Back to Journals » Research and Reports in Urology » Volume 10
Reduced sensitivity of multiparametric MRI for clinically significant prostate cancer in men under the age of 50
Authors Gielchinsky I , Scheltema MJ , Cusick T, Chang J, Shnier R, Moses D , Delprado W, Nguyen Q, Yuen C, Haynes A, Stricker PD
Received 22 March 2018
Accepted for publication 11 July 2018
Published 4 October 2018 Volume 2018:10 Pages 145—150
DOI https://doi.org/10.2147/RRU.S169017
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Ilan Gielchinsky,1,2 Matthijs J Scheltema,1 Thomas Cusick,1 John Chang,1,2 Ron Shnier,3,4 Daniel Moses,4,5 Warick Delprado,4,6 Quoc Nguyen,1 Carlo Yuen,4,7 Anne-Maree Haynes,1 Phillip D Stricker1,2,4,7
1Garvan Institute of Medical Research/The Kinghorn Cancer Centre, Darlinghurst, NSW, Australia; 2St Vincent’s Prostate Cancer Centre, Darlinghurst, NSW, Australia; 3Southern Radiology, Randwick, NSW, Australia; 4School of Medicine, University of New South Wales, Kensington, NSW, Australia; 5Spectrum Radiology, Randwick, NSW, Australia; 6Douglass Hanly Moir Pathology, Macquarie Park, NSW, Australia; 7St Vincent’s Clinic, Sydney, NSW, Australia
Introduction: Three percent of all new diagnosed prostate cancer (PC) patients are under the age of 50. Multiparametric MRI (mpMRI) is considered as increasingly powerful tool for decision-making in diagnosis of PC and in some active surveillance protocols. Since prostate architecture changes with age, we evaluated the sensitivity of mpMRI to detect clinically significant PC in patients under the age of 50 compared to pair-matched older patients.
Methods: Data from a prospective collected and ethics approved database were retrospectively analyzed. We reviewed 1,395 records of PC patients from the years 2012–2017, identifying those under the age of 50 who had radical prostatectomy as primary treatment, a pre-operative mpMRI, a full clinical data set and who had clinically significant cancer (N=51). Tumor size and International Society of Urological Pathology (ISUP) score pair-matching was performed for patients older than 55 years. Clinically significant cancer was defined as ISUP >2 or ISUP 2 with >5% Gleason 4. The sensitivity to detect clinically significant cancer with mpMRI was calculated using pre-operative Prostate Imaging Reporting and Data System (PI-RADS) score and whole-gland final pathology.
Results: The median patient age in the young and older groups was 47 and 62, respectively. Both cohorts matched significantly regarding tumor volume (P =0.91) and ISUP score (P =1.0). The median PI-RADS score for the young group was 3, and 4 for the older group. The sensitivity for mpMRI, for PI-RADS 3,4 and 5 was 80.3% (95% CI 66.8%–90.1%) in the young group and 84.3% in the older group (95% CI 71.4%–92.9%), demonstrating no statistically significant difference (P=0.603). Sensitivity of mpMRI for PI-RADS 4,5 was 49.0% (95% CI 34.7%–63.4%) for the young group and 72.5% (95% CI 58.2%–84.1%) for the older group, which differ significantly (P=0.014).
Conclusions: mpMRI may have a reduced sensitivity for detecting clinically significant PC in patients under the age of 50 for PI-RADS score 4,5 lesions. Many significant PC lesions were reported as PI-RADS 3 under the age of 50. We recommend that increased significance is placed on PI-RADS 3 lesions found in patients under the age of 50.
Keywords: mpMRI, prostate cancer, young, sensitivity
Introduction
Prostate cancer (PC) is the most commonly diagnosed cancer in the USA after skin cancer,1 and will affect one in six men during their lifetime. Since the introduction of prostate-specific antigen (PSA) in the 1980s, the detection of PC has risen.2 Although PC is considered to be a disease commonly affecting men over the age of 65, it is not rare for young men to be affected as well. The introduction of the PSA blood test resulted in a dramatic rise in PC incidence in men aged 40–49. Smith et al described PC incidence under the age of 50 in classic literature prior to the PSA era as 0.8%–1.1%,3 while Li et al found that in the USA during 2001–2007 the incidence was 3%.1 Primary diagnostic tools for PC in use today include blood PSA and its derivatives, digital rectal examination and mpMRI of the prostate. mpMRI is considered as an increasingly powerful tool for decision-making in patients suspected of having PC or for their active surveillance protocol in many centers. This young population not only has the most to gain from active surveillance but also the most to lose if significant cancer is missed. Thompson et al evaluated the performance of mpMRI in diagnosis of clinically significant PC, using transperineal template-guided mapping biopsies. In their study, they reported a sensitivity of 96% for the general population.4 Van Leeuwen et al created a nomogram to determine the risk of significant PC prior to biopsy. The nomogram combined mpMRI Prostate Imaging Reporting and Data System (PI-RADS) score along with other factors such as age, PSA level and prostate volume. The use of the nomogram can reduce 28% of prostate biopsies while missing 2.6% of significant PC cases.5 Siddiqui et al compared MRI-targeted biopsies to standard transrectal ultrasound (TRUS)-guided biopsies and found 30% higher diagnosis rate for high-risk cancers.6 The PROMIS (PRostate Magnetic resonance Imaging Study) trial reported diagnostic accuracy of mpMRI as 93% and found that if TRUS-guided biopsies were compared to mpMRI-guided biopsies, up to 18% more cases of clinically significant PC might be detected.7 In a review of the detection abilities of mpMRI for clinically significant PC by Futterer et al, mpMRI had the potential of excluding clinically significant disease. The sensitivity reported ranged from 58% to 96%.8 However, little is published on the diagnostic accuracy of mpMRI in PC detection in young patients. Since the architecture of the prostate changes with age (eg, size, hyperplasia, and glandular/fibrosis proportion), we aimed to evaluate the sensitivity of mpMRI in the subpopulation of patients under the age of 50. To the best of our knowledge, this has never been examined or published before.
Methods
Study design
Data from a prospective collected and ethics-approved database were retrospectively analyzed. Data were collected from the Garvan Institute of Medical Research Biobank in March 2017. We reviewed 1,395 cases of patients diagnosed with PC from 2012 to March 2017. From the original cohort, we identified the patients who had obtained a preoperative mpMRI (at 1.5 T or 3 T and mainly reported by one of our experienced uroradiologists – RS or DM – each of whom had reported more than 1,000 cases by then). Lesions were reported by PI-RADS (scans performed between 2012 and 2014 were reported by version 1, and scans performed between 2015 and 2017, by version 2). We then narrowed the cohort of patients to those who had radical prostatectomy as their primary treatment by three experienced surgeons (PDS, CY, and PB at St Vincent’s Hospital in Sydney, NSW, Australia) (N=1,248). Neoadjuvant-treated patients were excluded from the cohort because the effect on prostate architecture might be unexpected. This selection process resulted in a population of 796 patients. Of this cohort, 51 patients were under the age of 50 and had a full set of information that enabled pair-matching and had clinically significant cancer (Figure 1). Data collected from the bio-bank included patient age at diagnosis, prostate size, tumor volume, International Society of Urological Pathology (ISUP) grade from the pathology report, mpMRI PI-RADS version 1 or 2 score, and blood PSA value.
  | Figure 1 Flow chart of patients’ selection process. Abbreviations: ISUP, International Society of Urological Pathology; mpMRI, multiparametric MRI. |
Pathology
Clinically significant cancer was defined as ISUP >2 or ISUP 2 with >5% Gleason 4. The tumor volume was calculated from the radical prostatectomy whole mount specimen according to the 3D volume estimated method.9 The handling of the radical prostatectomy specimen was done according to the Royal College of Pathologists of Australasia cutup guide for examination of prostate.10
mpMRI acquisition
mpMRI was performed before prostate biopsy with a 1.5 T magnet or 3 T magnet. Axial, sagittal, and coronal TSE (turbo spin echo); TR: 4000, TE: 120–135, 3 mm thick slices with no gap, 16 cm field of view. Axial diffusion: B value: 1,500, 3 mm slices on same plane and slice location as the axial T2. Axial post contrast T1 dynamic series: 3D sequences, 5 second phases.
We also performed whole pelvis pre-contrast T1-weighted and post-contrast T1 fat saturated sequences. Scans were done using the gold standard European Society of Urogenital Radiology MRI protocol.11
Analysis
The 51 patients under 50 years of age were pair-matched to their most identical patients out of 601 potential candidates older than 55, using propensity score matching. Matching criteria included ISUP score and tumor volume on final pathology. Tumor aggressiveness and volume were selected as matching criteria as these parameters influence the detection ability of PC by mpMRI. The sensitivity of the mpMRI to diagnose clinically significant PC was calculated in each age group using the PI-RADS score and final pathology reports of the radical prostatectomy specimen, including 95% CIs. True positives were patients who had a lesion on mpMRI (PI-RADS 3–5 or PI-RADS 4–5) and had tumor on the prostatectomy section. False negatives were patients who had tumor on prostatectomy lesion but did not have a suspicious lesion on mpMRI (PI-RADS 1–2 or PI-RADS 1–3). The chi-squared test and Mann–Whitney U test were performed to evaluate matching characteristics and differences in sensitivity. Statistics were performed using SPSS version 23 (IBM) and significance was set at P<0.05.
Ethics
Patients were recruited and provided written informed consent, and data were collected at the time of surgery, as approved by the St Vincent’s Hospital Human Research Ethics Committee (HREC/12/SVH/231) in Sydney, NSW, Australia. Only de-identified data were used for analyses. Therefore, no further patient consent was required.
Results
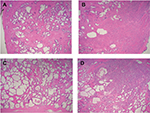
There were 51 patients in each group (see for patients’ characteristics). The median age for the young group was 47 (IQR 45–49) and 62 (IQR 59–67) for the older matched group. The median blood PSA level was 4.10 ng/mL (IQR 3.05–6.10) for the young group and 5.50 ng/mL (IQR 4.10–7.20) for the older age matched group. The median prostate size for the young and older groups was 37.0 mL (IQR 33.40–43.0) and 50.0 mL (IQR 42.25–61.50), respectively. The median tumor volume was 1.0 mL (IQR 0.70–1.70) for both groups (P=0.91). Median ISUP score was 2 for both groups (P= 1.0). The median PI-RADS score for the young group was 3, and 4 for the older group (Table 1). The sensitivity of mpMRI, for PI-RADS 3,4 and 5 was 80.3% (95% CI 66.8%–90.1%) in the young group and 84.3% in the older group (95% CI 71.4%–92.9%), demonstrating no statistically significant difference (P=0.603). Sensitivity of mpMRI for PI-RADS 4,5 was 49.0%–95% CI (34.7%–63.4%) for the young group and 72.5%–95% CI (58.2%–84.1%) for the older group, which differ significantly (P=0.014) (Table 2). Figure 2 illustrates potential undergrading of PI-RADS 2.
  | Table 2 Results summary Abbreviations: mpMRI, multiparametric MRI; PC, prostate cancer; PI-RADS, Prostate Imaging Reporting and Data System. |
Discussion
The preliminary work assessed, for the first time (to the best of our knowledge), the sensitivity of mpMRI in a subgroup of young patients under the age of 50 by comparing pre-surgical mpMRI and final pathology report after radical prostatectomy (considered as the gold standard) to pair-matched group of similar patients over the age of 55. These arbitrary age differences were chosen to create an age gap, with an aim to see whether there are age-dependent differences in the ability to detect PC with mpMRI. The pair-matching was performed on tumor volume on whole-mount pathology and the highest ISUP score, as these two parameters equate to architectural changes in the tissue, which are likely to be reflected on the mpMRI. We did not match blood PSA levels since even in a healthy population, the expected PSA for younger and older patients is not the same, thus, matching them will result in a bias toward lower PSA in the older group.
We found that the sensitivity of mpMRI for clinically significant PC, for a PI-RADS score of 4–5, in patients under the age of 50 was only 49.0%, compared to 72.5% for older patients (absolute sensitivity reduction of 23.5%). This phenomenon is also seen in breast cancer screening. Jatala et al reviewed screening timing and modalities for breast cancer. They described a comparison of contrast-enhanced MRI and digital mammography in high-risk young women, mentioning that this population was chosen because of the dense breast tissue present and the known decreased sensitivity of mammography in this age group.12 The prostate architecture dramatically changes with age (Figure 3). Ravoori et al attempted to optimize the MRI of the prostate in young and old mice and concluded that the differences in size and glandular hyperplasia with age imply that age should be an important determinant when choosing models of prostate biology and disease.13 One theoretical explanation for the reduced sensitivity of mpMRI under the age of 50 is that the tumor (Figure 3B) resembles the normal tissue (Figure 3A) more than it does in the older group (Figure 3C), which is more cystic. Nevertheless, one must take into account that, if considering PI-RADS score of 3,4 or 5 as a threshold for treatment or further biopsy, a significant difference between the groups was not found in the current study. This is because of more clinically significant tumors reported as PI-RADS 3 lesions in the younger age group. This implies that although PI-RADS 3 means equivocal cancer risk, in young patients, we must have a lower threshold for further investigation (eg, biopsy) in that “gray zone” score. We suggest that further studies might consider and validate a term “PI-RADS 3y” in the younger population of men under the age of 50, which might alert the clinician for that phenomenon. An important consideration is that radiologists may be biased when reviewing the mpMRI of younger patients toward a lower PI-RADS score (eg, prostatitis). One way to address this possible bias is to blind the radiologist to the patients’ age. This option is not straight forward because parameters of the prostate (size, adenoma, etc.) may unmask the patient age. It is important to mention that, in this trial, all MRI scans were done prior to biopsy and surgery, thus, there was no bias of the radiologist toward reporting of higher PI-RADS scores. Our two main readers (RS and DM) also collaborated on a trial evaluating mpMRI diagnostic performance.4 For that trial, a comparative accuracy was made and found the difference to be insignificant (95% CI –0.055 to 0.036, P= 0.676). There were a few limitations to our research. First, this is a retrospective study, which used the data of a single institution. Next, our sample size was relatively small due to very harsh selective criteria (eg, age under 50, mpMRI done after 2012 when our main radiologists had more than 1,000 reports, selecting our high-volume surgeons only). Furthermore, due to limited sample size, we were not able to stratify our outcomes by lesion location on mpMRI. Moreover, this is not a screening study because all the patients had PC. Finally, in 2015, the PI-RADS system was updated from version 1 to version 2. Since our cohort included scans done in years 2012–2017, we had mixed reporting. The major difference between the versions was downgrading the role of contrast-enhanced scans in the score, thus, if any bias resulted from the change, it would be that more PI-RADS 3 were reported as PI-RADS 2. We have ruled out any potential bias by checking that statistical significance between the patients reported by version 1 and version 2 in both groups demonstrated nonsignificance in both age groups (for patients under the age of 50, P= 0.79 and for patients over the age of 55, P=0.36).
Conclusions
mpMRI in patients under the age of 50 has a reduced sensitivity for diagnosing clinically significant PC when compared with patients over the age of 55. Using the cut-off of PI-RADS score 4,5 for prostate biopsy might result in missing half of the clinically significant cancers. We recommend that a greater weight be applied to PI-RADS score of 3 in this younger subgroup when deciding to progress to prostate biopsy. A future prospective screening study is recommended to evaluate our preliminary findings.
Acknowledgments
We wish to thank Dr Phillip Brenner for data use and the Garvan IT Department for ongoing infrastructure support of Cansto, the in-house built database. The Australian Commonwealth Department of Health and the St Vincent’s Prostate Cancer Center have funded this study through the Australian Prostate Cancer Research Centre-NSW (APCRC-NSW).
Disclosure
The authors report no conflicts of interest in this work.
References
Li J, German R, King J, et al. Recent trends in prostate cancer testing and incidence among men under age of 50. Cancer Epidemiol. 2012;36(2):122–127. | ||
Salinas CA, Tsodikov A, Ishak-Howard M, Cooney KA. Prostate cancer in young men: an important clinical entity. Nat Rev Urol. 2014;11(6):317–323. | ||
Smith CV, Bauer JJ, Connelly RR, et al. Prostate cancer in men age 50 years or younger: a review of the Department of Defense Center for Prostate Disease Research multicenter prostate cancer database. J Urol. 2000;164(6):1964–1967. | ||
Thompson JE, van Leeuwen PJ, Moses D, et al. The diagnostic performance of multiparametric magnetic resonance imaging to detect significant prostate cancer. J Urol. 2016;195(5):1428–1435. | ||
van Leeuwen PJ, Hayen A, Thompson JE, et al. A multiparametric magnetic resonance imaging-based risk model to determine the risk of significant prostate cancer prior to biopsy. BJU Int. 2017;120(6):774–781. | ||
Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390–397. | ||
Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. The Lancet. 2017;389(10071):815–822. | ||
Fütterer JJ, Briganti A, de Visschere P, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol. 2015;68(6):1045–1053. | ||
Chen ME, Johnston D, Reyes AO, et al. A streamlined three-dimensional volume estimation method accurately classifies prostate tumors by volume. Am J Surg Pathol. 2003;27(10):1291–1301. | ||
King S, Dimech M. Anatomical Pathology Macroscopic Cut-up Manual, Royal College of Pathologists of Australasia, Surry Hills NSW. 2015 [updated. 2017;12. Available from: http://www.rcpa.edu.au/Library/Practising-Pathology/Macroscopic-Cut-Up. Accessed May 03, 2018. | ||
Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–757. | ||
Jatala S, Fitzgerald S, Tietze P, et al. What are the recommended timing and screening modalities in women at higher risk for developing breast cancer? A Clin-IQ. J Patient Cent Res Rev. 2015;2(1):38–42. | ||
Ravoori M, Duggal J, Gagea M, et al. Visualizing the prostate gland by MR imaging in young and old mice. PLoS One. 2013;8(3):e55746. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.