Back to Journals » Clinical Ophthalmology » Volume 13
Refractive outcomes of implantation of an implantable phakic copolymer lens with peripheral holes in the intraocular posterior chamber in moderate to high myopia patients: a single-surgeon series
Authors Subudhi P , Patro S, Khan Z, Subudhi BNR, Sitaram S
Received 16 May 2019
Accepted for publication 26 August 2019
Published 23 September 2019 Volume 2019:13 Pages 1887—1894
DOI https://doi.org/10.2147/OPTH.S215821
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Supplementary video 1 of ID 215821.
Views: 297
Praveen Subudhi,1,2 Sweta Patro,1 Zahiruddin Khan,2 B Nageswar Rao Subudhi,3 Silla Sitaram4
1Department of Cornea and Refractive Surgery, Ruby Eye Hospital, Berhampur, Odisha, India; 2Ophthalmology Department, Hitech Medical College, Bhubaneswar, India; 3Ophthalmology Department, MKCG Medical College and Hospital, Berhampur, Odisha, India; 4Ophthalmology Department, SDH, Chatrapur, Odisha, India
Correspondence: Praveen Subudhi
Ruby Eye Hospital, Govinda Vihar, Sushruta Nagar, Ganjam, Berhampur 760001, Odisha, India
Email [email protected]
Purpose: To assess the safety and efficacy of implanting implantable phakic copolymer lenses (IPCLs) with peripheral optic holes in the intraocular posterior chamber in Indian patients with moderate to high myopia.
Methods: Seventy-five eyes of 50 patients who underwent IPCL implantation were retrospectively analyzed. Preoperative parameters, such as subjective refraction, anterior chamber depth (measured using a pentacam), and white-to-white diameter were measured. A custom-made IPCL using the aforementioned parameters was then implanted in the sulcus to correct moderate to high myopia. All patients had undergone neodymium-doped yttrium aluminum garnet peripheral iridotomy.
Results: Clinical outcome data were collated retrospectively from the medical case records of the patients. The mean age was 25.36 years (standard deviation [SD]: 3.60 years), and 55.55% of the patients were men. The mean preoperative best-corrected visual acuity (BCVA) was 0.367 logmar units (SD: 0.266, max: 0.0 and min: 1.2). The post-IPCL implantation mean uncorrected visual acuity was 0.225 logmar units (SD: 0.172, max: 0 and min: 0.7), which was significantly superior to the preoperative BCVA (P=<0.0001). Forty-three patients (86%; satisfaction scores of ≥4; scale 1–5) were “highly satisfied” to “extremely satisfied” with the outcome. The mean follow-up period was 1.8 years.
Conclusion: Implantation of the IPCL with peripheral holes in the intraocular posterior chamber resulted in a clinically significant improvement in unaided visual acuity. Long-term follow-up showed optimum stability of vision.
Keywords: IPCL, pathological myopia, peripheral optical hole
Introduction
The current prevalence of myopia in India is considerably higher than that reported in previous studies.1 Poor awareness, social taboo, and illiteracy are the most common causes of negligence and hesitation to receive visual acuity correction.2 Uncorrected visual acuity leads to unsatisfactory academic performance and constrained social interaction.3 Spectacle correction is a viable option, but it is underused owing to low social acceptance.4 Laser in-situ keratomileusis (LASIK) or laser vision correction is an effective method for correcting refractive errors; however, it is not feasible for high-power corrections. In patients who require high-power corrections, the phakic intraocular lens is a viable alternative. Phakic lenses have become increasingly popular in the current scenario of refractive surgery because they induce relatively few higher-order aberrations at the cornea level and preserve the natural accommodations of patients.5 STAAR surgical Visian ICL™ has been extensively studied by various surgeons globally, and its efficacy has been proven effectively.6–8 However, the implantable phakic copolymer lens (IPCL), manufactured by Care Group, Inc., has not been previously studied. This study retrospectively analyzed the safety and efficacy of the IPCL.
Materials and methods
Seventy-five eyes of 50 patients who had undergone an IPCL implantation operation from March 2015 to February 2017 were analyzed. Written informed consent was obtained from all the patients in tenets with declaration of Helsinki. The study was conducted in Ruby Eye Hospital after acquiring approval from the institutional review board. Patients aged 18–35 years with stable refraction were included in this study. In addition to high myopia (>−8 D), rejection for LASIK owing to thin corneas and stable post-LASIK regression were other indications for implantation. Exclusion criteria were as follows: advanced keratoconus, irregular corneal topography, an anterior chamber depth of <3.0 mm, narrow angles on gonioscopy, and endothelial guttae.
Preoperative examination
A detailed ophthalmic examination was performed in anterior and posterior segments through slit lamp biomicroscopy and indirect ophthalmoscopy, respectively. Visual acuity assessment was performed using Snellen charts; however, assessment results were converted to logmar units for statistical analysis. Corneal topography examination was performed using a Pentacam (software; Oculus, Wetzlar, Germany)”; the intraocular pressure was measured using noncontact tonometry and Goldman applanation tonometry, whereas the macular thickness and posterior pole status were evaluated through optical coherence tomography (Stratus OCT, software version, Carl Zeiss Meditech, Jena, Germany). The anatomy of the corneal endothelium was assessed using a slit lamp, and its functionality was determined through serial ultrasonic pachymetry (specular microscopy was not available at our center). A corneal endothelium with stable serial pachymetry and without evidence of guttae was considered healthy. Gonioscopy was performed in all the patients to assess angle anatomy. The patients with no manifestation of any abnormality were evaluated the next day for their refractive status by performing cycloplegia refraction. The white-to-white (WTW) diameter was measured manually by using digital calipers in the supine position under topical anesthesia. The WTW diameter was also measured using optical biometry (IOL Master, Zeiss Inc.); however, the manual measurement was considered the final measurement. Routine yttrium aluminum garnet peripheral iridotomy (YAG-PI) was performed at least 1 week prior to surgery. After peripheral iridotomy (PI), the patients were treated with topical steroids.
The IPCL is a customized lens that is manufactured after obtaining three preoperative parameters, namely subjective refraction, anterior chamber depth, and WTW diameter. Contact lens users were asked to discontinue usage for 15 days prior to the implantation procedure.
Description of lens
The IPCL is made of a hybrid acrylic hydrophilic material (Figure 1). It is a rectangular lens with eight holes; two in each haptic, four along the transitional zone, and two along the periphery of the optic, which determines the orientation of the lens inside the eye. The peripheral optical holes should always be directed upward inside the eye. The haptics of the lens has three curves, and the central curve is smaller in diameter than the other two curves. It has a central vault that obviates contact with the anterior capsule of the lens (Surgical procedure: video S1 and video S2)
 |
Figure 1 Implantable phakic copolymer lens over the butterfly cartridge. |
Under strict aseptic preparations, an IPCL was loaded in a butterfly cartridge containing balanced salt solution and a few drops of dispersive viscoelastic. The IPCL was placed in the inner groove of the cartridge, with the vault facing upward. The orientation of the IPCL inside the cartridge was identified using the peripheral holes provided in the optic. For right-eye implantations, the peripheral optical holes were on the left of the cartridge, and for left-eye implantations, the peripheral optical holes were on the right of the cartridge. Next, the haptics of the IPCL was taped to lock in the cartridge. Proper care was taken not to damage the optic portion. Then, the wings were folded and introduced into the groove of the handle. The plunger was pushed to visualize the smooth forward movement of the lens; any restriction and folding of the haptic inside the cartridge warranted reloading of the lens. This completed the loading of the lens. Then, a 2.8-mm temporal clear corneal incision was made in the patient’s eye, and two side ports were made at 6 and 9 o’clock positions diagonally opposite to each other. Intracameral dispersive viscoelastic fluid (hydroxypropyl methylcellulose) was introduced to create a space between the crystalline lens and corneal endothelium. The open end of the cartridge was introduced into the corneal incision. Then, using a slow and controlled push technique, the IPCL could unfold in the intracameral space. The unfolding of the lens occurred with the vault facing up, and correct unfolding was ensured by confirming that the peripheral optic holes were in a superior position. After complete unfolding of the lens, the leading haptic was tucked behind the iris by using a lens guide, followed by tucking of the trailing haptic. Finally, the viscoelastic fluid was washed out using a simcoe cannula, which ensured complete removal of the fluid from the intracameral space, including the inter-lens face, to prevent postoperative inflammation and intraocular pressure spikes.
Postoperative assessment
Visual acuity was assessed using Snellen visual acuity chart; however, it was converted to logmar units using standard conversion table for statistical assessment. Refraction was assessed using an auto refractometer to determine the amount of residual refractive error. The vault status of the IPCL was assessed through anterior segment optical coherence tomography (Cirrhus HD-OCT 5000, Zeiss Inc., Jena). ASOCT was performed at 1-month and 6-month postoperative period. Vault height (VH) was assessed in each post-op visit. VH was determined in photic and scotopic conditions. The anterior chamber reaction was assessed through slit lamp biomicroscopy by placing a 5 m×2-mm slit beam obliquely, preferably under dark light ambience.
Outcome assessment
Scheduled postoperative visits were conducted on postoperative days (PODs) 1, 7, 30, 90, and 180 s. During each visit, the patient was assessed for lens position (Figure 2), vault status, visual acuity, contrast sensitivity, intraocular pressure, and refractive status. On POD 30, the patients were asked to grade their satisfaction with the visual outcome on a scale of 1–5 provided on the satisfaction form (Table 1).
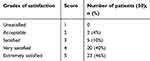 |
Table 1 Satisfaction scores |
 |
Figure 2 (A, B, and C) Postoperative lens position on pupillary dilatation in slit lamp. |
Results
In total, 75 eyes of 50 patients were included in the study. The mean age of the patients at the time of surgery was 25.36 years ([standard deviation [SD]: 3.64, min: 18 years and max: 34 years). Twenty-six were male patients and rest were female patients, respectively, were included in the study (Table 2).
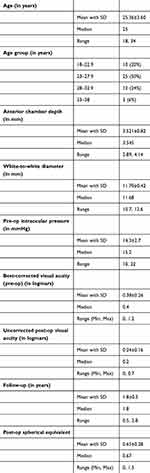 |
Table 2 Patient demographics and visual acuity (N-75) |
Visual acuity
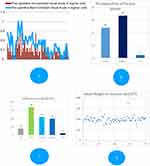
The mean preoperative best-corrected visual acuity (BCVA) was 0.367 logmar units (SD: 0.266, SEM- 0.031, min: 1.2 and max: 0.0). The average refractive error corrected was −19.57 D spherical equivalents (min: −5 D and max: −27.25 D). The mean cylindrical error corrected was −2.86 D (min: 1.5 D and max: −5.5 D). Approximately 64.4% of the recipients (48 eyes) received a spherical IPCL, whereas the remaining received a toric IPCL. The mode of insertion in both types of IPCLs was identical. The orientation of the toric IPCL was identical to that of the spherical IPCL. The rotation of the IPCL was not required along the steep axis of the cornea because the toricity was incorporated in the lens hence it needs to be placed along 0- and 180-degree meridian only. The mean unaided postoperative visual acuity in the POD 30 was 0.225 logmar units (SD: 0.172, SEM- 0.020). The mean uncorrected visual acuity in the POD 30 of follow-up was significantly superior to the preoperative BCVA (P≤0.0001) (Figure 3A). 89.33% of the patients attained either same or better visual acuity in comparison to preoperative BCVA (Figure 3A). Forty-four eyes achieved greater than 0.1 logmar improvement compared to their preoperative BCVA (Figure 3C). Eight eyes had comparatively less visual outcome. Five eyes of three patients exhibited poor outcomes owing to ametropic amblyopia, dull foveal reflex, and macular scar; these outcomes had been explained to the patients prior to the procedure. However, none of the patients experienced any deterioration of vision during the study period. No loss of lines occurred during the observation period (Table 2). A one-line improvement in contrast sensitivity was observed in 78.6% of the operated eyes.
Intraocular pressure
The mean preoperative intraocular pressure was 14.3±2.7 mmHg (Table 1) and 18.3±3.5 mmHg on POD 1. The intraocular pressure remained within the normal range during all the points of follow-up.
Anterior chamber cells and flare
Approximately, 86% of the eyes exhibited a clinically nonsignificant inflammatory reaction (≤2 cells) on POD 1. One patient experienced a severe inflammatory reaction with hypopyon on POD 1. However, complete remission of the inflammation was observed by POD 7, which remained stable thereafter. None of the patients required topical immunosuppressive therapy beyond 3 weeks.
Postoperative refraction assessment by using the subjective auto refractometer
The mean residual refractive power was 0.65 D (0–1.5 D, SD: 0.29). In total, 30 eyes (40%) had spherical equivalents between 0 and 0.5 D, 42 eyes (56%) of eyes had residual power of 0.5–1 D, and rest three eyes had>1D residual power (Figure 3B). However none of the patients required any form of spectacle correction. Furthermore, 86% of the patients were high to extremely satisfied with visual outcomes (Figure 4), thus obviating the need for further intervention. None of the patients required an explanation, a replacement, or a rotation of the IPCL during the follow-up period.
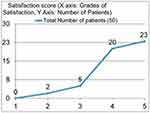 |
Figure 4 Satisfaction scores of the patients. |
Postoperative VH
Mean VH in ambient light condition was 296 microns SD, 43.59 microns median, 289 microns and in dark conditions were 323 microns SD, 56 microns median, 312 microns, the difference was statistically significant (p-value<0.001). Mean VH at 1 month and 6 months were 286 microns±35 microns and 285 microns±38 microns, respectively. There was no significant difference in the VH at 6-month interval. Scatter plot (Figure 3D) demonstrating VH in ambient light condition at 1-month follow-up.
Follow-up
The mean follow-up of the patients was 1.8 years (SD: 0.56 years, min: 0.5 and max: 2.8). The follow-up period was calculated until the end of the study period. Approximately 100% of the patients attended the follow-up on POD 7 and POD 30. However, all the patients did not attend the follow-up on PODs 90 and 180.
Complications (Table 3)
One eye in one patient exhibited a severe inflammatory reaction with hypopyon on POD 1. Ultra-sonogram evaluation showed acoustic free vitreous cavity with normal retinochoroidal scleral thickening; hence, endophthalmitis was ruled out. After a diagnosis of toxic anterior shock syndrome (TASS), aggressive topical immunosuppression (prednisolone eye drops) was administered to the patient. Within the next 2 days, an increase was observed in the reaction with a marginal increase in hypopyon, which subsequently started resolving and completely resolved over 2 weeks. As the inflammation decreased, the patient’s vision improved completely and remained stable until the last follow-up.
 |
Table 3 Complications |
One eye in one patient had pupillary block acute congestive glaucoma on POD 1 owing to non-patent PI and significant anterior vaulting of the lens. However, the YAG-PI procedure was re-performed on the same crater as that of the previous PI. With adequate medical management, normal intraocular pressure was restored.
Cataract formation is a significant postoperative complication of posterior chamber phakic intraocular lenses. We observed an anterior capsular cataract along the para-central area of crystalline lens in one eye, which was observed on dilatation of pupil 1 year postoperatively. However, it remained stable for the next 1 year and did not show progression. The patient had mild complaints of decreased vision but was not provided with any further intervention because of satisfactory binocular vision. Slit lamp examination of this patient revealed that the vault was normal, and no evidence of any contact with the anterior capsule of the lens was observed. We presumed that the cataract formation could be attributed to either manipulation during the intraocular procedure or to extremely high myopia. The patient was 22 years old and had received a −28 D IPCL intraocular lens. None of the patients had cystoid macular edema or retinal detachment during the observation period.
Discussion
The posterior chamber phakic intraocular lens has become the only type of intraocular lens for the correction of many refractive errors.9 The reasons for its considerable success are the biocompatibility of the materials used, preservation of pupillary activity, and far from the corneal endothelium.10 The safety and efficacy of the Visian ICL™, with central holes, have been demonstrated in multiple centers and have been extensively reported in the literature.11,12 However, to our knowledge, the IPCL with peripheral optic holes has not been described in the literature. Similar to the ICL, the IPCL can be implanted through a 2.8-mm incision, regardless of the amount of refractive correction and without any effect on the biomechanics of the central part of the cornea.
Spectacle correction of high myopia results in unsatisfactory vision correction because of higher-order aberrations.13 An intraocular lens at the focal point of the eye not only reduces the higher-order aberrations but also increases the field of vision.14 Thus, we presume that the phakic intraocular lens provides vision of a higher quality than spectacle correction (before surgery). In our study, the preoperative mean BCVA with spectacles was 0.38 logmar units, and the post-IPCL implantation mean unaided visual acuity was 0.24 logmar units. This difference was statistically significant (P=0.001).
With the IPCL, the refractive results are predictable and stable, unlike those obtained using LASIK for high myopic correction, because the implantation of the IPCL does not involve the risk of flap-related complications and myopic regression.15 However, in patients older than 45 years, the risks of cataract development and refractive shift increase.16 Our study results showed a predictable visual outcome in 82% of the patients and 98% of the patients if we exclude eyes with refractive correction of >−20 D. Additionally, in these eyes, uncorrected visual acuity exhibited two lines of improvement compared with the preoperative BCVA in 45% of the eyes.
Our safety results were comparable to those of corneal refractive surgery in our center. Three eyes had complications, one eye with each of the following: pupillary block, cataract, and TASS . Future pupillary blocks were obviated by ensuring PI through retro-illumination. Cataracts are potential complications of phakic intraocular lenses. The reported incidence of cataracts is 1.1–5% according to a meta-analysis. However, only 0–1.8% of the cases are clinically significant and require an explanation of the phakic lens and cataract surgery.17 The incidence of cataracts in our study was 1.5% (one case), which was similar to the incidence reported in a previous study on ICL. However, because it was far from the pupillary axis and caused mild visual defects, no further intervention was sought.
Conclusion
Thus, IPCL with peripheral optic holes is associated with highly satisfactory visual outcomes for patients with moderate to high myopia. Furthermore, it provides optimum long-term stability of vision. The follow-up period of our study was long, which enabled satisfactory assessment of postoperative stability.
We believe that with foreseeable long-term results, IPCL can be considered an effective alternative to ICL in developing countries, thus adding a crucial component to the refractive surgery armamentarium.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012 May 5;379(9827):1739–48. Review. PubMed PMID: 22559900. doi:10.1016/S0140-6736(12)60272-4
2. Wolffsohn JS, Calossi A, Cho P, et al. Global trends in myopia management attitudes and strategies in clinical practice. Cont Lens Anterior Eye. 2016;39(2):106–116. Epub 2016 Feb 16. PubMed PMID:26895773. doi:10.1016/j.clae.2016.02.005
3. Alió JL, Krueger RR, Bidgoli S. The World Burden of Refractive Blindness. J Refract Surg. 2016 Aug 1;32(9):582–4. PubMed PMID: 27598727. doi: 10.3928/1081597X-20160707-01
4. Malet F, Matos S, Meijome JM, et al. Global trends in myopia management attitudes and strategies in clinical practice. Cont Lens Anterior Eye. 2016;39(2):106–116. Epub 2016 Feb 16. PubMed PMID: 26895778. doi:10.1016/j.clae.2016.02.005
5. Uusitalo RJ, Aine E, Sen NH, et al. Implantable contact lens for high myopia. J Cataract Refract Surg. 2002;28(1):29–36. PubMed PMID: 11777707. doi:10.1016/s0886-3350(01)01218-4
6. ICL in Treatment of Myopia (ITM) Study Group. United States Food and Drug Administration clinical trial of the Implantable Collamer Lens (ICL) for moderate to high myopia: three-year follow-up. Ophthalmology. 2004;111:1683–1692. doi:10.1016/j.ophtha.2004.03.026
7. Implantable Contact Lens in Treatment of Myopia (ITM). Study Group U.S. Food and Drug Administration clinical trial of the implantable contact lens for moderate to high myopia. Ophthalmology. 2003;110:255–266. doi:10.1016/s0161-6420(02)01771-2
8. Kamiya K, Shimizu K, Igarashi A, et al. Four-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Arch Ophthalmol. 2009;127:845–850. doi:10.1001/archophthalmol.2009.67
9. Liang GL, Wu J, Shi JT, et al. Implantable collamer lens versus iris-fixed phakic intraocular lens implantation to correct myopia: a meta-analysis. PLoS One. 2014;9(8):e104649. eCollection 2014. PubMed PMID: 25115906. doi:10.1371/journal.pone.0104649
10. Pérez-Cambrodí RJ, Piñero DP, Ferrer-Blasco T, et al. The posterior chamber phakic refractive lens (PRL): a review. Eye (Lond). 2013;27(1):14–21. Epub 2012 Dec 7. Review. PubMed PMID:23222559. doi:10.1038/eye.2012.235
11. Moya T, Javaloy J, Montés-Micó R, et al. Implantable collamer lens for myopia: assessment 12 years after implantation. J Refract Surg. 2015;31(8):548–556. PubMed PMID: 26248348. doi:10.3928/1081597X-20150727-05
12. Du GP, Huang YF, Wang LQ, et al. Changes in objective vault and effect on vision outcomes after implantable collamer lens implantation: 1-year follow-up. Eur J Ophthalmol. 2012;22(2):153–160. PubMed PMID: 21607932. doi:10.5301/EJO.2011.8359
13. Hu JR, Yan ZH, Liu CF, et al. Higher-order aberrations in myopic and astigmatism eyes. Zhonghua Yan Ke Za Zhi. 2004;40(1):13–16. Chinese. PubMed PMID: 14989953.
14. Hosny MH, Shalaby AM. Visian implantable contact lens versus AcrySof cachet phakic intraocular lenses: comparison of aberrmetric profiles. Clin Ophthalmol. 2013;7:1477–1486. Epub 2013 Jul 22. PubMed PMID: 23901255; PubMed Central PMCID: PMC3726524. doi:10.2147/OPTH.S47909
15. Tse SM, Farley ND, Tomasko KR, et al. Intraoperative LASIK complications. Int Ophthalmol Clin. 2016;56(2):47–57. Spring. Review. PubMed PMID: 26938337. doi: 10.1097/IIO.0000000000000110
16. Vashist P, Talwar B, Gogoi M, et al. Prevalence of cataract in an older population in India: the India study of age-related eye disease. Ophthalmology. 2011;118(2):
17. Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. Clin Ophthalmol.2016;10:1059–1077. SFX The Knowledge Network [Context Link]. doi:10.2147/OPTH
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.