Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 10
Redefining face contour with a novel anti-aging cosmetic product: an open-label, prospective clinical study
Authors Garre A, Martinez-Masana G, Piquero-Casals J , Granger C
Received 8 August 2017
Accepted for publication 5 September 2017
Published 13 November 2017 Volume 2017:10 Pages 473—482
DOI https://doi.org/10.2147/CCID.S148597
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Video abstract presented by Aurora Garre
Views: 338
Aurora Garre,1 Gemma Martinez-Masana,1 Jaime Piquero-Casals,2 Corinne Granger1
1Innovation and Development, ISDIN S.A., Barcelona, Spain; 2Dermik Clinic, Barcelona, Spain
Background: Skin aging is accelerated by multiple extrinsic factors: ultraviolet radiation, smoking and pollution increase oxidative activity, damaging cellular and extracellular components such as DNA, proteins, and lipids. With age, collagen and hyaluronic acid levels decline, resulting in loss of elasticity and moisture of the skin. Over time this damage leads to characteristic signs that make the skin look older: altered facial contour, sagging skin, wrinkles, and an uneven complexion. This study evaluated the anti-aging effects of a new facial cream formulated with carnosine, Alteromonas ferment extract, crosspolymer hyaluronic acid, and a tripeptide.
Methods: An open-label intra-individual study to assess the anti-aging efficacy of the investigational product in 33 women aged 45 to 65 years. The product was applied twice daily for 56 days. Facial contour and skin deformation, elasticity, hydration, and complexion were measured with specialized equipment at baseline and days 28 and 56. Additionally, subjects completed questionnaires at days 28 and 56 on the perceived efficacy and cosmetic characteristics of the product.
Results: After 56 days of use of the investigational product, a redefining effect was observed, with a significant decrease in sagging jawline (7%). Skin was significantly more hydrated (12%), firmer (29%), and more elastic (20%) (P<0.001 for all). On complexion assessment, skin texture (a measure of skin smoothness) and spots (brown and red skin lesions) also improved significantly (12% and 6% decrease, respectively). In the subjective self-evaluation, the majority of subjects reported that the skin was visibly tightened and more elastic, flexible, and moisturized (91%, 88%, 91%, and 90%, respectively). The product was well tolerated with no adverse events reported during the study.
Conclusion: This new cosmetic product demonstrated anti-aging effects after 56 days of use, most notably a redefined facial contour and improved complexion. It is a safe and effective anti-aging product.
Keywords: skin aging, facial contour, redefining, carnosine, Alteromonas
Introduction
All body systems undergo age-related physiological changes,1,2 but unlike internal organ systems, the changes in our skin are visible. The skin has the potential to project an image of health and vitality, and a discord between one’s chronological age and apparent age is understandably undesirable. A 2015 survey by the American Society for Dermatologic Surgery reported that 72% of respondents were extremely bothered by skin texture and/or discoloration, and 67%, by sagging skin.3 With nonsurgical anti-aging alternatives gaining popularity4 and global population aging predicted to continue over the next 40 years,5 anti-aging solutions must be continually innovated to meet users’ needs.
The facial changes seen with aging involve multiple factors: skeletal changes and weakening and laxity of facial muscles6 accompanied by redistribution and loss of subcutaneous fat and skin laxity leads to wrinkles, prominent lines, and sagging skin.7 Changes in collagen play a key role in diminishing skin elasticity. Such changes involve reduced production, structural changes of disorganization, thickening, and fragmentation, and increased breakdown of collagen.8 At a biochemical level, numerous intracellular and extracellular changes are at play: not only are there fewer fibroblasts, but they express more matrix metalloproteinase (responsible for collagen breakdown) and less of its inhibitors.8,9 Glycosaminoglycans such as hyaluronic acid decrease, leading to loss of skin turgor.10 While intrinsic aging is genetically determined, photoaging is driven by the oxidative effects of ultraviolet radiation.11 Other factors that are recognized to impact skin aging are smoking, and environmental pollutants such as ozone and fine particulate matter.12,13 Exposure to infrared rays has been shown to increase MMP1 levels,9 and reactive oxygen species increase dramatically in response to UV radiation, in turn damaging cell DNA, RNA, proteins, and lipids.11 Air pollution reduces the skin’s antioxidant capacity, increases the generation of free radicals and reactive oxygen species, and via oxidative damage alters the function of lipids, DNA, proteins, and mitochondria.12,13 Advanced glycation end products (AGEs) are the result of nonenzymatic glycation reactions, with type I collagen and elastin being the most-affected proteins in intrinsic skin aging, and AGE production increases under conditions of oxidative stress.14 In the skin, glycation of collagen leads to cross-linked, stiff collagen fibers, affecting its ability to react with other matrix proteins.15
Nonsurgical options for facial contouring and sculpting, such as hyaluronic acid fillers and facial threading,16 are among the most popular and novel cosmetic anti-aging options in recent years. Although they are deemed safe and effective,17,18 for many consumers, such techniques are still unacceptably invasive, and the option of a cosmetic cream is far more appealing and practical than a needle.
An innovative facial cream was developed, based on the effective ingredients carnosine, Alteromonas ferment extract, crosspolymer hyaluronic acid, and tetradecyl aminobutyroylvalylaminobutyric urea trifluoroacetate (a tripeptide). The anti-aging effects of carnosine, an endogenous dipeptide, have been extensively studied and are well-documented in the literature,19 and include antioxidation, antiglycation,20 and anti-crosslinking of proteins.21 Alteromonas ferment extract, an exopolysaccharide obtained from marine microorganisms, is an innovative anti-pollution ingredient that chelates heavy metals22 and reduces adherence of PM2.5 (particulate matter up to 2.5 micrometers) to the skin.23 Crosspolymer hyaluronic acid has demonstrated greater benefits over linear hyaluronic acid in reducing transepidermal water loss, retaining and redistributing water within the epidermis, maintaining skin integrity, and improving skin barrier structure and function.24 It is also more resistant to breakdown by hyaluronidase than its linear counterpart.25,26 The manufacturers of the tripeptide tetradecyl aminobutyroylvalylaminobutyric urea trifluoroacetate report that in fibroblasts, it stimulates endogenous synthesis of hyaluron, proteoglycans, and collagen.
This article reports the results from a clinical study that tested the efficacy of this new anti-aging cosmetic facial cream for multiple skin parameters including facial contour, skin firmness, elasticity, hydration, and cutaneous imperfections.
Materials and methods
Clinical protocol
The study was performed by an independent clinical research organization in Lyon, France, from September 19 to November 15, 2016. Thirty-three healthy female subjects aged 45 to 65 years old were enrolled in this open-label, intra-individual study. The subjects were women with Fitzpatrick skin phototype I, II or III, who were following a daily skincare routine, and showed age-related skin alterations including fine wrinkles, slackened skin on the face, slight sagging of facial contour, imperfections on skin, and dry skin on forearms as determined by a Corneometer (Courage & Khazaka Electronic GmbH) reading of less than 50. The study excluded those who were pregnant, breastfeeding, or planning to get pregnant during the study, and subjects with cutaneous pathology in the studied zone. Other exclusion criteria were use of topical or systemic treatment in the weeks prior to the study that could interfere with cutaneous acceptability of the product, excessive exposure to sunlight/UV within the month prior to and during the study, and participation in another clinical trial during the study period or in the past 2 weeks involving the face. All subjects gave written informed consent. This study did not require approval from an ethics committee or competent authority because it was performed in France, involved a cosmetic product within the definition of article L. 5131-1 of the French Public Health Code, the product’s safety had already been established under dermatological control, it was conducted in healthy volunteers, and involved low-risk methods. However, it was conducted according to the Declaration of Helsinki (1964) and its successive updates. When collecting data, the investigators followed the study protocol, current internal procedures, and Good Clinical Practice guidelines CPMP/ICH/135/95 (R2).
All subjects were given a 50 mL sample of the investigational cosmetic product and instructed to apply it twice daily for 56 days to the face, neck, and one forearm. They were instructed to avoid excessive UV exposure, and not to modify their usual make-up and hygiene products, nor to use new products. Measurements of facial contour (cheek volume) and skin firmness, elasticity, hydration, and imperfections (definitions of texture and spots will follow) were taken at baseline and at visits at days 28 and 56 of the study. Subjects were asked not to apply any product of any sort on the morning of the measurements; showering with usual product was permitted. The areas assessed were the maxillary zone for elasticity, the cheek for firmness, and the forearm for hydration. The entire face was assessed for complexion. Prior to taking measurements, subjects spent 20 minutes in a temperature- and humidity-controlled environment. On day 28 and day 56 volunteers completed a questionnaire at home on the subjective efficacy and cosmetic attributes of the product. Adverse effects, if any, were reported.
Formulation
The investigational cosmetic product was a white oil in water emulsion cream containing 0.2% carnosine, 0.06% sodium hyaluronate, 0.02% Alteromonas ferment extract, and 0.0005% tetradecyl aminobutyroylvalylaminobutyric urea trifluoroacetate (a tripeptide).
Test methods
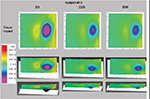
Face contour remodeling was measured using FaceScan (LMI Technologies GmbH, Berlin, Germany), a fringe projection system that compares 3D pictures and uses a color code to express changes in volume (Figure 1). To ensure that the subjects’ position was consistent throughout the study and did not interfere with the readings, the chair height, camera height, and headrest adjustment were recorded and reproduced at day 0, 28, and 56 measurements (Figure 2). The device’s analysis software ensures the 3D images are repositioned in an identical way for comparison.
Skin firmness was assessed using Dynaskin (EOTECH SA, Marcoussis, France), an add-on to the DermaTOP system. The device deforms the skin by blowing out air, and a 3D sensor captures the shape of the skin surface before, during, and after deformation. The software computes the difference to provide information on deformation of the skin and underlying tissues. Volume (mm3), surface (mm2), and maximum depth (mm) are measured. A decrease in any of these parameters indicates a firming effect.
Skin elasticity was measured using Cutometer (Courage & Khazaka Electronic GmbH, Cologne, Germany). This uses a vacuum to suck the skin into a measurement probe. The depth to which the skin penetrates the probe is used to produce a skin deformation curve (Figure 3). Each measurement is an average of two acquisitions, and three consecutive cycles were performed. From this curve, we can determine the cutaneous elasticity (termed R2) and viscoelasticity (termed R6). R2 is represented by the ratio Ua/Uf, where Ua is the total recovery to initial state (the degree to which the skin gets back to its initial state after suction and relaxation, total time 3 seconds) and Uf is the final distension (how deep into the probe the skin is at the end of suction). An increase in R2 indicates more elastic skin. R6 can also be quantified, from the ratio of delayed distension (Uv)/immediate extensibility (Ue). An increase in R6 indicates a greater level of skin viscoelasticity and hydration in the deeper layers of the skin.
Hydration was assessed on the forearm, using Corneometer, a capacitance measuring device that measures the humidity level of the most external cutaneous layers of the stratum corneum and records changes directly onto a computer. Prior to all measurements, subjects stayed in a temperature- and humidity-controlled environment (between 21°C and 25°C and between 35% and 55% relative humidity) for a minimum of 15 minutes to reduce variation in readings due to environmental effects.
The VISIA system (Canfield Scientific, Inc, Parsippany, NJ, USA) was used to evaluate skin complexion. The device takes rapid capture photos with different types of illumination which are overlaid to assess changes. The device has a chin rest and forehead rest which ensure the subject’s position is stable (Figure 4). The data processing screen directly controls repositioning using overlay visualization of the images at each time of acquisition. In this study, we looked specifically at two aspects of complexion: texture and spots. Texture represents the smoothness of the skin: the device identifies color gradations from the surrounding skin tone, and shows peaks and valleys on the skin surface (in yellow and blue) that indicate variation in surface texture. Spots are brown or red lesions of varying size including freckles, acne scars, hyperpigmentation, and vascular lesions. They have a distinct color and contrast from the background skin tone.28
Data analysis
Data were analyzed with Student’s paired t-test (2-tailed). A P-value <0.05 was considered statistically significant. Statistical analysis was performed using EXCEL (version 2016). Changes and percentage change of the different parameters were calculated using the following formula: ∆= (TZti - TZt0); ∆% = ((TZti - TZt0)x 100) / TZt0, where TZ = value obtained on treated zone, t0 = before product application, and ti = at each measurement time after application. Unless otherwise indicated, values are expressed as mean ± SEM.
Results
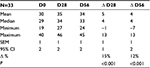
The study included 33 healthy female subjects with a mean age of 56 years (range, 45–65 years). Table 1 shows their baseline characteristics. All subjects completed the study to day 56, and there was no major protocol non-adherence. There was some minor protocol non-adherence, in six subjects, of applying the product only once a day (20 incidences in total). These non-adherences did not invalidate the data obtained from these subjects, and the results were analyzed on an intention-to-treat basis.
  | Table 1 Characteristics of the study subjects |
Cheek sagging decreased significantly (P<0.001) at day 56 with an average decrease of 7% (range –24% to +18%), indicative of a remodeling effect (Figure 5). A reduction was seen in 82% of subjects at day 56. Table 2 contains data on volume measurements at days 28 and 56.
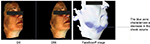
Skin deformation on Dynaskin measurement showed a significant decrease in volume, surface, and depth of skin deformations, both at day 28 and day 56 (Table 3). Average deformation volume decreased by 34% and 29% (P<0.001), average deformation surface decreased by 3% and 5% (P=0.028), and maximum deformation depth decreased by 34% and 29% (P<0.001), respectively, at days 28 and 56. A decrease in deformation is representative of increased firmness (Figure 6).
  | Table 3 Dynaskin skin deformation values and changes at days 0, 28, and 56 in 33 subjects applying the new anti-aging cream Note: Dynaskin; EOTECH SA, Marcoussis, France. |
  | Figure 6 Dynaskin images of deformation from one subject, showing a reduction in area, volume, and depth, at days 28 and 56. Note: Dynaskin; EOTECH SA, Marcoussis, France. |
At day 56, skin elasticity was significantly improved, as shown by a mean 20% (P<0.001) increase in R2 (Ua/Uf) and 12% increase in R6 (Uv/Ue) (P=0.028). These results are indicative of increased viscoelasticity and better hydration of the deeper layers of the skin (Figure 7).
The cutaneous hydration level of the superficial epidermis increased by 15% at day 28 and 12% at day 56, compared with the baseline state (P<0.001 for both), indicative of a moisturizing effect (Figure 8). Table 4 shows the data on cutaneous hydration at days 0, 28, and 56.
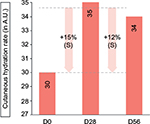  | Figure 8 Cutaneous hydration levels as measured with Corneometer at days 0, 28, and 56. Notes: S represents a significant result (P<0.01) (Corneometer; Courage & Khazaka Electronic GmbH). |
Assessment of skin complexion on VISIA showed that skin texture improved, becoming smoother at day 56 as shown by a reduction in skin texture count of 12% (P<0.001). Spots (brown or red skin lesions, including freckles, hyperpigmentation, and vascular lesions) were reduced by 6% (P=0.010) at day 56. Table 5 contains the data on texture and spots count.
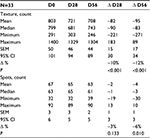  | Table 5 Values for skin texture and spots from VISIA analysis at days 0, 28, and 56 in 33 subjects applying the new anti-aging cream Note: VISIA; Canfield Scientific, Inc, Parsippany, NJ, USA. |
In the subjective assessment, subjects were asked their opinion on the cosmetic qualities of the product. The majority rated the application, fragrance, and texture as pleasant or very pleasant (94%, 81%, and 100%, respectively) and stated that the cream was absorbed quickly (85%) and was non-greasy (91%).
The product efficacy surveys completed by the subjects at day 28 and day 56 showed that the majority viewed their skin as visibly tightened, firmer, more moisturized, more elastic and denser, and more flexible. The questions and responses (percentage of subjects in agreement) from the efficacy questionnaire are presented in Table 6. The majority of subjects (88%) were satisfied with the product, and none reported an unpleasant or uncomfortable sensation. No adverse effects or serious adverse events were reported.
  | Table 6 Responses from subjective questionnaire on the efficacy of the investigational product, at days 28 and 56 Note: Closed questions, percentage of subjects who totally agree or rather agree. |
Discussion
In this clinical study, we set out to assess, using objective accepted methodology and instruments, the anti-aging effects of a finished investigational product formulated with ingredients that had been individually reported to affect skin aging. The results were very encouraging.
We wanted to establish whether such a product could induce a significant change after daily use for 8 weeks in a group of women older than 45 years. This age group was chosen because of the notable skin changes reported in peri-menopausal women, particularly sagging skin, dry skin, and complexion changes.29,30 Such changes are attributed in large part to a drop in estrogen levels at menopause, which in turn leads to reduced production and repair of collagen and elastin in the dermal connective tissue.31 The skin is also more susceptible to oxidative damage.32 Such changes result in noticeable effects in the skin such as reduced elasticity, lack of firmness, fine and coarse wrinkling, and dryness.33 Since estrogen is also known to be involved in regulating melanin levels, in menopausal women, a lack of regulatory estrogen is thought to be responsible for increased melanin synthesis, leading to uneven skin tone.34
One of the strengths of the study is the quantitative, state of the art methodology used. The methods and equipment used in this study were selected for their robustness. In a 2001 study on the technical validation of interference fringe projection systems, the authors concluded that the technique was accurate, reliable, and repeatable.35 FaceScan, which we used to determine facial contour, is such a system.
For skin firmness measurement, we used Dynaskin, a fast optical in vivo topometry method. This method has previously been described as an “accepted classical method” of assessing efficacy in vivo.36 Similarly, in a study in 2000, the author concluded that the Cutometer was suitable for objectively evaluating the effects of dermatological and cosmetic products on skin mechanics and hydration.37 In this study, the forearm was used for hydration measurements. It has previously been demonstrated that for the measurement of skin hydration and biomechanical properties, the forearm is representative of the face, and therefore appropriate for efficacy studies of facial cosmetic products.38 The VISIA system, which we used to assess complexion, has been used in multiple other studies on skin aging and cosmetic treatments.39–41
This study ran from September to November, with the expected seasonal weather changes of a drop in temperature. Usually, such change would lead to skin dryness, but in fact we observed significant increases in the cutaneous hydration rates with the product after 1 and 2 months of use, which we think attests to the product’s efficacy.
The study could have been strengthened by using a larger sample size and a placebo comparison group. It is possible that more pronounced anti-aging effects might have been seen if the study duration had been longer. The most striking result was the decrease in jawline sag. To better evaluate whether this result was meaningful to the subjects, we could have included in the questionnaire a specific question on this part of the face, to correlate objective and subjective results. We did not include objective assessment of rhytids in this study. While the subjective questionnaire results for this parameter were positive, the study could have benefited from an objective measurement, and this could be a focus for future studies.
In the existing literature, there is a growing body of evidence regarding the effects of the component ingredients of this cream, ranging from the “tried and tested” carnosine, to the more recently described Alteromonas ferment extract. Our results on this finished product lend clinical support to the evidence on the individual ingredients.
One study in a human explant model demonstrated that crosspolymer hyaluronic acid had greater benefits over linear hyaluronic acid in reducing transepidermal water loss, retaining and redistributing water within the epidermis, maintaining skin integrity, and improving skin barrier structure and function.24 In our study, we found that subjects’ skin was more hydrated and firmer after use of the investigational product, which contained crosspolymer hyaluronic acid.
Alteromonas ferment extract is an exopolysaccharide produced by an extremophile that lives in deep-sea hydrothermal vents; it has been found to exert anti-pollution effects. A recent paper23 looked at the multiple effects of this substance, and the authors reported many promising features: in in vitro keratinocyte models they showed that it protected against UV-induced lipid peroxidation, this was confirmed in explant models where it reduced MDA levels (MDA is the end product of lipid peroxidation). In vitro it was shown to strongly chelate cadmium and lead. In vivo they showed that it created a protective film on the skin that limited fine particle adhesion and improved barrier function.23 Heavy metal exposure and particle adhesion are associated with increased oxidative stress in the skin and pigmentation changes.12,13 Our findings of improved complexion and increased skin elasticity, hydration, and firmness and reduced sagging provide clinical evidence on the effects of the final product which incorporates this promising biomimetic ingredient.
Information provided by the manufacturer of the tripeptide ingredient tetradecyl aminobutyroylvalylaminobutyric urea trifluoroacetate states that it increases dermal hyaluron synthesis and proteoglycan, decorin, and lumican expression in dermal fibroblasts in vitro. In a clinical study from 2010 on an emulsion containing this ingredient, published in a cosmetics magazine, the reported results were reduced facial volume and sagging.42 The reduction in sagging jawline, remodeling effect, and increased skin firmness observed in the present study is in agreement with the effects reported in 2010.
Carnosine is a dipeptide with numerous functions, which include regulating enzyme activity and inhibiting oxidative reactions.19 The antioxidant mechanisms of carnosine include its ability to inactivate reactive oxygen species, scavenge free radicals, and chelate pro-oxidative metals.19,20 It also has been shown to stimulate collagen and prevent its crosslinking via anti-glycation.43 In our study, the increased skin firmness and reduction in sagging jawline observed add further evidence on the beneficial effects of this anti-aging ingredient.
Overall, the results from this study confirmed the anticipated anti-aging effects of this product on elasticity, hydration, complexion, and most notably, facial contour, after 8 weeks of use. As the global population ages, and consumer demand for a youthful appearance continues to increase, dermatological interventions of varying levels of invasiveness remain in high demand. A cosmetic product that produces clinically objective effects on the most-reported signs of aging is an attractive option for those unable to avoid extrinsic aging factors but wishing to improve their appearance without resorting to more invasive measures.
Conclusion
This novel cosmetic facial cream containing carnosine, Alteromonas ferment extract, sodium hyaluronate crosspolymer, and a tripeptide was shown to be safe and effective in its anti-aging effects on skin. Of particular interest was the redefining effect on facial contour which can be seen as a remodeling effect and improved complexion. Subject satisfaction on efficacy reflected these encouraging results, and the product was well tolerated. This product offers consumers an entirely non-invasive, safe and effective approach to counteract skin aging.
Acknowledgments
This study was funded by ISDIN S.A. and performed by Dermscan Laboratoire, France. Medical writing assistance was provided by J Marshall and M Narda.
Disclosure
AG, CG, and GMM are employees of ISDIN S.A. The authors report no other conflicts of interest in this work.
References
Khan SS, Singer BD, Vaughan DE. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell. 2017;16(4):624–633. | ||
Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–146. | ||
asds.net [homepage on the Internet]. ASDS Consumer Survey on Cosmetic Dermatologic Procedures. American Society for Dermatologic Surgery; 2015. Available from: https://www.asds.net/_Media.aspx?id=8963. Accessed September 8, 2017. | ||
asds.net [homepage on the Internet]. Cosmetic Surgery National Data Bank Statistics. American Society for Aesthetic Plastic Surgery; 2016. Available from: https://www.surgery.org/media/statistics Accessed July 17, 2017. Accessed September 8, 2017. | ||
United States Census Bureau. He W, Goodkind D, Kowal P. An Ageing World: 2015 International Population Reports. Report number P95-16-1. United States Census Bureau; 2016. Available from: https://www.census.gov/content/dam/Census/library/publications/2016/.../p95-16-1.pdf. Accessed September 8, 2017. | ||
Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90(4):592–600. | ||
Friedman O. Changes associated with the aging face. Facial Plast Surg Clin North Am. 2005;13(3):371–380. | ||
Fore J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manage. 2006;52(9):24–35. | ||
Schroeder P, Lademann J, Darvin M, et al. Infrared radiation-induced matrix metalloproteinase in human skin: implications for protection. J Invest Dermatol. 2008;128(10):2491–2497. | ||
Ghersetich I, Lotti T, Campanile G, Grappone C, Dini G. Hyaluronic acid in cutaneous intrinsic aging. Int J Dermatol. 1994;33(2):119–122. | ||
Panich U, Sittithumcharee G, Rathviboon N, Jirawatnotai S. Ultraviolet radiation-induced skin aging: the role of dna damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016;2016:7370642. | ||
Puri P, Nandar SK, Kathuria S, Ramesh V. Effects of air pollution on the skin: a review. Indian J Dermatol Venereol Leprol. 2017;83(4):415–423. | ||
Risom L, Møller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005;592(1–2):119–137. | ||
Crisan M, Taulescu M, Crisan D, et al. Expression of advanced glycation end-products on sun-exposed and non-exposed cutaneous sites during the ageing process in humans. PLoS One. 2013;8(10): e75003. | ||
Gkogkolou P, Böhm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinol. 2012;4(3):259–270. | ||
Villa MT, White LE, Alam M, Yoo SS, Walton RL. Barbed sutures: a review of the literature. Plast Reconstr Surg. 2008;121(3):102e-108e. | ||
Brandt F, Cazzaniga A. Hyaluronic acid gel fillers in the management of facial aging. Clin Interv Aging. 2008;3(1):153–159. | ||
Narins RS, Bowman PH. Injectable skin fillers. Clin Plast Surg. 2005;32(2):151–162. | ||
Prokopieva VD, Yarygina EG, Bokhan NA, Ivanova SA. Use of carnosine for oxidative stress reduction in different pathologies. Oxid Med Cell Longev. 2016;2016:2939087. | ||
Reddy VP, Garrett MR, Perry G, Smith MA. Carnosine: a versatile antioxidant and antiglycating agent. Sci Aging Knowledge Eviron. 2005;2005(18):pe12. | ||
Hobart LJ, Seibel I, Yeargans GS, Seidler NW. Anti-crosslinking properties of carnosine: significance of histidine. Life Sci. 2004; 75(11):1379–1389. | ||
Zhang Z, Cai R, Zhang W, Fu Y, Jiao N. A novel exopolysaccharide with metal adsorption capacity produced by a marine bacterium Alteromonas sp. JL2810. Mar Drugs. 2017;15(6). pii: E175. | ||
Borel M, Lamarque E, Loing E. Unique natural exopolysaccharides for biomimetic protective effect against urban pollution. J Soc Cosmet Chem. 2017;68(1):126–132. | ||
Sundaram H, Mackiewicz N, Burton E, Peno-Mazzarino L, Lati E, Meunier S. Pilot comparative study of the topical action of a novel, crosslinked resilient hyaluronic acid on skin hydration and barrier function in a dynamic, three-dimensional human explant model. J Drugs Dermatol. 2016;15(4):434–441. | ||
Lin CY, Peng HH, Chen MH, Sun JS, Liu TY, Chen MH. In situ forming hydrogel composed of hyaluronate and polygalacturonic acid for prevention of peridural fibrosis. J Mater Sci Mater Med. 2015; 26(4):168. | ||
Li CQ, Huang B, Luo G, Zhang CZ, Zhuang Y, Zhou Y. Construction of collagen II/hyaluronate/chondroitin-6-sulfate tri-copolymer scaffold for nucleus pulposus tissue engineering and preliminary analysis of its physico-chemical properties and biocompatibility. J Mater Sci Mater Med. 2010;21(2):741–751. | ||
Kapoor S, Saraf S. Assessment of viscoelasticity and hydration effect of herbal moisturizers using bioengineering techniques. Pharmacogn Mag. 2010;6(24):298–304. | ||
canfieldsci.com [homepage on the Internet]. Visia. Canfield. Available from: http://www.canfieldsci.com/imaging-systems/visia-complexion-analysis. Accessed September 8, 2017. | ||
Loutfy I, Abdel Aziz F, Dabbous NI, Hassan MH. Women’s perception and experience of menopause: a community-based study in Alexandria, Egypt. East Mediterr Health J. 2006;12 Suppl 2:S93-S106. | ||
Leitch C, Doherty V, Gebbie A. Women’s perceptions of the effects of menopause and hormone replacement therapy on skin. Menopause Int. 2011;17(1):11–13. | ||
Calleja-Agius J, Brincat M. The effect of menopause on the skin and other connective tissues. Gynecol Endocrinol. 2012;28(4):273–277. | ||
Bottai G, Mancina R, Muratori M, Di Gennaro P, Lotti T. 17β-estradiol protects human skin fibroblasts and keratinocytes against oxidative damage. J Eur Acad Dermatol Venereol. 2013;27(10):1236–1243. | ||
Thornton MJ. Estrogens and aging skin. Dermatoendocrinol. 2013; 5(2):264–270. | ||
Skoczyńska A, Budzisz E, Trznadel-Grodzka E, Rotsztejn H. Melanin and lipofuscin as hallmarks of skin aging. Postepy Dermatol Alergol. 2017;34(2):97–103. | ||
Lagarde JM, Rouvrais C, Black D, Diridollou S, Gall Y. Skin topography measurement by interference fringe projection: a technical validation. Skin Res Technol. 2001;7(2):112–121. | ||
Rorh M, Shrader A. FOITS (fast optical in vivo topometry of human skin): a classical method in modern efficacy testing. A history of Fringe Projection in Cosmetics. SÖFW Journal. 2009;135(8):2–10. | ||
Dobrev H. Use of cutometer to assess epidermal hydration. Skin Res Technol. 2000;6(4):239–244. | ||
Bazin R, Fanchon C. Equivalence of face and volar forearm for the testing of moisturizing and firming effect of cosmetics in hydration and biomechanical studies. Int J Cosmet Sci. 2006;28(6):453–460. | ||
Lee MC, Hu S, Chen MC, Shih YC, Huang YL, Lee SH. Skin rejuvenation with 1,064-nm Q-switched Nd:YAG laser in Asian patients. Dermatol Surg. 2009;35(6):929–932. | ||
Puccetti G, Nguyen T, Stroever C. Skin colorimetric parameters involved in skin age perception. Skin Res Technol. 2011;17(2):129–134. | ||
Ichibori R, Fujiwara T, Tanigawa T, et al. Objective assessment of facial skin aging and the associated environmental factors in Japanese monozygotic twins. J Cosmet Dermatol. 2014;13(2):158–163. | ||
personalcaremagazine.com [homepage on the Internet]. New Peptide Promotes Skin Firming and Remodelling. Personal Care; 2010. Available from: https://www.personalcaremagazine.com/story/6594/new-peptide-promotes-skin-firming-and-remodelling. Accessed September 8, 2017. | ||
Hipkiss AR, Michaelis J, Syrris P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 1995;371(1):81–85. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.