody CH12. Front Med. 2019;13(1):83–93. doi:10.1007/s11684-019-0682-z
44. Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies her2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1:508–523. doi:10.1158/2159-8290.CD-11-0109
45. Ross JS, Fakih M, Ali SM, et al. Targeting HER2 in colorectal cancer: the landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer. 2018;124(7):1358–1373. doi:10.1002/cncr.31125
46. Nakamura Y, Okamoto W, Kato T, et al. 526PD - TRIUMPH: primary efficacy of a phase II trial of trastuzumab (T) and pertuzumab (P) in patients (pts) with metastatic colorectal cancer (mCRC) with HER2 (ERBB2) amplification (amp) in tumour tissue or circulating tumour DNA (ctDNA): a GOZILA sub-study. Ann Oncol. 2019;30:v199–v200. doi:10.1093/annonc/mdz246.004
47. Strickler JH, Zemla T, Ou FS, et al. Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): initial results from the MOUNTAINEER trial. Ann Oncol. 2019;30:v200. doi:10.1093/annonc/mdz246.005
48. Siena S, Sartore-Bianchi A, Lonardi S, et al. Trastuzumab and lapatinib in HER2-amplified metastatic colorectal cancer patients (mCRC): the HERACLES trial. J Clin Oncol. 2015;33(15_suppl):3508. doi:10.1200/jco.2015.33.15_suppl.3508
49. Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(6):738–746. doi:10.1016/S1470-2045(16)00150-9
50. Siena S, Sartore-Bianchi A, Trusolino L, et al. Abstract CT005: final results of the HERACLES trial in HER2-amplified colorectal cancer. Cancer Res. 2017;77(13 Supplement):CT005–CT005.
51. Kanat O, Ertas H, Caner B. Dual HER2 inhibition strategies in the management of treatment-refractory metastatic colorectal cancer: history and status. World J Clin Cases. 2018;6:418–425. doi:10.12998/wjcc.v6.i11.418
52. Sartore-Bianchi A, Martino C, Lonardi S, et al. Phase II study of pertuzumab and trastuzumab-emtansine (T-DM1) in patients with HER2-positive metastatic colorectal cancer: the HERACLES-B (HER2 amplification for colo-rectaL cancer enhanced stratification, cohort B) trial. Ann Oncol. 2019;30:v869–v870. doi:10.1093/annonc/mdz394.024
53. Fondazione del Piemonte. Study of trastuzumab-emtansine in patients with HER2-positive metastatic colorectal cancer progressing after trastuzumab and lapatinib. Available from: https://ClinicalTrials.gov/show/NCT03418558. NLM identifier: NCT03418558.
54. Daiichi Sankyo. DS-8201a in human epidermal growth factor receptor2 (HER2)-expressing colorectal cancer (DESTINY-CRC01). Available from: https://ClinicalTrials.gov/show/NCT03384940. NLM identifier: NCT03384940.
55. Jacobs SA, Lee JJ, George TJ, et al. NSABP FC-11: a phase II study of neratinib (N) plus trastuzumab (T) or n plus cetuximab (C) in patients (pts) with “quadruple wild-type (WT)” (KRAS/NRAS/BRAF/PIK3CA WT) metastatic colorectal cancer (mCRC) based on HER2 status-amplified (amp), non-amplified (non-amp), WT, or mutated (mt). J Clin Oncol. 2019;37(4_suppl):TPS716.
56. Meric-Bernstam F, Hurwitz H, Raghav KPS, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20(4):518–530. doi:10.1016/S1470-2045(18)30904-5
57. Raghav KPS, McDonough SL, Tan BR, et al. A randomized phase II study of trastuzumab and pertuzumab (TP) compared to cetuximab and irinotecan (CETIRI) in advanced/metastatic colorectal cancer (mCRC) with HER2 amplification: S1613. J Clin Oncol. 2018;36(15_suppl):TPS3620–TPS3620. doi:10.1200/JCO.2018.36.15_suppl.TPS3620
58. Pietrantonio F, Di Nicolantonio F, Schrock AB, et al. ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer. J Natl Cancer Inst. 2017;109(12). doi:10.1093/jnci/djx089
59. Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019;30(Suppl_8):viii23–viii30. doi:10.1093/annonc/mdz282
60. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. doi:10.1056/NEJMoa1714448
61. Lassen UN, Albert CM, Kummar S, et al. Larotrectinib efficacy and safety in TRK fusion cancer: an expanded clinical dataset showing consistency in an age and tumor agnostic approach. Ann Oncol. 2018;29:viii133. doi:10.1093/annonc/mdy279.397
62. Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531–540. doi:10.1016/S1470-2045(19)30856-3
63. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271–282. doi:10.1016/S1470-2045(19)30691-6
64. Hyman D, Kummar S, Farago A, et al. Abstract CT127: phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi). Cancer Res. 2019;79(13 Supplement):CT127.
65. Drilon A, Ou S-HI, Cho BC, et al. Repotrectinib (TPX-0005) is a next generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent front mutations. Cancer Discov. 2018;8:1227–1236.
66. Drilon A, Zhai D, Deng W, et al. Abstract 442: repotrectinib, a next generation TRK inhibitor, overcomes TRK resistance mutations including solvent front, gatekeeper and compound mutations. Cancer Res. 2019;79(13 Supplement):442.
67. Seligmann JF, Fisher D, Smith CG, et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials. Ann Oncol. 2017;28(3):562–568. doi:10.1093/annonc/mdw645
68. Kopetz S, Desai J, Chan E, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015;33(34):4032–4038. doi:10.1200/JCO.2015.63.2497
69. Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20(20):5322. doi:10.1158/1078-0432.CCR-13-3045
70. Loupakis F, Cremolini C, Salvatore L, et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer. 2014;50(1):57–63. doi:10.1016/j.ejca.2013.08.024
71. Cremolini C, Antoniotti C, Lonardi S, et al. Primary tumor sidedness and benefit from FOLFOXIRI plus bevacizumab as initial therapy for metastatic colorectal cancer. Retrospective analysis of the TRIBE trial by GONO. Ann Oncol. 2018;29(7):1528–1534. doi:10.1093/annonc/mdy140
72. Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2(3):227–235. doi:10.1158/2159-8290.CD-11-0341
73. Rowland A, Dias MM, Wiese MD, et al. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer. 2015;112(12):1888–1894. doi:10.1038/bjc.2015.173
74. Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51(5):587–594. doi:10.1016/j.ejca.2015.01.054
75. Network NCC. Colon cancer (version 2.2020). Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
76. Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–1643. doi:10.1056/NEJMoa1908075
77. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi:10.1056/NEJMoa1500596
78. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi:10.1016/S1470-2045(17)30422-9
79. Bristol-Myers Squibb. A study of nivolumab, nivolumab plus ipilimumab, or investigator’s choice chemotherapy for the treatment of patients with deficient mismatch repair (dMMR)/microsatellite instability high (MSI-H) metastatic colorectal cancer (mCRC). Available from: https://ClinicalTrials.gov/show/NCT04008030. NLM identifier: NCT04008030.
80. Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26(4):566–576. doi:10.1038/s41591-020-0805-8
81. Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair–deficient/microsatellite instability–high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. doi:10.1200/JCO.2017.76.9901
82. Bailly C, Thuru X, Quesnel B. Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer. 2020;2(1). doi:10.1093/narcan/zcaa002
83. Wu Y, Deng Z, Wang H, Ma W, Zhou C, Zhang S. Repeated cycles of 5-fluorouracil chemotherapy impaired anti-tumor functions of cytotoxic T cells in a CT26 tumor-bearing mouse model. BMC Immunol. 2016;17(1):29. doi:10.1186/s12865-016-0167-7
84. Iwai T, Sugimoto M, Wakita D, Yorozu K, Kurasawa M, Yamamoto K. Topoisomerase I inhibitor, irinotecan, depletes regulatory T cells and up-regulates MHC class I and PD-L1 expression, resulting in a supra-additive antitumor effect when combined with anti-PD-L1 antibodies. Oncotarget. 2018;9(59):31411–31421. doi:10.18632/oncotarget.25830
85. Roche. A phase Ib study to evaluate the safety, efficacy, and pharmacokinetics of cibisatamab in combination with atezolizumab after pretreatment with obinutuzumab in participants with previously treated metastatic colorectal adenocarcinoma. Available from: https://ClinicalTrials.gov/show/NCT03866239. NLM identifier: NCT03866239.
86. David Wald, CCCC. Phase I trial of universal donor NK cell therapy in combination with ALT803. Available from: https://ClinicalTrials.gov/show/NCT02890758. NLM identifier: NCT02890758.
87. Sidney Kimmel CCC. SGI-110 in combination with an allogeneic colon cancer cell vaccine (GVAX) and cyclophosphamide (CY) in Metastatic Colorectal Cancer (mCRC). Available from: https://ClinicalTrials.gov/show/NCT01966289. NLM identifier: NCT01966289.
88. MDACC. M7824 in patients with metastatic colorectal cancer or with advanced solid tumors with microsatellite instability. Available from: https://ClinicalTrials.gov/show/NCT03436563. NLM identifier: NCT03436563.
89. Genentech. A study of RO7198457 as a single agent and in combination with atezolizumab in participants with locally advanced or metastatic tumors. Available from: https://ClinicalTrials.gov/show/NCT03289962. NLM identifier: NCT03289962.
90. Peter MacCallum Cancer Centre. MYPHISMO: MYB and PD-1 immunotherapies against multiple oncologies trial. Available from: https://ClinicalTrials.gov/show/NCT03287427. NLM identifier: NCT03287427.
91. National Cancer Institute. Ad/HER2/Neu dendritic cell cancer vaccine testing. Available from: https://ClinicalTrials.gov/show/NCT01730118. NLM identifier: NCT01730118.
92. Tong G, Xu W, Zhang G, et al. The role of tissue and serum carcinoembryonic antigen in stages I to III of colorectal cancer-A retrospective cohort study. Cancer Med. 2018;7(11):5327–5338. doi:10.1002/cam4.1814
93. Elekonawo FMK, Bos DL, Goldenberg DM, Boerman OC, Rijpkema M. Carcinoembryonic antigen-targeted photodynamic therapy in colorectal cancer models. EJNMMI Res. 2019;9(1):108. doi:10.1186/s13550-019-0580-z
94. Rijpkema M, Oyen WJ, Bos D, Franssen GM, Goldenberg DM, Boerman OC. SPECT- and fluorescence image-guided surgery using a dual-labeled carcinoembryonic antigen-targeting antibody. J Nucl Med. 2014;55(9):1519–1524. doi:10.2967/jnumed.114.142141
95. Koppe MJ, Soede AC, Pels W, et al. Experimental radioimmunotherapy of small peritoneal metastases of colorectal origin. Int J Cancer. 2003;106(6):965–972. doi:10.1002/ijc.11304
96. Dotan E, Cohen SJ, Starodub AN, et al. Phase I/II trial of labetuzumab govitecan (Anti-CEACAM5/SN-38 antibody-drug conjugate) in patients with refractory or relapsing metastatic colorectal cancer. J Clin Oncol. 2017;35(29):3338–3346. doi:10.1200/JCO.2017.73.9011
97. Akhter DT, Simpson JD, Fletcher NL, et al. Oral delivery of multicompartment nanomedicines for colorectal cancer therapeutics: combining loco-regional delivery with cell-target specificity. Adv Ther. 2020;3(2):1900171. doi:10.1002/adtp.201900171
98. Alkayyal AA, Tai L-H, Kennedy MA, et al. NK-cell recruitment is necessary for eradication of peritoneal carcinomatosis with an IL12-expressing maraba virus cellular vaccine. Cancer Immunol Res. 2017;5(3):211. doi:10.1158/2326-6066.CIR-16-0162
99. Foloppe J, Kempf J, Futin N, et al. The enhanced tumor specificity of TG6002, an armed oncolytic vaccinia virus deleted in two genes involved in nucleotide metabolism. Mol Ther Oncolytics. 2019;14:1–14. doi:10.1016/j.omto.2019.03.005
100. Amgen. Trial to evaluate the safety of talimogene laherparepvec injected into tumors alone and in combination with systemic pembrolizumab. Available from: https://ClinicalTrials.gov/show/NCT02509507. NLM identifier: NCT02509507.
101. Katz SC, Point GR, Cunetta M, et al. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther. 2016;23(5):142–148. doi:10.1038/cgt.2016.14
102. Katz SC, Burga RA, McCormack E, et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin Cancer Res. 2015;21(14):3149–3159. doi:10.1158/1078-0432.CCR-14-1421
103. Otsuka M, Koyama A, Matsuoka H, et al. Early palliative intervention for patients with advanced cancer. Jpn J Clin Oncol. 2013;43(8):788–794. doi:10.1093/jjco/hyt074
104. Delisle ME, Ward MAR, Helewa RM, Hochman D, Park J, McKay A. Timing of palliative care in colorectal cancer patients: does it matter? J Surg Res. 2019;241:285–293. doi:10.1016/j.jss.2019.04.009
105. Kopetz S, McDonough SL, Morris VK, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). J Clin Oncol. 2017;35(4_suppl):520. doi:10.1200/JCO.2017.35.4_suppl.520
106. Desai J, Markman B, Ananda S, et al. A phase I/II trial of combined BRAF and EGFR inhibition in patients (pts) with BRAF V600E mutated (BRAFm) metastatic colorectal (mCRC): the EViCT (Erlotinib and Vemurafenib in Combination Trial) study. J Clin Oncol. 2017;35(15_suppl):3557. doi:10.1200/JCO.2017.35.15_suppl.3557
107. Corcoran RB, André T, Atreya CE, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov. 2018;8(4):428.
108. van Geel R, Tabernero J, Elez E, et al. A phase Ib dose-escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF-mutant colorectal cancer. Cancer Discov. 2017;7:610–619. doi:10.1158/2159-8290.CD-16-0795
109. Bayer. Study on the effectiveness and safety of the combination of the two drugs regorafenib and nivolumab in patients with colorectal cancer (cancer of the colon or rectum classified as proficient mismatch repair and microsatellite stable). Available from: https://ClinicalTrials.gov/show/NCT04126733. NLM identifier: NCT04126733.
110. CanBas. CBP501, cisplatin and nivolumab in advanced refractory tumors. Available from: https://ClinicalTrials.gov/show/NCT03113188. NLM identifier: NCT03113188.
111. Merck. Study of MK-8353 in combination with pembrolizumab (MK-3475) in participants with advanced malignancies (MK-8353-013). Available from: https://ClinicalTrials.gov/show/NCT02972034. NLM identifier: NCT02972034.
112. MDACC. Encorafenib, cetuximab, and nivolumab in treating patients with microsatellite stable, BRAFV600E mutated unresectable or metastatic colorectal cancer. Available from: https://ClinicalTrials.gov/show/NCT04017650. Available from: NCT04017650.
113. University of California. Encorafenib, binimetinib, and nivolumab in treating patients with microsatellite stable BRAFV600E metastatic colorectal cancer. Available from: https://ClinicalTrials.gov/show/NCT04044430. NLM identifier: NCT04044430.
114. Roche. A study evaluating the efficacy and safety of multiple immunotherapy-based treatment combinations in patients with metastatic colorectal cancer (Morpheus-CRC). Available from: https://ClinicalTrials.gov/show/NCT03555149. NLM identifier: NCT03555149.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
