Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Reduced Synaptic Plasticity Contributes to Resistance Against Constant-Stimulus Electroconvulsive Treatment in a Rat Model of Stress-Induced Depression
Authors Wu B, Guo Y, Deng J, Chen Q, Min S
Received 27 January 2021
Accepted for publication 18 April 2021
Published 11 May 2021 Volume 2021:17 Pages 1433—1442
DOI https://doi.org/10.2147/NDT.S304075
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Bin Wu,* Yuanyuan Guo,* Jie Deng, Qibin Chen, Su Min
Department of Anesthesiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, 400016, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Su Min
Department of Anesthesiology, The First Affiliated Hospital of Chongqing Medical University, No. 1 Youyi Road, Yuzhong District, Chongqing, 400016, People’s Republic of China
Email [email protected]
Purpose: Depression is a common mood disorder in humans worldwide. Electroconvulsive therapy (ECT) remains the most effective treatment for patients with drug-resistant or severe depression; however, during ECT, electrical resistance can occur, antagonizing ECT efficacy. We aimed to investigate how depressed patients develop resistance to electric shocks during ECT.
Methods: Rats exposed to chronic unpredictable stress exert similar impairments in hippocampal synaptic plasticity as those in depressed humans, including hippocampal neuronal atrophy and reduced synaptic function and synapse-related proteins. Therefore, a rat model was used to model depressive-like behaviors in the current study. Depression-like behavior was stimulated in Sprague Dawley (SD) rats that were then randomized into six groups: control group (C); a rat model of stress-induced depression group (D); and four groups in which a rat model of stress-induced depression received one, three, five, or seven electroconvulsive shocks (ECS; DE1, DE3, DE5, and DE7). The sucrose preference test (SPT) and Morris water maze (MWM) were utilized to evaluate anhedonia and spatial learning and memory in rats, respectively. Synaptic plasticity was recorded electrophysiologically in terms of field excitatory postsynaptic potential (fEPSP) and long-term potentiation (LTP).
Results: The rat model of stress-induced depression triggered a decrease in the sucrose preference percentage (SPP) and the baseline fEPSP slope relative to those observed for the C group, and these changes were significantly rescued by ECT in a shock number-dependent manner within five shocks. However, the rat model of stress-induced depression displayed an increase in the escape latency and a decrease in space exploration time, in addition to decreased LTP relative to those in the C group, which was further augmented by ECT in a shock number-dependent manner within five shocks.
Conclusion: Changes in synaptic plasticity might be responsible for the development of resistance against constant-stimulus ECT in a rat model of stress-induced depression.
Keywords: depression, electroconvulsive shocks, electrical resistance, synaptic plasticity
Introduction
Depression has been predicted to be the second most frequent mood disorder through 2020 by the World Health Organization (WHO).1 Over 350 million people are estimated to suffer from depression, which is associated with over half of the 800,000 suicides/year worldwide.2
Our previous study indicated that electroconvulsive therapy (ECT), which remains the most effective therapeutic method for patients with drug-resistant or severe depression, is ineffective in up to 30% of depressed patients3 due to inefficacy caused by electrical resistance during ECT.4,5 As ECT progresses, the seizure threshold increases by 40–125%; consequently, the electrical charge and number must also increase to maintain the anti-depressant effects, particularly in older patients,4 which can severely damage the learning and memory functions in treatment-resistant depression patients.6
However, the underlying mechanisms that are responsible for the development of electrical resistance against ECT are complex. One possible reason is synaptic plasticity, which is altered in depression and may underlie the ability of ECT to relieve depression in patients.7,8 For instance, similar to depressed patients, rats exposed to chronic unpredictable stress present with impaired hippocampal synaptic plasticity, as indicated by the atrophy of hippocampal neurons and reduced synaptic function and synapse-related protein expression;9,10 consequently, the rat model was used to model depressive-like behaviors in the current study. In addition, repeated ECSs have been shown to enhance the synaptic function of the hippocampus, as reflected in increased nerve fiber growth, synapse numbers, and electrophysiological function observed in animal models.11,12 Altogether, synaptic enhancement may at least partially underlie the anti-depressive effects of ECT.
Here, we explored whether synaptic plasticity contributes to the development of electrical resistance in ECT using a rat model of stress-induced depression.
Materials and Methods
Rats
Adult male (2–3 months, 200–250 g) Sprague Dawley (SD) rats were maintained in the Laboratory Animal Centre of the First Affiliated Hospital of Chongqing Medical University (Chongqing, China) under controlled conditions of 22–24 °C, 60–64% humidity, and a 12-h day/night cycle, with lights on/off at 8:00/20:00. One week before the experiments, the animals were adapted to the new environment. The procedures of the current study were approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University and were performed in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the suffering and the number of rats used in the current study.
Stress-Induced Depression
Rats exposed to chronic unpredictable stress present with similar hippocampal synaptic plasticity impairments as are observed in depressed patients, including the atrophy of hippocampal neurons and reductions in synaptic function and synapse-related proteins;9,10,13 consequently, a rat model of chronic unpredictable mild stress, as described in our previous study, was used to model depressive-like behaviors in the current study.14 The stress procedure is comprised of the following 9 types of stressors: 1) swimming for 5 min in 4 °C cold water; 2) swimming for 5 min in 45 °C hot water; 3) pinching the tail for 60 sec; 4) food deprivation for 1 day; 5) water deprivation for 1 day; 6) shaking for 20 min; 7) continuous lighting for 1 day; 8) being caged in a cage containing damp sawdust for 1 day; 9) being in a cage tilted 45° from horizontal for 1 day. Animals were housed one rat per cage and were randomly subjected to one of the described stressors once/day for 28 consecutive days. The randomization was applied individually to each rat. The same stressor was not used on successive days to ensure the unpredictability of the stimulation.
Electroconvulsive Shock (ECS)
Shocks were delivered under propofol anesthesia (10 mg/mL, 9 mL/kg, intraperitoneal; FX061, AstraZeneca, UK), as described in our previous report.14 Shocks featuring bidirectional square wave pulses of 120 mC were delivered once/day for seven consecutive days by ear clip electrodes on the Niviqure ECT system (Niviqure Meditech, Bangalore, India). Sham shocks were delivered as described above, except without the current. Oxygen was supplied to rats before, during and after the shocks, and before the recovery of body movement, the blood oxygen saturation (SpO2) was monitored to maintain at least 95%. During the whole process of ECS and the recovery period after ECS, there was no obvious inhibition of respiration. As described by our previous report,15 all rats in the current study also experienced tonic-clonic seizures for at least 10 s in each group after treatment with ECS, indicating the success of ECS treatment. Tonic-clonic seizures in rats weighing over 300 grams can cause spinal cord injuries (SCIs), resulting in bilateral lower-limb palsy. Fortunately, none of the rats (n =15 per group) developed SCI in the present study.
Grouping
The SD rats were randomly allocated to six groups (n = 15 per group). The rats in the C group were subjected to neither chronic unpredictable stress nor ECS, whereas those in the D group were subjected to chronic unpredictable stress and sham shocks (as described above, except without the current), and those in the DE1, DE3, DE5, and DE7 groups were subjected to chronic unpredictable stress, followed by the delivery of one, three, five, or seven ECSs. All rats were exposed to oxygen during the experiment. In brief, at the end of the experimental period, nine rats were subjected to behavioral tests, and six rats underwent electrophysiological measurements.
The experimental timeline of the procedure was similar to that used in our previous study16 and is summarized in Figure 1. Starting the day after the completion of the chronic unpredictable stress model (day 29), baseline measurements were established in nine rats using the sucrose preference test (SPT), and the Morris water maze test (MWM) was performed on days 30–35. Subsequently, the rats received one, three, five, and seven ECS once per day, on days 36, 36–38, 36–40, and 36–42, respectively. The rats were subjected to SPT the day after the last ECS was administered and subjected to the MWM for six consecutive days, starting two days after the last ECS.
 |
Figure 1 Experimental timeline. |
Sucrose Preference Test (SPT)
SPT was used to assess anhedonia, as we previously described.16 On the first day, all rats were supplied with two bottles of 1% (w/w) sucrose to learn how to drink. On the second day, one bottle of sucrose was replaced with sterile water. On the third day, rats were deprived of water and food for 23 h and then provided with access to one bottle containing 1% sucrose and one bottle containing sterile water for 1 h. All rats were free to drink for 30 min, then the position of two bottles were exchanged to prevent position preference. The weight of each bottle was weighed before and after the experiment to determine the sucrose preference of each rat. The SPP was calculated using the following equation: SPP = {sucrose consumption (mL)/[sucrose consumption (mL) + sterile water consumption (mL)]} × 100%.
Morris Water Maze (MWM)
The MWM is widely used to evaluate spatial learning and memory in rodents, which depends on activity in the hippocampus and N-methyl-D-aspartate (NMDA) receptor-mediated Hebbian plasticity. The MWM was performed as we previously reported.17 The MWM was conducted in a circular water tank with a diameter of 150 cm, divided into four quadrants (SE, SW, NW, and NE). Water at a temperature of 22 ± 1 °C was rendered opaque using dark, nontoxic, and washable paint. The ZH0065 (Zhenghua Instruments, China) was utilized to record the swimming velocity, trajectory, and time required to reach the platform and the time spent in each quadrant.
Rats were released in each quadrant and allowed as long as 60 sec to find a platform with a diameter of 11 cm that was placed 1.5 cm beneath the water surface in the center of the NE quadrant. Rats were subjected to four trials in different quadrants daily (one from each quadrants) for five consecutive days and placed onto the platform for 15 sec after each trial. For those rats that successfully reached the platform within 60 sec, the time necessary was recorded as the escape latency, which was each day’s average of all four trials. For those rats that failed to find the platform within 60 sec, the escape latency was recorded as 60 sec after rats were guided onto the platform.
On the sixth day, the platform in the center of NE was removed. Rats were released to SW and allowed to swim for 60 sec. The time spent swimming in NE was recorded as space exploration time.
Electrophysiology
At 24 h after the completion of ECS treatment, the rats were anesthetized by intraperitoneal injection of sodium pentobarbital (2%, 50 mg/kg). Afterward, cardiac perfusion was performed with artificial cerebrospinal fluid (ACSF) at 0–4 °C, as we previously reported.17 The perfused brain was placed into ice-cold ACSF equilibrated with 95% O2 and 5% CO2 for 2 min. Next, brain slices were transferred to a perfusion trough and immobilized in the center of the visual field using nylon thread. The hippocampal CA3 and CA1 regions were placed under the microscope, and bipolar stimulator electrodes were placed in the Schaffer collaterals of the CA3 region.18 We performed the electrophysiology experiments in the rats from one group per day.
Tissues were stimulated by a 0.2-msec square wave with gradually increasing power until the induced fEPSP no longer rose. The stimulation power was adjusted to 50% of this maximum power to induce a baseline fEPSP. When the induced fEPSP waveform and slope were stable, the baseline fEPSP was recorded every 2 min. Three traces were recorded with an interval of 28 sec. The baseline fEPSP was recorded for half an hour, during which time the average slope was recorded as the baseline fEPSP value.
LTP was stimulated by high-frequency stimulation of 200 pulses at 100 Hz. Post-fEPSP was recorded every 2 min for 60 min. Three traces were recorded with an interval of 28 sec. After 30 min of high-frequency stimulation, the average fEPSP slope was recorded as the post-fEPSP value. The magnitude of LTP was calculated using the formula: LTP (%) = [(mean slope post-fEPSP − mean slope baseline fEPSP)/mean slope baseline EPSP] × 100%.
Statistical Analysis
Data were analyzed by SPSS 22.0 (IBM, Chicago, IL, USA) and are presented as the mean ± SD. All data were tested for normal distribution and homogeneity of variance. Intergroup differences in escape latency were assessed by repeated-measures analysis of variance (ANOVA). Post-mortem variables were evaluated using the Student–Newman–Keuls (SNK)-q test, while differences in SPP, space exploration time, baseline fEPSP, and LTP were analyzed by one-way ANOVA, and the groups were compared using the SNK-q test. P < 0.05 indicated a significant difference.
Results
Sucrose Preference Test (SPT)
As shown in Figure 2 and Table 1, before ECT treatment in SD rats, the SPP differed significantly among the C, D, DE1, DE3, DE5, and DE7 groups (F = 19.33, P < 0.001). SPP was significantly reduced by approximately 30% in the D, DE1, DE3, DE5, and DE7 groups compared with that in the C group (P < 0.01). In addition, no significant difference was observed between the D group and any of the DE groups (P > 0.05).
 |
Table 1 SPP of Each Rat Before and After ECS |
 |
Figure 2 SPP before and after ECSs. *P < 0.05 vs C. #P < 0.05 vs D. ∇P < 0.05 vs DE1. ΔP < 0.05 vs DE3. |
After the ECT treatment of rats exposed to chronic unpredictable stress, SPP differed significantly among the six groups (F = 22.52, P < 0.001), which was reversed by ECS application in a shock number-dependent manner; it was significantly higher in the DE5 group and DE7 groups (P < 0.01) compared with the D group, but no significant difference was observed between the D group and the DE1 or DE3 groups (P > 0.05). Additionally, no significant differences in SPP were observed between the DE1 and DE3 groups (P > 0.05) or between the DE5 and DE7 groups (P > 0.05).
Morris Water Maze (MWM)
No significant difference was observed in the swimming speeds among the six groups (P > 0.05, Figure 3A).
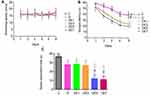 |
Figure 3 MWM outcomes for different groups. (A) Swimming speed. (B) Escape latency. (C) Space exploration time. *P < 0.05 vs C. #P < 0.05 vs D. ▽P < 0.05 vs DE1. ΔP < 0.05 vs DE3. |
As shown in Figure 3B, the escape latency for each group decreased over the course of the five-day training period and differed significantly among the six groups (F = 8.58, P < 0.001), with significantly longer escape latencies recorded for the D group than for the C group (P < 0.05). In rats exposed to chronic unpredictable stress and treated with ECT, the escape latency was significantly lengthened by ECS applications in a shock-number-dependent manner. The escape latency increased significantly in the DE5 and DE7 groups compared with that in the D group (P < 0.05), but these increases were not significant for the DE1 or DE3 groups (P > 0.05). No significant differences in escape latency were observed between the DE1 and DE3 groups (P > 0.05) or between the DE5 and DE7 groups (P > 0.05).
On day 6 of the MWM, the space exploration time was measured (Figure 3C and Table 2), which differed significantly among the six groups (F = 10.229, P < 0.001). The space exploration time was significantly shorter in the D group compared with that in the C group (P < 0.05), which was further significantly shortened by ECS application when using the increased shock numbers in the DE5 and DE7 groups (P < 0.05) but was not significantly shortened in the DE1 or DE3 groups (P > 0.05) compared with that in the D group. No significances in space exploration time were found between the DE1 and DE3 groups (P > 0.05) or between the DE5 and DE7 groups (P > 0.05).
 |
Table 2 Space Exploration Time of Each Rat |
Constant-Stimulus ECSs and Baseline Field Excitatory Postsynaptic Potential (fEPSP) in the Hippocampal SC-CA1 Pathway
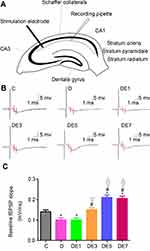
Diagrammatic representation of a hippocampal cross-section, showing the placement of the stimulating and recording electrodes, is presented in Figure 4A.
As exhibited in Figure 4B and C and Table 3, baseline fEPSP differed significantly among the six groups (F = 66.127, P < 0.05). Baseline fEPSP was significantly lower in the D group than in the C group (P < 0.05), which was rescued by ECS application in a shock number-dependent manner in the DE3, DE5, and DE7 groups (P < 0.01) but not in the DE1 group (P > 0.05) in comparison with the D group. Moreover, the baseline fEPSPs in DE5 and DE7 groups (P < 0.05) but not in the DE3 group (P > 0.05) were significantly higher than that in the C group. No significance was observed in baseline fEPSP between the DE5 and DE7 groups (P > 0.05).
 |
Table 3 Baseline fEPSP of Each Rat |
Constant-Stimulus ECSs and Long-Term Potentiation (LTP) in the Hippocampal SC-CA1 Pathway
As shown in Figure 5A and B and Table 4, LTP was stimulated using a high-frequency stimulation procedure, which resulted in significantly different outcomes among the six groups (F = 43.522, P < 0.05). LTP was significantly reduced in the D group compared with the C group (P < 0.05), which was further lowered with the use of increasing ECS numbers in the DE3, DE5, and DE7 groups (P < 0.05) but not DE1 group (P > 0.05). Additionally, no significant differences in LTP were found between the DE5 and DE7 groups (P > 0.05).
 |
Table 4 fEPSP of Each Rat |
Discussion
Until now, synaptic plasticity, which is altered during depression, has been considered to underlie the ability of ECT to relieve depression and represent a potential factor in the development of electrical resistance against ECT,7,8 although the precise underlying mechanisms remain undiscovered. To the best of our knowledge, the present study provides the most detailed insights thus far into the potential mechanisms underlying electrical resistance against ECT in a rat model of stress-induced depression, which was manifested in the progressively improved SPP and the increased synaptic function (fEPSP) observed with the increase from one to five shocks, peaking at five shocks and not increasing further at seven shocks. In addition, MWM and LTP gradually worsened with increasing shocks, likely reflecting the increasingly severe damage to learning and memory functions.
The current study utilized a chronic, unpredictable, mild stress paradigm to stimulate depression-like symptoms in SD rats, which is a model of “reactive” depression that presents with similarities to depression observed in humans.13 Anhedonia remains the core symptom of depression,19 which was assessed using the SPT in the current study and identified a significantly lower SPP among the rats in the D group compared with the rats in the C group, indicating the successful establishment of a rat model of stress-induced depression. In addition, multiple ECSs significantly increased SPP, indicating the ability of ECS applications to produce anti-depressive effects, consistent with the existing global literature and our previous report.17,20,21 Consistently, the present study indicated that the use of an increasing number of ECSs resulted in an increased anti-depressive effect, as observed by the upregulation of SPP with the increasing number of shocks, although the effect peaked at five shocks and did not further increase at seven shocks.
As we previously reported, MWM is commonly used for the examination of hippocampus-dependent spatial learning and memory functions in rodents.17 Consistently, in the current study, damage to the hippocampus was indicated by longer escape latencies and shorter space exploration times. In rats exposed to chronic unpredictable stress, the escape latency was significantly prolonged by ECS application for up to five shocks, with no further prolongation observed at seven shocks. The space exploration times were dramatically shortened in rats exposed to chronic unpredictable stress and treated with five ECSs, but no further shortening was observed at seven ECSs. According to the results of the current study, the application of ECSs using five and seven shocks reduced the symptoms in a rat model of stress-induced depression by reducing anhedonia, which was accompanied by impaired learning and spatial memory.
In animal models exposed to chronic unpredictable stress, ECS application promotes synaptic growth in the hippocampus and induces changes that resemble functional LTP.22,23 Moreover, synaptic changes have been suggested to represent a potential mediator of the anti-depressant effects of ECSs.7,8 In the rat model of stress-induced depression used in the present study, the baseline fEPSP slope significantly increased with the increased number of shocks, peaking at five shocks; analogously, LTP was remarkably decreased with increasing shocks, reaching a minimum value at five shocks. These results further suggested that reduced synaptic plasticity is positively associated with electrical resistance against anti-depressive ECT at five shocks, and no significant difference was observed between the DE5 and DE7 groups. Moreover, ECS application using five and seven shocks to a rat model of stress-induced depression reduced anhedonia, as measured by SPT, while simultaneously exhibiting impaired learning and spatial memory, as measured by the MWM, which corresponds well with the observed reduction of LTP magnitude.
Taken together, these results indicated the exiting of a “ceiling effect” of five ECSs applied with constant power in the present study, which was observed in the SPT, fEPSP, LTP, and MWM analyses and mirrors the electrical resistance against ECT observed in patients with depression, as reported by previous studies4,5 Furthermore, the findings of the present study were consistent with a previous study which demonstrated the limited ability of ECSs to amplify the progenitors required to increase the generation of neurons when applied at 10 shocks, without observable effects on depression-like behaviors.24 In addition, another report showed significantly more apoptotic cells in animals treated with 10 ECSs compared with those in sham animals, but no significant difference was observed in the numbers of apoptotic cells between animals treated with 20 ECSs and sham animals.25
In the current study, ECS application exhibited a shock number-dependent effect on the alleviation of a rat model of stress-induced depression up to five shocks, with larger numbers of ECSs associated with a higher SPP, longer escape latency, shorter space exploration time, higher baseline fEPSP slope, and reduced induction of LTP in rats exposed to chronic unpredictable stress. In addition, in the current study, the rats in the C and D groups were not exposed to propofol. In our previous study, propofol was not found to significantly affect brain plasticity in rats exposed to chronic unpredictable stress (16). In the current study, we aimed to demonstrate the effects of different numbers of ECSs on synaptic plasticity among the DE1, DE3, DE5, and DE7 groups, with only one variable: the number of ECS applied. Therefore, we acknowledge that the impacts of propofol were not considered; however, the findings in the current study support our view. We provide strong evidence indicating that reduced synaptic plasticity contributes to resistance against ECS during constant-stimulus ECT in a rat model of stress-induced depression, as no significant differences were observed between the DE5 and DE7 groups.
However, three limitations exist in the current study: 1) the changes in the body weight in each group were not measured; 2) although the rat model used in this study resembles the human model of depression, these findings must be applied with caution due to rheological differences in depression between rodents and humans; and 3) the rats exposed to chronic unpredictable stress in our study were initially healthy, whereas depression in humans is often comorbid with other diseases; therefore, our findings should be verified and extended in studies with a larger number of animals and in preclinical and human trials in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
1. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi:10.1016/S0140-6736(17)32154-2
2. Bradvik L, Mattisson C, Bogren M, Nettelbladt P. Mental disorders in suicide and undetermined death in the Lundby Study. The contribution of severe depression and alcohol dependence. Arch Suicide Res. 2010;14(3):266–275. doi:10.1080/13811118.2010.494146
3. Hao X, Zhu X, Li P, Feng L, Min S. NMDA receptor antagonist enhances anti-depressant efficacy and alleviates learning-memory function impairment induced by electroconvulsive shock with regulating glutamate receptors expression in hippocampus. J Affect Disord. 2016;190:819–827. doi:10.1016/j.jad.2015.11.021
4. Plakiotis C, Chin LF, O’Connor DW. The change in electrical energy delivered to aged patients over a course of moderate dose unilateral electroconvulsive therapy. Psychogeriatrics. 2010;10(4):187–190. doi:10.1111/j.1479-8301.2010.00340.x
5. Tor PC, Bautovich A, Wang MJ, et al. A systematic review and meta-analysis of brief versus ultrabrief right unilateral electroconvulsive therapy for depression. J Clin Psychiatry. 2015;76(9):e1092–e1098. doi:10.4088/JCP.14r09145
6. Desbeaumes Jodoin V, Richer F, Miron JP, Gosselin MF, Lespérance P. Long-term sustained cognitive benefits of vagus nerve stimulation in refractory depression. J ECT. 2018;34(4):283–290. doi:10.1097/YCT.0000000000000502
7. Marsden WN. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuropsychopharmacol. 2013;43:168–184. doi:10.1016/j.pnpbp.2012.12.012
8. Singh A, Kar SK. How electroconvulsive therapy works? Understanding the neurobiological mechanisms. Clin Psychopharmacol Neurosci. 2017;15(3):210–221. doi:10.9758/cpn.2017.15.3.210
9. Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62(1):63–77. doi:10.1016/j.neuropharm.2011.07.036
10. Qiao H, An SC, Ren W, Ma X. Progressive alterations of hippocampal CA3-CA1 synapses in an animal model of depression. Behav Brain Res. 2014;275:191–200. doi:10.1016/j.bbr.2014.08.040
11. Chen AC, Shin KH, Duman RS, Sanacora G. ECS-induced mossy fiber sprouting and BDNF expression are attenuated by ketamine pretreatment. J ECT. 2001;17(1):27–32. doi:10.1097/00124509-200103000-00006
12. Chen F, Madsen TM, Wegener G, Nyengaard JR. Repeated electroconvulsive seizures increase the total number of synapses in adult male rat hippocampus. Eur Neuropsychopharmacol. 2009;19(5):329–338. doi:10.1016/j.euroneuro.2008.12.007
13. Li DJ, Wang FC, Chu CS, et al. Significant treatment effect of add-on ketamine anesthesia in electroconvulsive therapy in depressive patients: a meta-analysis. Eur Neuropsychopharmacol. 2017;27(1):29–41. doi:10.1016/j.euroneuro.2016.11.008
14. Ren L, Hao X, Min S, et al. Anesthetics alleviate learning and memory impairment induced by electroconvulsive shock by regulation of NMDA receptor-mediated metaplasticity in depressive rats. Neurobiol Learn Mem. 2018;155:65–77. doi:10.1016/j.nlm.2018.06.013
15. Zhang F, Luo J, Min S, Ren L, Qin P. Propofol alleviates electroconvulsive shock-induced memory impairment by modulating proBDNF/mBDNF ratio in depressive rats. Brain Res. 2016;1642:43–50. doi:10.1016/j.brainres.2016.03.020
16. Luo J, Min S, Wei K, et al. Propofol prevents electroconvulsive-shock-induced memory impairment through regulation of hippocampal synaptic plasticity in a rat model of depression. Neuropsychiatr Dis Treat. 2014;10:1847–1859. doi:10.2147/NDT.S67108
17. Ren L, Zhang F, Min S, et al. Propofol ameliorates electroconvulsive shock-induced learning and memory impairment by regulation of synaptic metaplasticity via autophosphorylation of CaMKIIa at Thr 305 in stressed rats. Psychiatry Res. 2016;240:123–130. doi:10.1016/j.psychres.2016.03.053
18. Sun X, Zhang J, Li H, et al. Propofol effects on excitatory synaptic efficacy in the CA1 region of the developing hippocampus. Brain Res Dev Brain Res. 2005;157(1):1–7. doi:10.1016/j.devbrainres.2005.02.011
19. Li Y, Wu Y, Li R, et al. Propofol regulates the surface expression of GABAA receptors: implications in synaptic inhibition. Anesth Analg. 2015;121(5):1176–1183. doi:10.1213/ANE.0000000000000884
20. Stewart CA, Reid IC. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology. 2000;148(3):217–223. doi:10.1007/s002130050045
21. Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress. 2017;6:78–93. doi:10.1016/j.ynstr.2016.08.002
22. Wang D, Sun T. Neural plasticity and functional recovery of human central nervous system with special reference to spinal cord injury. Spinal Cord. 2011;49(4):486–492. doi:10.1038/sc.2010.124
23. Nabavi S, Fox R, Proulx CD, et al. Engineering a memory with LTD and LTP. Nature. 2014;511(7509):348–352. doi:10.1038/nature13294
24. Nakamura K, Ito M, Liu Y, et al. Effects of single and repeated electroconvulsive stimulation on hippocampal cell proliferation and spontaneous behaviors in the rat. Brain Res. 2013;1491:88–97. doi:10.1016/j.brainres.2012.10.052
25. Ito M, Seki T, Liu J, et al. Effects of repeated electroconvulsive seizure on cell proliferation in the rat hippocampus. Synapse. 2010;64(11):814–821. doi:10.1002/syn.20796
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.