Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Reduced Plasma Estradiol Levels are Associated with Sleep Apnea in Depressed Peri- and Post-Menopausal Women
Authors Cai L , Huang L , Wei L, Yao J, Xu L, Chen W
Received 8 September 2021
Accepted for publication 18 November 2021
Published 2 December 2021 Volume 2021:17 Pages 3483—3488
DOI https://doi.org/10.2147/NDT.S333154
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Li-Qiang Cai,1,2 Lei Huang,1,2 Li-Li Wei,1,2 Jia-Shu Yao,1,2 Luo-Yi Xu,1,2 Wei Chen1,2
1Department of Psychiatry, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Center for Sleep Medicine, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Wei Chen
Department of Psychiatry, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, East Qingchun Road 3, Hangzhou, Zhejiang, 310016, People’s Republic of China
Tel +86-571-87887800
Email [email protected]
Objective: This study aimed at investigating the correlation between estradiol and sleep apnea among women with major depressive disorders during the perimenopausal and postmenopausal periods.
Methods: A total of 84 perimenopausal and postmenopausal women diagnosed with depression, and who had been subjected to whole-night polysomnography (PSG) were retrospectively studied. They were assigned into two groups based on the presence of OSA (apnea–hypopnea index (AHI)≥ 5) (OSA vs non-OSA). The correlation between estradiol levels and apnea–hypopnea index were assessed by logistic regression models after adjusting for age, body mass index (BMI), Hamilton Depression Rating Scale (HAMD), Pittsburgh Sleep Quality Index (PSQI), apnea frequency and progesterone.
Results: Among the 84 patients, 45.23% had OSA. Estradiol levels were significantly elevated in non-OSA than in OSA patients (p< 0.05). Univariate analysis revealed that elevated estradiol levels are associated with reduced odds of OSA (odds ratio [OR] 0.92, 95% confidence interval [CI] 0.875– 0.966, p = 0.001). Multivariate analyses showed that low estradiol levels (OR = 0.859, 95% CI 0.826– 0.991, p = 0.031), higher HAMD scores (OR = 1.212, 95% CI 1.012– 1.453, p = 0.037), higher apnea frequency (OR = 2.493, 95% CI 1.389– 4.473, p = 0.002) and higher BMI (OR=1.635, 95% CI 1.136– 2.353, p = 0.008) are correlated with OSA.
Conclusion: The ratio of depressed perimenopausal to postmenopausal women comorbid OSA was high. Higher BMI, low estradiol levels, high apnea frequency and high HAMD scores were correlated with OSA diagnosis and could be potential diagnostic markers for OSA in depressed perimenopausal and postmenopausal women. Reduced estradiol levels were correlated with an increased risk of OSA among depressed perimenopausal and postmenopausal women.
Keywords: major depressive disorder, estradiol, obstructive sleep apnea, menopause, polysomnography
Introduction
Major depressive disorders (MDD) among midlife women is a major public health concern. The global annual prevalence of MDD in women is estimated to be around 5.5% in 2010.1 In China, the prevalence is 5.3% in 2017.2 However, these incidences are elevated among women undergoing menopausal transition.2,3 Bromberger et al. found that women are two to four times more likely to suffer from major depressive disorders (MDD) during the perimenopausal or early postmenopausal periods.4 Freeman et al studied 231 women undergoing menopausal transition and found that MDD is present in 26% of women with perimenopausal and postmenopausal.5
Major depressive disorder (MDD) in women is frequently accompanied by sleep disturbance. Hall et al6 reported that the prevalence of sleep disorders in menopausal women is 50%. Moreover, menopausal women are more likely to suffer from subjective or objective sleep quality impairment.7–9 Perger and Lindberg et al reported that snoring and obstructive sleep apnea (OSA) are risk factors for sleep disturbance during the peri- and postmenopausal period.9–11 More than 90% of peri- and postmenopausal women with OSA are not clinically diagnosed.11–13 This maybe because, menopausal women hardly complain about snoring, gasping, witnessed apneas and other stereotypic symptoms when compared to men with similar obstructive sleep apnea severity.14 However, women with OSA are more likely to complain of insomnia, restless legs and depression.15 Multiple studies have found that women during pregnancy, in those with polycystic ovarian syndrome, during the late menopause transition, and in the postmenopause are risk factors for OSA.15–18 While, reported risk factors for OSA among women include aging, body mass index (BMI), waist circumference and pharyngeal abnormalities.15 After adjusting for increasing age and higher BMI scores, women in menopause transition and postmenopause are 3 times more likely to have OSA compared to premenopausal women.15,18–20 This suggests that reduced estradiol levels may affect OSA occurrence during menopause transition and in early postmenopause.15
Compared to men with OSA, women with OSA are more likely to suffer from depressive symptoms and disorders.21 Additionally, in women with or without a history of depression, there is an increased prevalence of MDD.22 Furthermore, estrogen deficiency is a risk factor for MDD in women.23 Therefore, changes in female reproductive hormone levels maybe associated with increased exposure to both OSA and MDD during peri- and postmenopause. It has been reported that during the peri- and postmenopause periods, there is an association between OSA and reduced estradiol (E2) levels in depressed women.24 However, after adjusting for age and BMI, the association was not significant. Moreover, the association between the degree of depressive symptoms, apnea frequency determined by the polysomnography recordings and progesterone(PRG) levels was not evaluated.24 A limited number of studies have evaluated the association between OSA and plasma E2 levels in depressed peri- and postmenopausal women. Therefore, in this study, our objective is to investigate the association between OSA and estradiol levels in depressed perimenopausal and postmenopausal women, controlling for different potential confounding variables, in order to help test hypotheses that lower estradiol levels would correlate with greater likelihood of OSA.
Methods
Subjects
A total of 84 female patients who had undergone in-room polysomnography, from December 2019 to December 2020, were recruited. The inclusion criteria were: I. All patients aged between 40 and 65 years; ii. Those diagnosed with major depressive or bipolar disorders (in depressive episode) based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria; iii. Perimenopause or postmenopausal women, as defined by the STRAW menstrual bleeding criteria;25 iv. Those who agreed to sign a consent form and v. Women whose scores on the Hamilton Depression Rating Scale (17-items) ≥ 10. Patients with underlying respiratory diseases, a history of taking drugs that can affect normal sleep architecture and ANS, psychotic symptoms, suicide ideas, restless leg syndrome, those diagnosed with other sleep related disorders or other clinical phases of bipolar disorder, and a previous history of treatment for OSA were excluded. Then, patients were assigned into normal (N=46) and OSA groups (N=38). The normal group was defined as patients with apnea–hypopnea index (AHI)<5, whereas the OSA group was defined as AHI≥5, which was the criteria for diagnosing obstructive sleep apnea.
All subjects provided written informed consent to be included in the study, and the study protocol that was written according to the Helsinki Declaration was approved by the ethics committee of Sir Run Run Shaw Hospital. Before obtaining the consent, the patients were given sufficient time and opportunity to inquire about the details of the study and decide to study participation or not.
Polysomnography
Polysomnographic (PSG) recording was performed using Nox A1 (Iceland Nox Medical company). The recorded signals were electroencephalography, electrocardiogram, electromyography of submentum and leg, arterial oxygen saturation (SaO2, including airflow at nose and mouth, chest, and abdominal movement) as well as body position. Measurements were performed from 21.00 h to 06.00 h the following morning in an air-conditioned, sound insulated dark room. Apnea events were based on complete cessation or near-complete cessation of airflow for at least 10 seconds or longer while hypopnea events were defined as a decrease of 30% in airflow for at least 10 seconds accompanied by SaO2 reduction of > 3% or an arousal response. Apnea and hypopnea counts were manually revised by trained technicians after automatic scoring. The apnea–hypopnea index was calculated as the number of episodes of apnea and hypopnea per h during total sleep time.
Assessment
We performed the Hamilton Depression Rating Scale (HAMD) and Pittsburgh Sleep Quality Index (PSQI) scoring for the recruited patients after they had undergone PSG.
Serum E2 and PRG levels were evaluated at diagnosis. Venous blood samples were drawn after an overnight fast of at least 8 h. However, blood samples were not collected during the menstrual period for women undergoing menopause transition. E2 and PRG levels were assayed using chemiluminescence (BECKMAN COULTER UniCel DxI800). The minimal detectable limit for Estradiol and progesterone levels were: 0.1 ug/L (progesterone) and 10.17 pg/mL (E2). The inter-assay coefficients of variation were 7.5% (progesterone), 9.6% (E2). The intra-assay coefficients of variation were 6.2% (progesterone), 7.1% (E2).
Statistical Analysis
The Shapiro–Wilk test was used to determine whether data were normally distributed. Normally distributed continuous data were expressed as mean ± standard deviation, and analyzed by a t test. The chi square test was used for classification data while the Mann–Whitney test was used to analyze continuous data of non-normal distribution. The correlation between Apnea frequence and HAMD score, BMI, serum estradiol levels were analyzed using the Spearman correlation coefficient. Logistic regression models were used to evaluate the correlation between serum estradiol levels and OSA. Logistical regression models were built using OSA as the dependent measure and serum estradiol levels as the primary independent measure. Multivariable analyses were performed using logistic regression models (forced entry) to adjust for estradiol, HAMD score, PSQI score, Apnea frequency, progesterone, age and BMI. Statistical analyses were performed using SPSS 22.0 (SPSS Inc, Chicago, IL), and p ≤ 0.05 was considered statistically significant.
Results
Sample Characteristics
The mean age for these patients was 55.29 ± 6.72 years. A total of 62 patients were postmenopausal while 22 were in the perimenopausal period. Mean serum E2 levels were 22.72 ± 6.01 pg/mL. On PSG, 38 patients were diagnosed as OSA (AHI ≥ 5) while 46 were diagnosed as non-OSA (AHI < 5). Compared to women without OSA, serum E2 levels were significantly reduced in women with OSA (p <0.05). A total of 54 women were diagnosed with major depressive disorders while 30 were diagnosed with bipolar affective disorder. Women with OSA had higher BMI than those without OSA (p < 0.001). OSA group differed significantly in apnea frequency, HAMD and PSQI scores (p < 0.001) with more depression symptoms and sleep problems than those in the non-OSA group. With regards to menopause status, progesterone or depressive disorder diagnoses, there were no significant differences between those with and without OSA (Table 1).
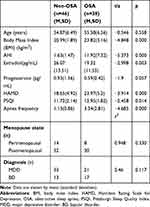 |
Table 1 Comparison Between Characteristics of the Study Groups |
Correlations Between Serum Estradiol Levels and OSA
OSA severity was negatively correlated with E2 levels in perimenopausal and postmenopausal women (OR=0.92, 95% CI 0.875–0.966, p = 0.001). The risk for OSA reduced by 8% as E2 levels increased by 1 pg/mL. Although a greater proportion of postmenopausal women had OSA when compared to those in menopause transition (48.4% vs 36.4%, respectively), the association of menopause status with OSA was rendered nonsignificant (p = 0.33). The statistical results were consistent for postmenopausal women (n =62), exhibiting a negative correlation between reduced E2 levels and OSA (OR 0.94, 95% CI 0.892−0.987, p=0.014). For peri- and postmenopausal women, there was a negative correlation between reduced progesterone levels and OSA (OR 0.487, 95% CI 0.169–1.402, p=0.182). E2 levels inclined to be reduced in those with, compared to those without OSA (median estradiol 19.32 pg/mL vs 26.07 pg/mL in OSA vs non-OSA, p = 0.003).
The correlations between apnea frequency and BMI (rs=0.156, p=0.157), and apnea frequency and estradiol (rs=−0.084,p=0.447) were not significantly. While apnea frequency was positively correlated with higher HAMD scores (rs=0.318,p=0.003) (Figure 1).
After simultaneously adjusting for E2, HAMD scores, PSQI scores, PRG, apnea frequency, age and BMI, multivariate analyses showed that E2 levels (OR = 0.859, 95% CI 0.826–0.991, p = 0.031), higher HAMD scores (OR = 1.212, 95% CI 1.012–1.453, p = 0.037), apnea frequency (OR = 2.493, 95% CI 1.389–4.473, p = 0.002) and higher BMI (OR=1.635, 95% CI 1.136–2.353, p = 0.008) were significantly associated with OSA (Table 2).
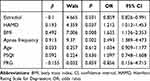 |
Table 2 Variables Associated with OSA versus Non-OSA |
Discussion
In this study, we found that 45.23% of the 84 patients with depression during the peri- and postmenopause period met the inclusion criteria for OSA. Also, we explored that OSA was definitively associated with E2 levels in depressed perimenopausal and postmenopause women, independent of age and BMI. In addition, the present study extends prior work linking OSA, depression and menopause transition24 and demonstrates an association between OSA and HAMD scores, apnea frequency and BMI indices. Together, these findings indicate that there were many undiagnosed OSA cases with reduced E2 levels in depressed perimenopausal and postmenopause women.
For depressed women in peri- and postmenopause periods, serum E2 levels were significantly lower in those diagnosed with OSA when compared to those without OSA in our study. Furthermore, after adjusting for E2 levels, HAMD scores, apnea frequency, PSQI scores, PRG levels, age and BMI, it was found that E2 levels and higher BMI were significantly correlated with OSA diagnosis. Therefore, a higher BMI (obesity) score is highly associated with OSA occurrence. These findings suggest that E2 reduction may induce OSA. Our findings are consistent with those of a study that reported an inverse relationship between E2 levels and OSA,24,26 and are indirectly supported by reports that postmenopausal women using hormone therapy have a lower prevalence of OSA than those not taking hormone therapy.19,27 Depressed women in late menopause transition and postmenopause periods and who have higher BMI scores and lower estradiol levels should be referred to a sleep clinic for OSA screening by polysomnography.
In our results, there was higher HAMD scores and apnea frequency for women with OSA than that for women without OSA. Moreover, there was a significant correlation between HAMD scores, apnea frequency and OSA diagnosis, implying that higher HAMD scores greatly increase OSA risks or possibility of having higher apnea frequency. It would be logical to assume that higher apnea frequency or OSA might also exacerbate depression symptoms. A significant correlation was shown between OSA, apnea frequency and depressive severity, as revealed by HAMD scores and apnea frequency in women with depression who were in menopause transition and postmenopause periods.
Although our sample size was comparable to those of other studies, it was relatively small. Studies with larger sample sizes should determine whether OSA treatment may further improve sleep quality and mood in depressed peri- and postmenopause women.
Conclusion
In conclusion, there are many cases of undiagnosed OSA among depressed peri- and postmenopause women. Moreover, there is a correlation between reduced E2 levels and OSA, with higher HAMD scores, higher apnea frequency and higher BMI scores being associated with OSA diagnosis in depressed women during the peri- and postmenopause periods. Treatment of sleep and depression disorders among women should not overlook OSA treatment. OSA screening and providing the necessary treatment can improve therapeutic response rates for depression.28 When treating mood disorders complicated by OSA, psychiatrists should be cautious of using drugs with a muscle relaxant effect, such as benzodiazepines.
Acknowledgments
The study was supported by grants from Research supports from the Special Project of the modernization of traditional Chinese medicine of Zhejiang Province (No. 2020ZX012), the Chinese Sleep Research Society Hansoh Project (2019HSB01) and a Key Project of the Medical and Health Science and Technology Plan of Hangzhou Municipality (No. Z20200051).
Disclosure
All authors declare no competing interest.
References
1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586.
2. Ren X, Yu S, Dong W, et al. Burden of depression in China, 1990-2017: findings from the global burden of disease study 2017. J Affect Disord. 2020;268:95–101.
3. Tang R, Luo M, Li J, et al. Symptoms of anxiety and depression among Chinese women transitioning through menopause: findings from a prospective community-based cohort study. Fertil Steril. 2019;112:1160–1171.
4. Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41:1188–1879.
5. Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63:375–382.
6. Hall MH, Kline CE, Nowakowski S. Insomnia and sleep apnea in midlife women: prevalence and consequences to health and functioning. F1000Prime Rep. 2015;26(7):63.
7. Nevšímalová S. Sleep and sleep-related disorders in women. Cas Lek Cesk. 2019;158:321–322.
8. Valiensi SM, Belardo MA, Pilnik S, et al. Sleep quality and related factors in postmenopausal women. Maturitas. 2019;123:73–77.
9. Perger E, Mattaliano P, Lombardi C. Menopause and sleep apnea. Maturitas. 2019;124:35–38.
10. Lindberg E, Bonsignore MR, Polo-Kantola P. Role of menopause and hormone replacement therapy in sleep-disordered breathing. Sleep Med Rev. 2020;49:101225.
11. Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706.
12. Cai L, Xu L, Wei L, Sun Y, Chen W. Evaluation of the risk factors of depressive disorders comorbid with obstructive sleep apnea. Neuropsychiatr Dis Treat. 2017;16(13):155–159.
13. Heinzer R, Marti-Soler H, Marques-Vidal P, et al. Impact of sex and menopausal status on the prevalence, clinical presentation, and comorbidities of sleep-disordered breathing. Sleep Med. 2018;51:29–36.
14. Redline S, Kump K, Tishler PV, et al. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149(3 Pt 1):722–726.
15. Young T, Rabago D, Zgierska A, et al. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–672.
16. Veasey SC, Rosen IM. Obstructive sleep apnea in adults. N Engl J Med. 2019;380(15):1442–1449.
17. Sanders MH. Increased risk of obstructive sleep apnea in obese women with polycystic ovary syndrome (a review of two related articles). Articles reviewed: ‘Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome’ and ‘Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance’. Sleep Med. 2002;3:287–289.
18. Dancey DR, Hanly PJ, Soong C, et al. Impact of menopause on the prevalence and severity of sleep apnea. Chest. 2001;120:151–155.
19. Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613.
20. Atsma F, Bartelink ML, Grobbee DE, et al. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–279.
21. Freeman EW. Associations of depression with the transition to menopause. Menopause. 2010;17(4):823–827.
22. Maartens LW, Knottnerus JA, Pop VJ. Menopausal transition and increased depressive symptomatology: a community based prospective study. Maturitas. 2002;42:195–200.
23. Joffe H, de Wit A, Coborn J, et al. Impact of estradiol variability and progesterone on mood in perimenopausal women with depressive symptoms. J Clin Endocrinol Metab. 2020;105:e642–e650.
24. Galvan T, Camuso J, Sullivan K, et al. Association of estradiol with sleep apnea in depressed perimenopausal and postmenopausal women: a preliminary study. Menopause. 2017;24:112–117.
25. Harlow SD, Gass M, Hall JE, et al. STRAW + 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168.
26. Netzer NC, Eliasson AH, Strohl KP. Women with sleep apnea have lower levels of sex hormones. Sleep Breath. 2003;7:25–29.
27. Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–1192.
28. Waterman L, Stahl ST, Buysse DJ, et al. Self-reported obstructive sleep apnea is associated with nonresponse to antidepressant pharmacotherapy in late-life depression. Depress Anxiety. 2016;33:1107–1113.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

