Back to Journals » Infection and Drug Resistance » Volume 15
Recurrent Skin and Soft Tissue Infections Caused by Ureaplasma urealyticum in an Immunocompromised Adult Patient: A Case Report
Authors Zhang M, Huang H, Yang H, Yang G, Wang W, He J, Wang G, Yang X, Zhai Z
Received 16 September 2022
Accepted for publication 23 November 2022
Published 28 November 2022 Volume 2022:15 Pages 6863—6868
DOI https://doi.org/10.2147/IDR.S390096
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Min Zhang,1,* Hui Huang,1,* Haichao Yang,1 Ge Yang,2 Wenwen Wang,1 Juying He,2 Guiyu Wang,3 Xichuan Yang,1 Zhifang Zhai1
1Department of Dermatology, the First Affiliated Hospital, Army Medical University, Chongqing, People’s Republic of China; 2Department of Pharmacy, the First Affiliated Hospital, Army Medical University, Chongqing, People’s Republic of China; 3Department of Clinical Laboratory, the First Affiliated Hospital, Army Medical University, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhifang Zhai, Department of Dermatology, the First Affiliated Hospital, Army Medical University, No. 30 Gaotanyan Street, Chongqing, 400038, People’s Republic of China, Tel +86-2368766344, Email [email protected]
Abstract: Ureaplasma urealyticum (U. urealyticum) is a normal commensal that colonizes the human genital tract and usually of low virulence; however, it can trigger serious extragenital infections in immunocompromised patient. In this case, a 48-year-old female immunocompromised patient with a four-year history of recurrent ulcer on extremities was presented to our hospital due to aggravation of lesions 10 months before. She was initially diagnosed as Pseudomonas aeruginosa infection secondary to lupus panniculitis and slightly responded to ceftazidime treatment; however, a new rash appeared on her left hip 16 days after admission, which was aggravated even under antibiotic treatment. After multiple negative cultures, U. urealyticum was identified in her left hip tissue using metagenomic next-generation sequencing (mNGS). U. urealyticum was also confirmed in her secretion samples from left hip, left thigh, right calf and uterine neck using mycoplasma culture and quantitative real-time polymerase chain reaction. Her lesions, especially the new rash, were positively responded to sensitive antibiotic treatment. To the best of our knowledge, this is the first case of U. urealyticum induced recurrent skin and soft-tissue infections (SSTIs) in an immunocompromised adult patient. This case suggests that the prevalence of this kind of infections may be underestimated because of the limitation of routine culture. mNGS may be considered to look for atypical pathogens to improve the antimicrobial treatment of complicated infections.
Keywords: Ureaplasma urealyticum, skin and soft-tissue infection, extragenital infection, immunocompromised, metagenomic next-generation sequencing
Introduction
Ureaplasma urealyticum, a normal commensal that colonizes the genital human tract, is an opportunistic pathogen of human genitourinary tract infections. Generally, this microorganism is of low virulence; yet, it can trigger serious diseases in immunocompromised patient. Although rare, U. urealyticum can also cause extragenital infections such as septic arthritis,1 perinephric abscess,2 brain abscess,3 hyperammonemia4 and necrotizing soft tissue infection.5
Skin and soft-tissue infections (SSTIs) are a group of diseases with different aetiology, clinical presentation and prognosis. In recent years, the prevalence of SSTIs has greatly increased, mainly because of human life prolongation and the increased number of patients with civilization disorders such as obesity and diabetes mellitus. SSTIs are usually caused by bacteria such as Staphylococcus aureus, Pseudomonas aeruginosa and Enterococcus spp;6 in unusual circumstances, it may be also caused by mycoplasma.7 Herein, we reported the first case of recurrent SSTIs mainly caused by U. urealyticum in an immunocompromised adult patient.
Case Presentation
A 48-year-old female patient presented to our hospital due to “four-year history of recurrent ulcer on her arms and legs, which was aggravated 10 months before”. The patient’s past medical history was notable for seven years of systemic lupus erythematosus (SLE); besides, she claimed that unprovoked erythema appeared on her left thigh and both hands 4 years ago, which gradually spread to four limbs and developed into recurrent ulcers. These painful skin lesions were improved after topical medications’ treatment; but, new lesions appeared again later and aggravated 10 months before her admission.
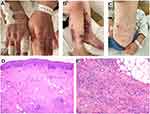
On admission, physical examination revealed numerous massive infiltrative erythema on her four limbs, some of which had been developed into ulcers covered with yellow-white purulent secretions or depressed scar with brown pigmented spots (Figure 1A–C). Laboratory results showed abnormal neutrophil percentage of 76.6% (normal reference range: 50%~70%), monocyte percentage of 10.1% (normal reference range: 3%~8%) and erythrocyte sedimentation rate of 59 mm/h (normal reference range: 0–20mm/h). Typical SLE symptoms such as facial butterfly erythema and nail deformation were observed. SLE diagnosis was confirmed by her positive autoantibody spectrum and medical history review. Her SLE was controlled using drugs such as prednisone, hydroxychloroquine sulfate and cyclophosphamide. Histopathology of the right calf erythema showed dermal perivasculitis and subcutaneous fat inflammation (Figure 1D and E). Pseudomonas aeruginosa was identified in her wound secretion and tissue cultures four days after admission. Based on these information, the patient was initially diagnosed as Pseudomonas aeruginosa infection secondary to lupus panniculitis. Pyogenic fluids of the wounds were decreased after ceftazidime (2g, bid, iv) treatment.
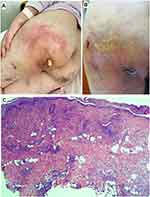
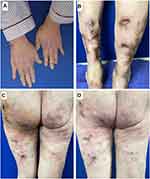
Sixteen days after admission, a new rash appeared on her left hip with obvious inflammatory symptoms of redness, swelling, heat and pain. The rash expanded gradually into a mass. A furuncle-like lump was suspected after ultrasound examination showed an abscess in the subcutaneous soft tissue of the patient’s left hip. Abscess incision and drainage were then performed, yet the range of redness and swelling was further enlarged under the condition of ceftazidime treatment (Figure 2A). The anti-infection regimen was changed to ceftriaxone sodium (2g, q12h, ivgtt), amikacin (15 mg/Kg, qd, ivgtt) and metronidazole (0.5 g, q8h, ivgtt) after consultation with pharmacists. The range of redness and swelling was enlarged again, and the treatment regimen was changed to linezolid (0.6 g, q12h, iv), piperacillin/tazobactam (4.5g, q8h, iv) and levofloxacin (0.75 g, qd, iv). The rash did not aggravate in the next three days, but more serious pain and redness were presented in the 4th day after change of the treatment regimen (Figure 2B). Tissue biopsy was performed on her left hip and histopathology showed features of purulent inflammation that involved with dermis and subcutaneous fatty tissue (Figure 2C). Metagenomic next-generation sequencing (mNGS) was employed to identify potential pathogens after multiple negative secretion and tissue cultures. Briefly, DNA was extracted using the QIAamp® UCP Pathogen DNA Kit (Qiagen) and library was constructed using the IDseq TM DNA Library Prep Kit (Vision medicals, Guangdong, China). After library quality assessment, sequencing was completed on the Illumina NextSeq 550 instrument (Illumina). Data analysis was performed using the IDseq™ commercial bioinformatic pipeline (Vision Medicals). One day later, mNGS detected 2286 sequences that can be mapped to the genome of U. urealyticum. BLAST alignment results were listed in Supplementary Table 1. Therapeutic regimen was empirically changed to piperacillin/tazobactam (4.5 g, q8h, iv), levofloxacin (0.75 g, qd, iv) and minocycline (loading dose of 200 mg, followed by 100 mg, q12h, iv). To verify mNGS result, secretion samples from the patient’s left hip, left thigh, right calf and uterine neck were collected. U. urealyticum identification was observed in mycoplasma culture using the mycoplasma IES test kit (Zhuhai Encode Medical Engineering, Ltd, Guangdong, China) and quantitative real-time polymerase chain reaction (qRT-PCR) using a U. urealyticum Fluorescence Diagnostic Kit (Sansure Biotech, China). Five days after empirical treatment, skin lesions of the patient were improved (Figure 3A–C) and she discharged from our hospital with drugs changed to josamycin, doxycycline and moxifloxacin because the susceptibility testing using the mycoplasma IES test kit (Zhuhai Encode Medical Engineering, Ltd, Guangdong, China) indicated this stain was susceptible to josamycin, doxycycline and minocycline. Two weeks after discharge, re-examination showed further improvements in her skin lesions (Figure 3D). Six months later, a telephone follow-up confirmed that the patient was in good recovery without recurrent of SSTIs.
Discussion
Genital mycoplasmas, often refer to Mycoplasma hominis, M. genitalium, M. primatum, M. spermatophilum, U. urealyticum and U. parvum, are groups of cell wall-lacking bacteria belonging to the class of Mollicutes that colonize the genitourinary tract of humans. Although rare, genital mycoplasmas can induce the development of SSTIs. For instance, Cho et al reported one case of M. hominis SSTIs in an immunocompromised pediatric patient.7 Here, we described the first case of U. urealyticum SSTIs in an immunocompromised adult patient. Considering genital mycoplasmas culture requiring specialized media and incubation conditions, most clinical departments do not have a routine examination for these microorganisms; thus, the prevalence of SSTIs caused by genital mycoplasmas, including U. urealyticum, may be underestimated.
SSTIs are frequently polymicrobial in nature.8 Our patient was initially diagnosed as Pseudomonas aeruginosa infection, which was susceptible to ceftazidime; however, a new rash appeared and aggravated even under ceftazidime treatment, suggesting other microorganisms may be responsible for the pathogenesis of her skin lesions. mNGS is a high-throughput sequencing technique that can rapidly detect a variety of pathogens, such as bacteria, fungi and mycoplasma, in clinical specimens. In a study performed by Ruan et al, authors have evaluated the diagnostic value of mNGS in U. urealyticum pneumonia.9 In this case, U. urealyticum was identified by mNGS and confirmed by mycoplasma culture and qRT-PCR. After then, U. urealyticum sensitive drugs were used and the skin lesions of the patient were significantly improved without recurrence. We speculated that this case of recurrent SSTIs was mainly caused by U. urealyticum infection.
To date, there is no consensus on the treatment of U. urealyticum infection. In a study performed by Wang et al, authors analyzed 6051 outpatient genital specimens and found 1889 cases of U. urealyticum infection, and more than 94.6% of the U. urealyticum isolates were susceptible to doxycycline, minocycline and tetracycline.10 In another in vitro antimicrobial susceptibility study, Samra et al found moxifloxacin was the most active agent against U. urealyticum, followed by levofloxacin.11 In this case, the U. urealyticum isolate was susceptible to josamycin, doxycycline and minocycline but resistant to tetracycline, levofloxacin, erythromycin, ciprofloxacin, ofloxacin, roxithromycin, azithromycin, clarithromycin and sparfloxacin. Our patient responded rapidly to empirical treatment which includes minocycline and her skin condition was significantly improved after josamycin, doxycycline and moxifloxacin treatment.
Conclusions
This is the first case of recurrent SSTIs mainly caused by U. urealyticum in an immunocompromised adult patient. The prevalence of U. urealyticum SSTIs may be underestimated because of the difficulty in identifying this microorganism with routine culture. mNGS may be considered to look for atypical pathogens, such as U. urealyticum, to improve the antimicrobial treatment of complicated infections.
Ethics Approval
The local institutional review board of the Army Medical University has approved the publication of this case.
Informed Consent Statement
Written informed consent has been provided by the patient to have the case details and any accompanying images published.
Acknowledgments
We sincerely appreciate Ms. Huan Xu (Vision Medicals Co. Ltd., Guangzhou, China) for providing the technical support of mNGS.
Funding
The authors declare that no funds were received during the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mahlouly J, Lhopitallier L, Suttels V, et al. Septic arthritis of the shoulder due to Ureaplasma urealyticum after emergency caesarean section: a case report. BMC Infect Dis. 2020;20(1):1–6. doi:10.1186/s12879-020-05497-3
2. Diaz Pallares C, Griener T, Vaughan S. Ureaplasma urealyticum disseminated multifocal abscesses in an immunocompromised adult patient: a case report. BMC Infect Dis. 2020;20(1):1–4. doi:10.1186/s12879-020-4771-z
3. Deetjen P, Maurer C, Rank A, Berlis A, Schubert S, Hoffmann R. Brain abscess caused by Ureaplasma urealyticum in an adult patient. J Clin Microbiolo. 2014;52(2):695–698. doi:10.1128/JCM.02990-13
4. Fleming D, Cunningham SA, Patel R. Contribution of uremia to ureaplasma-induced hyperammonemia. Microbiol spect. 2022;10(1):e01942–21. doi:10.1128/spectrum.01942-21
5. Gassiep I, Gore L, Dale JL, Playford EG. Ureaplasma urealyticum necrotizing soft tissue infection. J Infect Chemother. 2017;23(12):830–832. doi:10.1016/j.jiac.2017.07.007
6. Rennie RP, Jones RN, Mutnick AH; SENTRY Program Study Group. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000). Diagn Micr Infect Dis. 2003;45(4):287–293. doi:10.1016/S0732-8893(02)00543-6
7. Cho H, Park KG, Han SB, Chung NG, Park YJ. First Case of skin and soft tissue infection caused by mycoplasma hominis in a pediatric immunocompromised patient. Ann Lab Med. 2017;37(4):346–348. doi:10.3343/alm.2017.37.4.346
8. Esposito S, Ascione T, Pagliano P. Management of bacterial skin and skin structure infections with polymicrobial etiology. Expert Rev Antiinfe. 2019;17(1):17–25. doi:10.1080/14787210.2019.1552518
9. Ruan X, Li M, Qin X. Diagnostic value of metagenomic next generation sequencing for ureaplasma urealyticum infection: a case report. Lab Med. 2022;53(4):e74–e76. doi:10.1093/labmed/lmab091
10. Wang QY, Li RH, Zheng LQ, Shang XH. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in female outpatients, 2009–2013. J Microbiol Immunol. 2016;49(3):359–362. doi:10.1016/j.jmii.2014.06.007
11. Samra Z, Rosenberg S, Dan M. Susceptibility of Ureaplasma urealyticum to tetracycline, doxycycline, erythromycin, roxithromycin, clarithromycin, azithromycin, levofloxacin and moxifloxacin. J Chemother. 2011;23(2):77–79. doi:10.1179/joc.2011.23.2.77
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.