Back to Journals » Research and Reports in Urology » Volume 13
Recurrent Incontinence After Transvaginal Partial Sling Excision in Patients with Prior Mid-Urethral Sling
Authors Shapiro R , Dueñas-Garcia OF, Vallejo M , Trump T, Sufficool M, Zaslau S
Received 25 September 2020
Accepted for publication 16 December 2020
Published 12 January 2021 Volume 2021:13 Pages 9—15
DOI https://doi.org/10.2147/RRU.S281697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Robert Shapiro,1,2 Omar Felipe Dueñas-Garcia,1 Manuel Vallejo,1 Tyler Trump,2 Makenzy Sufficool,3 Stanley Zaslau1,2
1Department of Obstetrics & Gynecology, West Virginia University School of Medicine, Morgantown, WV 26506, USA; 2Department of Urology, West Virginia University School of Medicine, Morgantown, WV 26506, USA; 3West Virginia University School of Medicine, Morgantown, WV 26506, USA
Correspondence: Robert Shapiro
Department of Obstetrics & Gynecology, West Virginia University School of Medicine, Morgantown, WV 26506, USA
Email [email protected]
Introduction: Patients may develop recurrent urinary tract infections, pain syndromes, dyspareunia, and voiding difficulty after mid-urethral sling placement that can be treated by partial sling excision.
Objective: The primary objective of this study was to evaluate the incidence of de novo incontinence and voiding difficulty after partial sling excision. A secondary objective was to assess risk factors associated with future incontinence surgery in this subset of patients.
Methods: From 2009 to 2017, 95 female patients with subjective complaints of pelvic pain, dyspareunia, or voiding difficulty following synthetic mid-urethral sling placement for stress urinary incontinence underwent partial sling excision at a single institution. The incidence of urinary incontinence was assessed 6 months after partial sling excision. Patients were also assessed for resolution of voiding difficulty and future incontinence surgery. Primary endpoints were examined by Pearson’s Chi-square test and interval data by t-test. A p < 0.05 was significant.
Results: About 72% of patients were more likely to be continent after partial sling excision irrespective of initial symptoms prior to surgery. No difference was seen in voiding difficulty between the continent and incontinent patients after partial sling excision (p=0.09). Patients with a retropubic mid-urethral sling were more likely to be continent after partial sling excision (p=0.03). Preoperative maximum flow rate > 16 mL/sec was associated as an independent variable to develop incontinence surgery after partial sling excision (p=0.009).
Conclusion: In conclusion, partial sling excision poses a low risk for de novo urinary incontinence regardless of preoperative symptoms. Stress urinary incontinence may be less likely to reoccur in those patients having a retropubic approach. A preoperative maximum flow rate of > 16 mL/sec is a risk factor for future incontinence surgery after partial sling excision and should be taken into consideration when formulating a treatment plan.
Keywords: sling excision, mesh, incontinence, mid-urethral sing
Introduction
Stress urinary incontinence (SUI) is defined as the involuntary loss of urine with provocative maneuvers, such as coughing, laughing, sneezing, etc.1 It is a common problem among women and leads to a decline in quality of life. The standard of care to improve SUI, as designated by the American Urogynecologic Society, is synthetic mid-urethral slings.2 This treatment has been highly studied, with greater than 2000 publications in the scientific literature describing its use and efficacy.3 Although most patients experience significant relief after this common surgical treatment, as with any surgical procedure, complications can occur. Besides mesh exposure, patients may develop recurrent urinary tract infections, pain syndromes, dyspareunia, and voiding difficulty after mid-urethral sling placement that can require further surgical intervention.3,4
While conventional thinking has been to treat these complications conservatively, it has been shown that treatment such as medications, behavioral therapy, and physical therapy frequently do not result in improvement of the patient’s symptoms.5 Various surgical options exist for treatment including sling explant, which has been considered the procedure of choice to address symptoms refractory to medical treatment.6 This procedure involves removing the vaginal portion as well as the lateral arms that extend into either the obturator/adductor muscles or retropubic space of which either can be associated with substantial morbidity.7 Transvaginal partial sling excision is much less invasive as it only involves incising the sling and associated scar tissue underneath the urethra.8 Limited information exists in the literature on the incidence of incontinence after partial sling excision or the improvement of subjective symptoms of chronic pelvic pain, dyspareunia and other voiding symptoms.
The primary objective of this study was to evaluate the incidence of de novo incontinence and voiding difficulty after partial sling excision. A secondary objective was to assess risk factors associated with future incontinence surgery in this subset of patients.
Patients and Methods
This retrospective observational study was performed at Ruby Memorial Hospital, West Virginia University, Morgantown, WV. Approval for this study was obtained from The Institutional Review Board of West Virginia University. All patients provided informed consent to participate in this study, and that it was conducted in accordance with the Declaration of Helsinki. Potential patients were identified by Current Procedural Terminology (CPT) codes of pelvic pain, dyspareunia, and/or voiding dysfunction for greater than 3 months following a mesh-based mid-urethral sling procedure. Pelvic pain was defined as pain that occurred below the umbilicus including the groin area for at least 3 months and not associated with the menstrual cycle. Dyspareunia was defined as genital pain that occurred during or immediately following intercourse. Voiding difficulty was defined broadly as any perceived voiding difficulty that was not present prior to mid-urethral sling placement. These included voiding symptoms such as urinary hesitancy, slow stream, incomplete bladder emptying, abdominal straining to void, double-voiding, and position-dependent voiding.
From January 2009 to December 2017, 108 patients with symptoms including voiding difficulty, pelvic pain, and/or dyspareunia following synthetic mid-urethral sling placement for SUI subsequently underwent partial sling excision at our institution. These patients had all failed a trial of the conservative treatment that included either a trial of behavioral therapy, physical therapy, and/or medications.
During this study period, three surgeons trained in female pelvic medicine/reconstructive surgery performed the partial sling excision. Preoperative assessment with urodynamics testing was performed on all patients. The surgery was performed under general anesthesia. In most cases, either a gynecologic or urologic resident assisted with the procedure under the direct supervision of the primary surgeon. All attending surgeons used the same standard technique for the transvaginal partial sling excision.
An approximate two 3-cm vertical incision was made just under the mid-urethra through the vaginal wall mucosa. Careful sharp and blunt dissection was performed to expose the underlying sling and scar tissue. The sling was dissected away from the urethra and lysed in the midline (Figures 1 and 2). The sling and scar tissue were partially excised just under the bony pelvis in either direction (Figures 3 and 4).
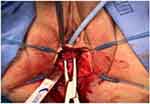 |
Figure 1 The sling is identified and undermined with a right-angle clamp just below the mid-urethra. |
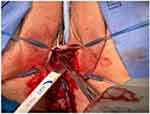 |
Figure 2 The sling is incised in the midline. The free edge of the sling is grasped with an allis clamp and undermined laterally to the inferior pubic rami on both sides. |
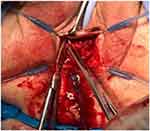 |
Figure 3 The sling is excised at the level of the inferior pubic rami on both sides. |
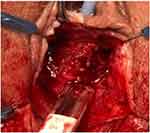 |
Figure 4 The vaginal portion of the sling underneath the mid-urethra is now completely removed. |
The incidence of urinary incontinence was assessed 6 months after partial sling excision by an objective cough stress test. Patients were also assessed for resolution of voiding difficulty. Primary endpoints were examined by Pearson’s Chi-square test and interval data by t-test. When p-values were <0.05, an effect was considered statistically significant. Data were analyzed using JMP software Version Pro 12.2, SAS Institute Inc., Copyright 2015.
Results
We identified 108 patients who underwent transvaginal partial sling excision. Thirteen patients were lost to follow up. Mean age of the patient was 53 years of age (range 27–84). Approximately half (52.2%) of these patients were post-menopausal. Mean BMI of the patient was 32 (range 22.3–57.9). In our case series, we only had one case of urethral injury during partial sling excision. The injury was recognized intra-operatively and was repaired without any long-term sequelae.
At 6 months after partial sling excision, 72% (68/95) of patients were continent irrespective of initial symptoms prior to surgery (Table 1). Sixty-five % (62/95) rated their pain as improved. Approximately half (48/95) had less voiding difficulty. No differences were noted in post-void residual (PVR) before and after partial sling excision (Table 1). Neither postoperative continence nor need for future incontinence surgery after partial sling excision was associated with the patient’s primary complaint. No differences were seen in voiding difficulty and maximum uroflow rate between the continent and incontinent patients after partial sling excision. (p=0.09) (Table 1). Similarly, no difference was noted in pain perception after partial sling excision (p=0.93) (Table 1). Patient’s primary complaint was also not predictive of future incontinence surgery after partial sling excision.
 |
Table 1 De Novo Urinary Incontinence After Partial Sling Excision |
Patients with a retropubic mid-urethral sling were more likely to continue to be continent after partial sling excision (p=0.03) (Table 2). A preoperative uroflow >16 mL/sec was associated with future incontinence surgery after partial sling excision. (p=0.009) (Table 2).
 |
Table 2 Risks Factors for Subsequent Incontinence Surgery After Partial Sling Excision |
Discussion
While rates of complications and re-operation after synthetic mid-urethral slings are low, it is important to determine the safest and most effective option to resolve complications for patients. Literature and formal recommendations from governing societies regarding the most appropriate management for this situation are lacking. Our study has aimed to fill in this gap by focusing on rates of incontinence and voiding difficulty after partial sling excision as well as predictive factors for future surgery to treat incontinence.
In our study, partial sling excision was not shown to cause de novo urinary incontinence in a majority of patients, regardless of preoperative symptoms. Prior research has hypothesized that removal of mesh completely underneath the urethra may play a key role in the recurrence of SUI.9 This has led to sling revision rather than explant as the preferred treatment for acute urinary retention after a mid-urethral sling.10 Molden et al retrospectively assessed outcomes after sling revision for both retropubic and transobturator slings. Techniques included stretching of the sling, incision to release the sling, and resection of the central portion of the sling. While voiding dysfunction resolved in 80% of patients, 21% developed de novo SUI.11 Segal et al explored outcomes after sling release for various incontinence procedures. Of 44 patients who underwent a sling release, 34% (15/44) of subjects developed de novo SUI.8 A study by Geller found 34% of patients developed de novo incontinence following sling revision.12 Tse and Chan’s study quotes recurrent incontinence rates around 19%.13 Unlike these prior studies which involved either incising or removing a small portion of the sling, our study showed similar rates of recurrent incontinence with excision of most of the suburethral mesh.
Patients with a retropubic mid-urethral sling were more likely to be continent after partial sling excision. This outcome in part may be due to the etiology of SUI in our patient population. Although difficult to quantify, impaired urethral sphincter function likely played a larger role than urethral support regarding the etiology of SUI within our patient population. Impaired urethral sphincter function is more likely to occur in menopausal patients with estrogen insufficiency.14 On average, women in our study were in their early to mid-fifties at the time of partial sling excision and hence more likely to be menopausal. As the mesh arms were largely left intact during partial sling excision, the lateral support to the urethra remained. Thus, the lateral mesh arms provided continued support to the urethral sphincter resulting in continued continence within our patient population.
Impaired urethral sphincter function may have also accounted for uroflow being predictive of future incontinence procedures. With impaired urethral sphincter function, the coaptation mechanism no longer functions properly. This type of urethral sphincter dysfunction is referred to as intrinsic sphincter deficiency (ISD).15 Thus, regardless of the anatomical location of the urethra, urinary incontinence occurs in the dysfunction of the urethral sphincter itself. Consequently, patients with this type of urethral sphincter dysfunction can have higher uroflow rates because the urethral sphincter loses its ability to narrow. According to McGuire, it was reported that 75% of total patients who underwent urinary incontinence surgery more than once had ISD.16 Based on these findings, uroflow rates and history of prior retropubic sling should play a role in determining treatment options and counseling regarding the risk of future incontinence procedures, particularly with patients that share the same demographic characteristics as those in our study.
Other small studies on the efficacy of sling revision show a high variation on outcome following the procedure.17,18 The variation of efficacy may be attributed to a lack of uniformity regarding sling revision surgery technique and timing after the initial mesh sling surgery. An advantage of our study was the uniformity in surgical technique which resulted in a favorable outcome, regardless of the initial synthetic sling that was placed.
Although patients with a retropubic mid-urethral sling were more likely to be continent after partial sling excision, the actual number of patients confirmed to have a retropubic sling (N=17) was low. On account of difficulty in obtaining all prior operative records from our patient population, the type of sling procedure (retropubic, transobturator, and single incision) used during initial placement was largely unknown. We view this as a strength of the study rather than a weakness. As most of the current literature concentrates on a single type of sling technique in relation to partial sling excision, the fact that our postoperative sling excision continence rates were high (72%) without knowing the specific type of sling used in the initial surgery makes our results more generalizable and clinically relevant.
An interesting finding of our study was that no significant difference was noted in pelvic pain after partial sling excision. Pain symptoms after surgery are often attributed to either scarring or a foreign body.18 It would therefore seem likely that removal of the foreign body would help alleviate pain. In our study, the patients with pain all had their sling partially excised which should have resulted in a significant improvement. This was not seen within our patient population suggesting that mesh material may not be a pain generator when used for incontinence treatment as other literature has suggested.19
The fact that no differences were seen in voiding difficulty and maximum flow rate between the continent and incontinent patients after partial sling excision suggests that the patient’s voiding difficulty was unlikely to be directly due to the sling. If it were, an obstructive voiding pattern should demonstrate lower maximum flow rates that would statistically improve after partial sling excision. This should even be more pronounced in the subset of patients that were incontinent after partial sling excision if the patient’s symptoms were on account of the sling. This, however, was not seen. The reason is likely because of the multifactorial etiology of voiding difficulty. Voiding difficulty was defined widely to include symptoms such as urinary hesitancy, slow stream, incomplete bladder emptying, abdominal straining to void, double-voiding, and position-dependent voiding. Many of these symptoms can result from not only obstructive voiding but also a neurologic problem or medication that alters nervous system function. While approximately half of patients felt improvement in these symptoms, analysis of objective voiding parameters (maximum flow rate and PVR) demonstrated no statistical differences after partial sling excision. In a study by Petrou et al urethrolysis outcomes were not significantly different when urodynamic parameters were used instead of clinical criteria.20 Because of the multifactorial etiology of voiding difficulty, there does not yet seem to be conclusive evidence for partial sling excision as a clear treatment. Patients should be counseled regarding the potential limited benefit partial sling excision affords for voiding difficulty and may actually worsen symptoms by causing subsequent de novo incontinence.
This study has several limitations. With any study relying on electronic medical records, coding discrepancies could exist causing misclassification with data collection. Also, with limited post-operative follow up, it is difficult to discern whether the improvements were long lasting or based primarily on the placebo effect. It is well documented within the literature that surgery has a profound placebo effect. A 2014 Cochrane review of 53 trials that compared elective surgical procedures to placebos found that sham surgeries provided some benefit in 74% of the trials and worked as well as the real surgery in about half.21 Finally, since all the data were collected from a single academic, tertiary care hospital, this may limit the generalizability to non-academic/community hospitals.
In conclusion, partial sling excision poses a low risk for de novo urinary incontinence regardless of preoperative symptoms. Stress urinary incontinence may be less likely to reoccur in those patients having a retropubic approach. A preoperative maximum flow rate >16 mL/sec is a risk factor for future incontinence surgery after partial sling excision and should be taken into consideration when formulating a treatment plan. This study contributes to the existing literature on treating complications related to mid-urethral sling surgery. Further research is warranted regarding the exact mechanism and underlying pathophysiology of pain and voiding difficulty after mid-urethral sling placement.
Data Sharing Statement
Data supporting the results reported are available upon request.
Consent
Written informed consent was obtained from the patient for publication of these images in this article and any accompanying images.
Acknowledgments
An abstract of this paper was presented at the American Urogynecologic Society Annual Meeting in October 2020. Female Pelvic Medicine & Reconstructive Surgery: October 2020 – Volume 26 – Issue 10S – p S89-S189. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 5U54GM104942-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have obtained written consent from the patients to publish their respective cases/figures.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr. Shapiro is a proctor for Boston Scientific. Dr. Zaslau is a consultant for Johnson and Johnson and was an expert witness for Ethicon. The authors report no other conflicts of interest in this work.
References
1. Nager CW. The urethra is a reliable witness: simplifying the diagnosis of stress urinary incontinence. Int Urogynecol J. 2012;23(12):1649–1651. doi:10.1007/s00192-012-1892-y
2. American Urogynecologic Society, Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction. Position Statement on Mesh Midurethral Slings for Stress Urinary Incontinence Silver Spring (MD): AUGS. Schaumburg (IL): SUFU; 2016.
3. Jonsson F, Levin P, Wu J. Trends in the surgical management of stress urinary incontinence. Obstet Gynecol. 2012;119(4):854.
4. Nguyen J, Jakus-Waldman S, Water A, White T, Menefee SA. Perioperative complications and reoperations after incontinence and prolapse surgeries using prosthetic implants. Obstet Gynecol. 2012;3(119):539–546. doi:10.1097/AOG.0b013e3182479283
5. Brown E, Cohn J, Kaufman M, Dmochowski R, Reynolds WS. Evaluation and management of mid-urethral sling complications. Curr Bladder Dysfunct Rep. 2016;11(2):160–168. doi:10.1007/s11884-016-0365-4
6. Blaivas JG, Purohit RS, Weinberger JM, et al. Salvage surgery after failed treatment of synthetic mesh sling complications. J Urol. 2013;190(4):1281–1286. doi:10.1016/j.juro.2013.03.044
7. Stachowicz A, Hubb AJ, Wood SC, Veronikis DK. Complete midurethral sling removal: an analysis of mesh explantation by length. AJOG. 2017;216(3):S610. doi:10.1016/j.ajog.2016.12.109
8. Segal J, Steele AC, Vassallo BJ, et al. Various surgical approaches to treat voiding dysfunction following anti-incontinence surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(4):372–377. doi:10.1007/s00192-005-0018-1
9. Syan R, Peyronnet B, Drain A, et al. Exploring stress urinary incontinence outcomes after sling excision for perforation or exposure. Low Urin Tract Symptoms. 2019;1–5.
10. Management of mesh and graft complications in gynecologic surgery. Committee opinion no. 694. american college of obstetricians and gynecologists. Obstet Gynecol. 2017;129(4):e102–8. doi:10.1097/AOG.0000000000002022
11. Molden S, Bracken J, Nguyen A, et al. A retrospective multicenter study on outcomes after midurethral polypropylene sling revision for voiding dysfunction. Female Pelvic Med Reconstr Surg. 2010;16(6):340–344. doi:10.1097/SPV.0b013e3181f5ac07
12. Geller E. Prevention and management of postoperative urinary retention after urogynecologic surgery. Int J Womens Health. 2014;6:829–838. doi:10.2147/IJWH.S55383
13. Tse V, Chan L. Outlet obstruction after sling surgery. BJU Int. 2011;108:24–28. doi:10.1111/j.1464-410X.2011.10712.x
14. Horbach NS, Ostergard DR. Predicting intrinsic urethral sphincter dysfunction in women with stress urinary incontinence. Obstet Gynecol. 1994;84(2):188–192.
15. Haab F, Zimmern PE, Leach GE. Female stress urinary incontinence due to intrinsic sphincteric deficiency: recognition and management. J Urol. 1996;156(1):3–17. doi:10.1016/S0022-5347(01)65925-1
16. McGuire EJ. Urodynamic findings in patients after failure of stress incontinence operations. Prog Clin Biol Res. 1981;78:351–360.
17. Karram MM, Siddighi S. Surgical and nonsurgical approaches to treat voiding dysfunction following antiincontinence surgery. Curr Opin Obstet Gynecol. 2007;19(5):490–495. doi:10.1097/GCO.0b013e3282efdc32
18. Tijdink MM, Vierhout ME, Heesakkers JP, Withagen MI. Surgical management of mesh-related complications after prior pelvic floor reconstructive surgery with mesh. Int Urogynecol J. 2011;22(11):1395–1404. doi:10.1007/s00192-011-1476-2
19. Petrou SP, Brown JA, Blaivas JG. Supramental transvaginal urethrolysis. J Urol. 1999;161(4):1268–1271. doi:10.1016/S0022-5347(01)61655-0
20. Lee D, Chang J, Zimmern P. Iatrogenic pelvic pain: surgical and mesh complications. Phys Med Rehabil Clin N Am. 2017;28(3):603–619. doi:10.1016/j.pmr.2017.03.010
21. Wartolowska K, Hopewell S, Hopewell S. Use of placebo controls in the evaluation of surgery: a systematic review. BMJ. 2014;348(may21 2):g3253. doi:10.1136/bmj.g3253
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
