Back to Journals » Journal of Pain Research » Volume 12
Rectal enema of bupivacaine in cancer patients with tenesmus pain – case series
Authors Kowalski G, Leppert W , Adamski M , Szkutnik-Fiedler D , Baczyk E, Domagalska M , Bienert A, Wieczorowska-Tobis K
Received 26 October 2018
Accepted for publication 15 March 2019
Published 11 June 2019 Volume 2019:12 Pages 1847—1854
DOI https://doi.org/10.2147/JPR.S192308
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Grzegorz Kowalski,1,2 Wojciech Leppert,3 Michal Adamski,2 Danuta Szkutnik-Fiedler,4 Ewa Baczyk,1 Malgorzata Domagalska,5 Agnieszka Bienert,4 Katarzyna Wieczorowska-Tobis1
1Chair and Department of Palliative Medicine, Poznan University of Medical Sciences, Poznan, Poland; 2Department of Anaesthesiology, Józef Strus Multiprofile Municipal Hospital, Poznan, Poland; 3Laboratory of Quality of Life Research, Chair and Department of Palliative Medicine, Poznan University of Medical Sciences, Poznan, Poland; 4Chair and Department of Clinical Pharmacy and Biopharmacy, Poznan University of Medical Sciences, Poznan, Poland; 5Department of Anesthesiology, Gynecology - Obstetrics Clinical Hospital, Poznan, Poland
Introduction: Rectal tenesmus pain in cancer patients most frequently appears in patients with colon cancer, and as a consequence of radiotherapy of the hypogastrium region. Treatment with opioids and adjuvant analgesics is often ineffective.
Patients and methods: Here, we report on two female patients diagnosed with colon and ovary cancer, respectively, who had very severe tenesmus pain (numerical rating scale 8–10) despite using high doses of opioids, including methadone with corticosteroids, anticonvulsants, antidepressants and ketamine.
Results: In both patients, bupivacaine was administered via a rectal enema. In the first patient, bupivacaine was administered at a dose of 100 mg 0.1% (100 mL), and subsequently 100 mg 0.2% (50 mL), leading to effective analgesia for 8 and 12 hrs, respectively. In the second patient, 100 mg 0.1% (100 mL) was initially administered, followed by 100 mg 0.2% (50 mL), leading to effective analgesia for 12 and 17 hrs, respectively, with only dull abdominal pain reported that was relieved by 100 mg IV ketoprofen and complete disappearance of tenesmus pain. Rectal bupivacaine administration did not cause neurologic adverse effects, heart function disturbances or decreased blood pressure. A volume of 50 mL was enough to cover a painful area in the colon. Initial bupivacaine concentrations in the blood serum did not exceed 50 ng/mL and eventually dropped to 20 ng/mL and below.
Conclusions: Administration of 100 mg bupivacaine as a rectal enema is safe and provides effective analgesia, and this procedure may be conducted in hospital departments and out-patient clinics. Furthermore, this procedure in the case of pain recurrence, can be repeated, and by providing effective pain relief often allows time for the patient to be transferred to a specialized pain center.
Keywords: analgesia, bupivacaine, rectal enema, tenesmus pain
Introduction
Cancer patients with tumours localized to the rectum, prostate in men, cervix in women, or lymph node metastases in the pelvis, infiltration of sacra bone, and/or the lower lumbar spine, may experience a very severe, often recurring pain, known as rectal tenesmus pain, which is not associated with the presence of stool in the rectum.1,2 Rectal tenesmus pain is not dependent on time of day, patient activity, or body position. The pain appears unexpectedly several times a day, sometimes every few minutes. The pain is induced by irritation and damage to the gut wall, neighboring tissues, and/or nerve fibers covering the pelvic organs. There is often a neuropathic pain component present: lancinating, dragging, stinging, tingling, and burning sensations in the perineum, inguinal region, hypogastrium, and left hip region. The pain may also be a consequence of treatment procedures, especially radiotherapy of the hypogastrium.3
Rectal tenesmus pain is often not relieved despite using high doses of opioids,4 antidepressants,5 anticonvulsants, and corticosteroids. Increasing doses of analgesics and the addition of ketamine6 or methadone7–9 are often ineffective. Pain management is a significant issue not only for patients but also for families (caregivers) and medical staff (doctors and nurses) caring for the patients. The use of bupivacaine administered via a deep rectal enema may decrease the pain intensity and sometimes fully relieve the pain for a few hours or longer.2 Conducting this procedure is possible in each hospital department and in out-patient clinics and in the case of pain recurrence, it can be repeated, which allows time for the patient to be transferred to a specialized pain center.
Ethics approval for this study was obtained from the Bioethics Committee at Poznan University of Medical Sciences, Poland. Patients were informed about the use of bupivacaine and its mode of delivery and gave written informed consent to have the case details published and undergo blood sampling for testing the level of bupivacaine in their blood serum.
Case presentations
Case 1
A 59-year-old lady (body mass 102 kg, height 168 cm) diagnosed with recurrence and dissemination of cancer of sigmo-rectal flexure, after frontal resection, radiotherapy, chemotherapy, hysterectomy with adnexa removal, and partial resection of the greater omentum. The patient was also diagnosed with depression. A computed tomography scan showed recurrence with infiltration of the front sacral region.
The patient was admitted to a palliative medicine in-patient unit in a relatively good general condition – Eastern Cooperative Oncology Group 2 due to rectal tenesmus pain of a very severe intensity – numerical rating scale (NRS) 8–10, which was appearing every few minutes and felt like a dragging pain in the sacral region that radiated to the back, hip, and left lower extremity. Tenesmus had been present for 1 month, with increasing intensity and frequency, and it did not respond to treatment despite use of increasing doses of analgesics for background pain treatment: transdermal fentanyl 150 µg/hr every 3 days, controlled-release oxycodone tablets 40 mg po twice daily, and methylprednisolone 8 mg po once daily. On the second day of hospital stay the following drugs were used: dexamethasone 8 mg IV twice daily, pregabalin 150 mg twice daily; clomipramine 75 mg twice daily; zofenopril 30 mg once daily, bromazepam 3 mg twice daily; zolpidem 10 mg once daily; drotaverine 80 mg once daily; pantoprazole 40 mg once daily; and lactulose 15 mL twice daily. The following additional doses of rescue analgesics were used for breakthrough pain episodes: ketoprofen 100 mg and morphine 10 mg SC or 5 mg IV. On the third day of the stay, the patient was offered bupivacaine administered via a deep rectal enema. After obtaining patient consent, 100 mL bupivacaine 0.1% (1 mg/mL) was administered.
After several minutes, the pain intensity significantly decreased. Before bupivacaine administration, the pain was at NRS 8 and in subsequent hours, it decreased to 3-2-1 and finally, the patient was pain-free. After 8 hrs, the pain reappeared (NRS 7). Bupivacaine was re-administered at a dose of 50 mL 0.2% (2 mg/mL). After a few minutes, pain intensity again started to lessen, decreasing during the subsequent hours to NRS 6-5-1-0. After approximately 17 hrs, tenesmus pain reappeared NRS 8. The patient was again treated with a deep rectal enema of 50 mL bupivacaine 0.2%. Pain intensity decreased for the next 15 h. However, after this period, the tenesmus pain reappeared. After obtaining patient consent, a neurolytic block of the superior hypogastric plexus was conducted.
During sedation induced by propofol, when lying on the right side, using X-ray for guidance, a 22G 15-cm needle was inserted, 7–8 cm laterally from the midline of the spine, on the left side, to the caudal-medially to a level of medial-lateral area L5–S1 (Figure 1A and B). The patient reported paresthesia in the formerly painful hypogastrium. 0.25% bupivacaine with methylprednisolone (DepoMedrol) 40 mg (8 mL) was administered. After approximately 10 mins, 6 mL 95% alcohol was administered. Rectal tenesmus and hip pain were relieved, and a dull hypogastric pain of a lower intensity (NRS 3–4) remained.
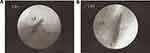 | Figure 1 Blockade and neurolytic block of the superior hypogastric plexus: (A) lateral and (B) frontolateral.Abbreviation: L5, fifth lumbar vertebra. |
Initially, there was no need of rescue analgesic administration. Basic drugs were continued as follows: transdermal fentanyl 100 µg/hr every 3 days, prolonged-release oxycodone/naloxone 20 mg/10 mg po twice daily, and dexamethasone 8 mg IV twice daily, pregabalin 150 mg twice daily, zofenopril 30 mg once daily, bromazepam 1.5 mg twice daily, clomipramine 75 mg once daily, pantoprazole 40 mg once daily, and lactulose 15 mL twice daily (all drugs were administered orally except fentanyl and dexamethasone). Due to a periodic intensification of dull abdominal pain, ketoprofen 100 mg IV was administered as a rescue analgesic, and pain was effectively relieved.
Laboratory test results
Full blood count: WBC, 6.68×10e9/L; %NEUTR, 76.3%; %LYMPH, 15.3%; %MONO, 5.7%; %EOS, 0.5%; %BASO, 0%; %LUC, 2.0%; #NEUT, 5.10×10e9/L; #LYMPH, 1.02×10e9/L; #MONO, 0.38×10e9/L; #EOS, 0.04×10e9/L; #LUC, 0.14×10e9/L; RBC, 3.55×10e12/L; HGB, 4.90 mmol/L; HCT, 0.26 L/L; MCV, 74 fL; MCH, 1.38 fmol; MCHC, 18.52 mmol/L; RDW, 16.60%; PLT, 377.01×10e9/L; MPV, 7.70 fL; PCT, 0.003 L/L; PDW, 54.2%.
Biochemistry: albumins, 22.2 g/L; chloride, 100.0 mmol/L; glucose, 5.57 mmol/L; creatinine, 62.9 mmol/L; urea, 2.57 mmol/L; potassium, 4.6 mmol/L; sodium, 137.0 mmol/L; calcium, 1.93 mmol/L, APTT time, 27.9 s; PT, 11.4%.
Case 2
A 50-year-old female patient (body mass 56 kg, height 172 cm) diagnosed with ovary cancer IIB according to Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) staging, G3 serous adenocarcinoma with dissemination, after resection of the uterine with adnexa, greater omentum, and right pelvis lymph nodes. Recurrence occurred after chemotherapy. The patient underwent exploratory laparotomy and sigmoid colostomy. During her hospital stay, a gut–vagina fistula was diagnosed. Subsequent chemotherapy V–VIII courses (gemcitabine as monotherapy) were administered.
The patient was admitted to a palliative medicine in-patient unit due to troublesome rectal tenesmus pain. The patient was not cognitively impaired and able to walk. Pain was appearing every few minutes, and did not respond to the following analgesics: transdermal fentanyl 150 µg/hr every 3 days, controlled-release morphine tablets 130 mg po twice daily, controlled-release oxycodone tablets 40 mg po twice daily , and amitriptyline 25 mg po twice daily, pregabalin 150 mg po twice daily, duloxetine 60 mg po once daily, furosemide 40 mg po once daily, spironolactone 100 mg po once daily, potassium 600 mg po once daily, enoxaparin 0.4 mg SC once daily, and levothyroxine 56 µg po once daily.
Multiple doses of rescue analgesics were provided: fentanyl buccal tablets 200–400 µg, methadone syrup 10-20 mg po, and morphine 5–10 mg SC.
Laboratory test results
Full blood count: WBC, 8.52×10e9/L; %NEUT, 75.3%; %LYMPH, 14.8%; %MONO, 7.3%; %EOS, 1.4%; %BASO, 0.1%; %LUC, 1.1%; #NEUT, 6.41×10e9/L; #LYMPH, 1.2710e9/L; #MONO, 0.62 ×10e9/L; #EOS, 0.12×10e9/L; #BASO, 0.01×10e9/L; #LUC, 0.09×10e9/L; RBC, 2.81×210e9/L; HGB, 5.8 mmol/L; HCT, 0.27 L/L; MCV, 95 fL; MCH, 2.05 fmol; MCHC, 21.53 mmol/L; RDW, 17.10; PLT, 312.00×10e9/L; MPV, 9.10 fL; PCT, 0.003 L/L; PDW, 52.0%.
Biochemistry: Albumins, 27.8 g/L; aminotranspherase alanine, 16 U/L; aminotranspherase asparginate, 19 U/L; total bilirubin, 10.77 mmol/L; chloride, 95.0 mmol/L; glucose, 4.82 mmol/L; creatinine, 82.4 mmol/L; urea, 6.01 mmol/L; potassium, 4.6 mmol/L; sodium, 135.0 mmol/L; PT time, 11.6 s; PT 94.0%; INR 1.1; CRP high sensitivity, 55.10 mg/L.
The patient was offered a deep rectal enema of bupivacaine at a dose of 100 mL 0.1% (1 mg/mL). After several minutes, the pain intensity decreased and the frequency of pain attacks decreased to 2–3 episodes per 12 hrs. After this time, the patient reported an increase in pain intensity. Because the pain did not completely disappear during the next day, the patient was re-administered bupivacaine, although at a higher concentration of 0.2% (2 mg/mL) and a lower volume (50 mL). Several minutes after the drug administration, the pain disappeared for 12 hrs. The patient reported mild pain (a blood sample was taken for laboratory tests), which quickly disappeared without any intervention and in subsequent hours and days did not reappear.
As rectal tenesmus pain disappeared, there was initially no need to administer rescue analgesics. However, on subsequent days the patient had dull hypogastric pain NRS 4-5. This pain was also present earlier, but it was masked by tenesmus pain and was significantly less intense. Rescue analgesics did not provide a satisfactory effect and an increase of basic analgesics doses induced excessive drowsiness. It was decided to conduct a neurolytic block of the superior hypogastric plexus.
A neurolytic block was conducted during sedation induced by propofol, using X-ray for guidance. A 22G 15-cm needle was inserted 7–8 cm laterally from the midline of the spine, over the wing of ilium, on the left side, caudal-medially (Figure 2, position 1). The patient reported paresthesia in the formerly painful hypogastrium. Subsequently, the needle was inserted approximately 1 cm further (Figure 2, position 2). 8 mL 0.25% bupivacaine with methylprednisolone (Depo-Medrol) 40 mg was administered and after approximately 10 mins, 6 mL 96% alcohol was administered. The pain disappeared and the patient was discharged home after a few days, with the recommendation to continue the basic analgesic regimen with fentanyl 200 µg buccal tablets as a rescue analgesic.
 | Figure 2 Blockade of the superior hypogastric plexus - lateral view. Notes: 1, Initial needle position; 2, Appropriate needle position.Abbreviation: L5, fifth lumbar vertebra. |
After 4 weeks, the patient was readmitted to the palliative medicine in-patient unit due to a recurrence of tenesmus pain. Severe pain (NRS 8–10) was appearing every few minutes, after a gynecologic examination was conducted. A neurolytic block of the superior hypogastric plexus was conducted again, and the pain then disappeared. The patient was discharged to her home without any need of increasing doses of analgesics.
Measurement method of bupivacaine concentration in the blood serum
Bupivacaine in patient plasma samples was analyzed by high-performance liquid chromatography (Waters 2,695 Separations Module with autosampler; Waters, Milford, MA, USA) with UV detection (Waters 2,487 Dual l Absorbance Detector; wavelength: 245 nm) after a liquid–liquid extraction.10 An analytical SYMMETRY C18 column (3.5 μm; 4.6×150 mm; Waters) was used with a mobile phase of acetonitrile and potassium dihydrogen phosphate buffer (24:76, v/v) and a flow rate of 1.0 mL/min. Retention times for bupivacaine and the internal standard (lidocaine) were 7.6 and 2.9 mins, respectively. The method was validated according to published European Medicines Agency (EMA) guidelines (EMA data collection and processing were carried out using Empower™ Pro software v. 1,154; Waters). The relationship was found to be linear for a bupivacaine concentration range of 20–500 ng/mL. Intra- and inter-day precision and accuracy were <14%.11
Discussion
In both patients, inoperable advanced cancer with dissemination and involvement of abdominal organs was diagnosed. Both patients reported a neuropathic pain – a painful tenesmus with lancinating and burning sensations suggesting infiltration of the hypogastric plexus. Rectal tenesmus pain in cancer patients is not very frequent. In our center, 1–3 patients are diagnosed with this type of pain each year. These patients are most frequently diagnosed with a local recurrence and dissemination of rectal cancer (patient 1), and less frequently with other primary tumor locations and dissemination, eg, ovary cancer (patient 2). Pain is induced by a direct stimulation or damage of tissues and/or nerve fibers innervating pelvic organs. Pain may be persistent and unresponsive to typical pharmacology treatment with opioids and adjuvant analgesics.1,2,4
Sometimes nerve fibers may be involved in edema diffusing into adjacent tissues, and the pain may be transient, with increasing or decreasing intensity depending on edema increasing or decreasing, respectively. In patient 2, the pain disappeared after the second bupivacaine administration. Tissue edema may accompany metastases due to tumor dissemination, but it may also appear due to treatment procedures, especially radiotherapy to the hypogastrium.3 This occurred in patient 2, in that pain occurred after mechanical irritation of tissues due to gynecology examination.
Tenesmus pain is often present in diseases with underlying infection pathology. These diseases include bacterial, virus, and parasite infections, and sometimes adjacent organ and tissue infections in the course of Crohn’s disease and endometriosis.12,13 If treatment directed at the underlying pathology is possible, pain quickly disappears and pain management usually involves complementary therapy. In advanced cancer patients and those with dissemination, pain management is one of the main elements determining patient quality of life (QoL). In these patients tenesmus pain responds weakly to analgesics including opioids,4,14 anticonvulsants, antidepressants,5 and corticosteroids. Additional ketamine6 and methadone7–9 often do not decrease pain intensity (patients 1 and 2). A trial of calcium channel blockers, which decrease gut wall muscle tension, indicated that they may provide relief and disappearance of tenesmus pain in some patients.15 When these drugs are unavailable, botulin toxin has been utilized.16 This type of pain requires experienced pain and palliative medicine specialists working in special pain clinics.17
Epidural or subarachnoid continuous blockade is the most frequently conducted blockades.2,17,18 Local anesthetics with opioids and/or other adjuvant analgesics are administered via a catheter inserted into the epidural or subarachnoid space. This technique requires appropriate drug choice and doses titrated individually for each patient. A drawback is the possibility of the catheter falling out when the patient moves and the necessity of its reinsertion. Sometimes a neurolytic block is conducted in the epidural or subarachnoid space.19–23 More frequently, neurolytic blocks are conducted of the superior hypogastric plexus, usually with satisfactory effects.1,24 In patients with pain limited to the anus and perineum regions, a neurolytic block of the lower hypogastric plexus is conducted.25,26 Efforts involving radiofrequency are also undertaken.27
Patient transfer and/or contact with an experienced pain or palliative medicine specialist normally takes a few days. During this period, the patient still experiences tenesmus pain, often in spite of using high doses of analgesics.28,29 The use of bupivacaine administered via a deep rectal enema is a non-standard but effective and safe procedure, which can be undertaken in hospital departments and out-patient clinics.1
Bupivacaine is a widely used local anesthetic, which, unfortunately, may evoke neuro- and cardiotoxic effects.30,31 There is little information concerning its use via the rectal route.1,2 Bupivacaine administered as a rectal enema in the aforementioned patients provided effective analgesia for 8–17 hrs. After rectal bupivacaine administration, neither neurologic nor cardiovascular disturbances such as heart arrhythmias or decreased blood pressure were observed. A dose of 100 mg at a concentration of 0.2% (50 mL) seems to be most effective as it caused the pain to disappear completely for 12–17 hrs. A dose of 100 mg at a concentration of 0.1% (100 mL) provided slightly less effective analgesia, sometimes failing to provide complete pain relief. A volume of 50 mL seems to be sufficient to cover the painful area, and a volume of 100 mL is probably not necessary.
When using a volume of 100 mL, the initial bupivacaine concentrations in the blood serum are higher, in comparison to when using a volume of 50 mL with twice the concentration (Table 1). For patient 1 (bupivacaine dose of approximately 1 mg/1 kg of body weight), the initial concentration in the blood serum after administration of 100 mL was 25 ng/mL and this quickly dropped below 20 ng/mL (the lower limit of detection). After administration of a volume of 50 mL, the concentration did not exceed 20 ng/mL and it was maintained under the lower limit of detection.
 | Table 1 Bupivacaine concentration in the blood serum (ng/mL) at 30 mins, 2 hrs, and 4 hrs after administration, and at time of pain reoccurrence |
For patient 2 (bupivacaine dose of approximately 1.7 mg/kg of body weight), after administration of 100 mL bupivacaine, the initial concentration in the blood serum was approximately 47 ng/mL, and this subsequently decreased and then fluctuated in the range of 20-25 ng/mL (Table 2). After administration of 50 mL, the initial concentration in the blood serum was 26 ng/mL, and this decreased in the subsequent consecutive measurements to approximately 20 ng/mL (Figure 3).
 | Table 2 Bupivacaine concentration in the blood serum (ng/mL) at 30 min, 2 hrs, and 4 hrs after administration, and at time of pain reoccurrence |
 | Figure 3 Bupivacaine concentrations in the blood serum (ng/mL) (Table 2). Abbreviation: LLOQ, lower limit of detection. |
A larger volume is associated with a bigger surface for drug absorption, which may explain the higher bupivacaine concentration in the blood serum. The dose of bupivacaine per kg of body weight also influences the concentration in the blood serum. In subsequent hours, concentrations of the drug in the blood serum were nearly stable, slightly exceeding 20 ng/mL. Patients reported pain when the concentration was 20 ng/mL. Toxic concentrations of bupivacaine in the blood serum are in the range of 0.5–5 µg/mL.30,31 The observed concentrations after rectal administration of 100 mg are multiple times lower.
Possible mechanisms of bupivacaine action include a local anesthetic effect, blocking nerve fibers serving the gut wall, a probable mild flaccidity effect, and a spasmolytic effect of the gut wall muscles. No problems with bowel movements were observed in either patient.
The use of bupivacaine at the aforementioned doses and concentrations is safe, and it effectively decreases pain intensity or provides complete relief of tenesmus pain for 8–17 hrs. In the case of pain recurrence, the drug may be re-administered, which allows time for patients to be transferred to specialist pain and palliative medicine units.
Conclusion
A rectal enema of 50 mL 0.2% bupivacaine provides effective relief or complete disappearance of tenesmus pain for 12–17 hrs within a few minutes. A rectal enema of 100 mL 0.1% bupivacaine decreases the intensity and frequency of tenesmus pain for 8–12 hrs. A rectal enema of bupivacaine at aforementioned concentrations and volumes did not induce symptoms of neurotoxicity or cardiotoxicity. Bupivacaine concentrations in the blood serum after rectal administration of 100 mL 0.1% and 50 mL 0.2% did not exceed 50 ng/mL.
A rectal enema of bupivacaine may be repeated after several hours. The use of a rectal enema of 100 mg bupivacaine at concentrations of 0.1% and 0.2% is a safe and effective method of decreasing pain intensity or achieving complete relief of tenesmus pain. These observations of effectiveness and safety require confirmation in a larger group of patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Laoire AN, Fettes L, Murtagh FEM. A systematic review of the effectiveness of palliative interventions to treat rectal tenesmus in cancer. Palliat Med. 2017;31:975–981. doi:10.1177/0269216317697897
2. Zaporowska-Stachowiak I, Kowalski G, Łuczak J, et al. Bupivacaine administered intrathecally versus rectally in the management of intractable rectal cancer pain in palliative care. Onco Targets Ther. 2014;7:1541–1550.
3. Do NL, Nagle D, Poylin VY. Radiation proctitis: current strategies in management. Gastroenterol Res Pract. 2011;2011:917–941.
4. Mercadante S, Portenoy RK. Opioid poorly–responsive cancer pain. Part 3. Clinical strategies to improve opioid responsiveness. J Pain Symptom Manage. 2001;21:338–354. doi:10.1016/S0885-3924(01)00250-0
5. Livovsky DM, Adar T, Lysy J. Tricyclic antidepressants for the treatment of tenesmus secondary to rectal prolapse. Gastroenterology. 2014;146:S–720. doi:10.1016/S0016-5085(14)62612-3
6. Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Pharmacol. 2014;77:357–367. doi:10.1111/bcp.12094
7. Mercante S, Fulfaro F, Dabbene M. Methadone in treatment of tenesmus not responding to morphine escalation. Support Care Cancer. 2001;9:129–130.
8. Alter N, Dion D, Boulanger A, Choiniere M. Management of chronic neuropathic pain with methadone: a review of 13 cases. Clin J Pain. 2005;21:364–369. doi:10.1097/01.ajp.0000125247.95213.53
9. Leppert W, Kowalski G. Methadone as an additional opioid for cancer patient with severe neuropathic and bone pain not responsive to other opioids and adjuvant analgesics. J Palliat Care. 2013;29:119–121. doi:10.1177/082585971302900209
10. Qin WW, Jiao Z, Zhong MK, et al. Simultaneous determination of procaine, lidocaine, ropivacaine, tetracaine and bupivacaine in human plasma by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1185–1189.
11.
12. Bielefedt K, Davis B, Birion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:778–788.
13. Solomon ML, Middleman AB. Abdominal pain, constipation, and tenesmus in an adolescent female: consider Chlamydia proctitis. J Pediatr Adolesc Gynecol. 2013;26:e77–e79.
14. Leppert W, Kowalski G. Long-term administration of high doses of transdermal buprenorphine in cancer patients with severe neuropathic pain. Onco Targets Ther. 2015;8:3621–3627. doi:10.2147/OTT.S91347
15. Stowers KH, Hartman AD, Gustin J. Diltiazem for the management of malignancy-associated perineal pain and tenesmus. J Palliat Med. 2014;17:1075–1077. doi:10.1089/jpm.2013.0250
16. Hawley PH. Botulinum toxin for severe anorectal pain. J Pain Symptom Manage. 2002;24:11–13.
17. Chambers WA. Nerve blocks in palliative care. Br J Anaesth. 2008;101:95–100.
18. Kedlaya D, Reynolds L, Waldman S. Epidural and intrathecal analgesia for cancer pain. Best Pract Res Clin Anaesthesiol. 2002;16:651–665.
19. Candido K, Stevens RA. Intrathecal neurolytic blocks for the relief of cancer pain. Best Pract Res Clin Anaesthesiol. 2003;17:407–428.
20. Porges P, Zdrahal F. Intrathecal alcohol neurolysis of the lower sacral roots in inoperable rectal cancer. Anaesthesist. 1985;34:627–629.
21. Watanabe A, Yamakage M. Intrathecal neuorolytic block in a patient with refractory cancer pain. J Anesth. 2011;25:603–605.
22. Poddar K, Dasgupta S, Gulati R. Epidural alcohol neurolysis – a good option for cancer pain management in developing countries. J Anesth Crit Care. 2016;6:244. doi:10.15406/jaccoa.2016.06.00244
23. Bameshki A, Hashemian A, Jahanbakhsh S. Neurolytic epidural block with 5% phenol for cancer pain. Reg Anesth Pain Med. 2007;32:65.
24. Stogicza A, Trescot AM, Racz E, Lollo L, Magyar L, Keller E. Inferior hypogastric plexus block affects sacral nerves and the superior hypogastric plexus. ISRN Anesthesiol. 2012;686:82. doi:10.5402/2012/686082
25. Choi HS, Kim YH, Han JW, Moon DE. A new technique for inferior hypogastric plexus block: a coccygeal transverse approach – a case report. Korean J Pain. 2012;25:38–42. doi:10.3344/kjp.2012.25.1.38
26. Mohamed SA, Ahmed DG, Mohamad MF. Chemical neurolysis of the inferior hypogastric plexus for the treatment of cancer–related pelvic and perineal pain. Pain Res Manag. 2013;18:249–252.
27. De Conno F, Saita L, Ripamonti C, Ventafridda V. Control of tenesmus by radiofrequency. Pain. 1987;S144. doi:10.1016/0304-3959(87)91360-1
28. Walker SM, Goudas LC, Cousins MJ, Carr DB. Combination spinal analgesic chemotherapy: a systemic review. Anesth Analg. 2002;95:674–715.
29. Togal T, Demirbilek S, Köroğlu A, Yapici E, Ersoy O. Effects of S(+) ketamine added to bupivacaine for spinal anaesthesia for prostate surgery in elderly patients. Eur J Anaesthesiol. 2004;21:193–197. doi:10.1097/00003643-200403000-00005
30. Cox B, Durieux ME, Marcus MA. Toxicity of local anesthetics. Best Pract Res Clin Anaesthesiol. 2003;17:111–136. doi:10.1053/bean.2003.0275
31. Dillane D, Finucane BT. Local anesthetic systemic toxicity. Can J Anaesth. 2010;57:368–380.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
