Back to Journals » Cancer Management and Research » Volume 14
Recombinant Oncolytic Adenovirus Combined with Cyclophosphamide Induces Synergy in the Treatment of Breast Cancer in vitro and in vivo
Authors Wang J, Zuo S, Zhang Y, Li S, Shi Y, Du T, Han J, Jin N, Li Y , Li X
Received 4 May 2022
Accepted for publication 8 September 2022
Published 15 September 2022 Volume 2022:14 Pages 2749—2761
DOI https://doi.org/10.2147/CMAR.S373271
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Jing Wang,1 Shuting Zuo,1 Yan Zhang,1 Shanzhi Li,2 Ying Shi,1 Tonghua Du,1 Jicheng Han,2 Ningyi Jin,2– 4 Yiquan Li,2 Xiao Li2,3
1Department of Breast Surgery, The Second Hospital of Jilin University, Changchun, 130000, People’s Republic of China; 2Academician Workstation of Jilin Province, Changchun University of Chinese Medicine, Changchun, 130117, People’s Republic of China; 3Changchun Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Changchun, 130122, People’s Republic of China; 4Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou, 225009, People’s Republic of China
Correspondence: Yiquan Li; Xiao Li, Academician Workstation of Jilin Province, Changchun University of Chinese Medicine, Liuying West Road, 666, Jingyue Economic & Technological Development Zone, Changchun, People’s Republic of China, Tel +86-431-86985923, Fax +86-431-87985861, Email [email protected]; [email protected]
Purpose: Oncolytic virus therapy has gradually become an integral approach in cancer treatment. We explored the therapeutic effects of the combination of a dual cancer-selective anti-tumor recombinant adenovirus (Ad-Apoptin-hTERTp-E1a) and cyclophosphamide on breast cancer cells.
Methods: The inhibition of MCF-7 and MDA-MB-231 breast cancer cells by Ad-Apoptin-hTERTp-E1a (Ad-VT), cyclophosphamide, and Ad-VT + Cyclophosphamide was investigated using the CCK-8 assay. The combination index (CI) was calculated using CalcuSyn software to determine the best combination based on the inhibition rates of the different treatment combinations. The CCK-8 assay and crystal violet staining were used to detect the cytotoxicity of the combined Ad-VT and cyclophosphamide in breast cancer cells and breast epithelial cells. Subsequently, Hoechst staining, annexin V flow cytometry, and JC-1 staining were used to analyze the inhibitory pathway of Ad-VT plus cyclophosphamide on breast cancer cells. Cell migration and invasion of breast cancer cells were assessed using the cell-scratch and Transwell assays. The anti-tumor effects of different treatment groups in a tumor-bearing nude mouse model also were analyzed.
Results: The treatment combination of Ad-VT (40 MOI) and cyclophosphamide (400 μM) significantly inhibited MCF-7 and MDA-MB-231 cells and reduced the toxicity of cyclophosphamide in normal cells. Ad-VT primarily induced breast cancer cell apoptosis through the endogenous apoptotic pathway. Apoptosis was significantly increased after treatment with Ad-VT plus cyclophosphamide. The combination significantly inhibited the migration and invasion of MCF-7 and MDA-MB-231 cells. The in vivo experiments demonstrated that exposure to Ad-VT plus cyclophosphamide significantly inhibited tumor growth and extended the survival time of the nude mice.
Conclusion: Ad-VT plus cyclophosphamide reduced toxicity and exhibited increased efficacy in treating breast cancer cells.
Keywords: cyclophosphamide, recombinant adenovirus, breast cancer, synergy, toxicity
Introduction
According to the latest World Cancer Report, the number of newly diagnosed cancer cases is increasing and cancer deaths have exceeded one million annually.1 The report noted that the number of breast cancer (BC) patients exceeded the number of lung cancer patients, and breast cancer now exhibits the highest cancer incidence. Breast cancer still ranks first among women in terms of mortality.
Currently, breast cancer treatment mainly uses surgery combined with chemotherapy such as cyclophosphamide, adriamycin, raltitrexed, fluorouracil, capecitabine, and paclitaxel. Among them, Cyclophosphamide (CTX) is an alkylating drug with anti-tumor and immunosuppressive activities. CTX serves as an antineoplastic agent for malignant lymphoma, multiple myeloma, breast cancer, small cell lung cancer, ovarian cancer, neuroblastoma, retinoblastoma, Ewing’s sarcoma, soft tissue sarcoma, acute leukemia, and chronic lymphocytic leukemia. Its immunosuppressive activity centers on the direct control of the body’s immune response to CRAds. Control of CPA effects through precise administration helps reduce circulating regulatory T cells, thereby avoiding extensive immunosuppression.2 Most breast cancer patients are sensitive to chemotherapeutic drugs, but some patients relapse after the first-line treatment is completed. Numerous patients also have severe reactions to chemotherapy. Therefore, alternative types of therapies are needed.
The clinical application of viruses to treat cancer was initiated in the middle of the 20th century. However, it was not until recombinant DNA technology and virus genome engineering emerged in the 1990s that a new wave of virus treatments was triggered. Viral therapy is the strategy of using live viruses as a treatment.3–5 There are two kinds of oncolytic virus therapy, (1) tumor gene therapy with a non-replicating virus as a carrier and (2) the use of replicating virus as the oncolytic agent.6–11 Among them, the emergence of oncolytic adenovirus therapy has dramatically reduced the side effects of chemotherapy.12
Apoptin is a small protein consisting of 121 amino acids that was first isolated and identified from the chicken anemia virus (CAV) in Japan in 1974 and is encoded by the VP3 gene of CAV.13–16 Phosphorylated apoptin is located in the nucleus of tumor cells and the cytoplasm of normal cells, and apoptin can only cause cell death when it is located in the nucleus.17 Therefore, apoptin has the ability to kill tumor cells selectively.18–21
Transcription of human telomerase reverse transcriptase is a major component in regulating telomerase activity. Telomerase is essential for cancer cells to maintain immortality. Thus, interference with telomerase activity can inhibit the growth of cancer cells.22,23
Ad-VT (Ad-Apoptin-hTERTp-E1A) includes a tumor-specific promoter (hTERTp, human telomerase reverse transcriptase) and the apoptin gene. Therefore, Ad-VT is a specific, dual oncolytic adenovirus with tumor-specific killing activity and tumor-specific replication ability.24 This adenovirus replicates specifically in numerous tumor cells, leading to tumor cell death that is mediated by effective apoptin.25–28 In addition, we found in a previous study that Ad-VT combined with other chemotherapy drugs exhibited a significant synergistic effect on lung cancer cells.29,30
In this study, we investigated the inhibitory pathway of Ad-VT combined with cyclophosphamide on breast cancer cells and its toxic effect on normal breast epithelial cells. This study also analyzed the optimal concentration of Ad-VT in combination with cyclophosphamide using a range of assays to reduce the toxicity of cyclophosphamide and improve its therapeutic effects in BC cells.
Materials and Methods
Cells and Viruses
The human breast cancer cell lines MCF-7 and MDA-MB-231 cells and human normal breast epithelial cell lines MCF-10A cells were obtained from Type Culture Collection of the Chinese Academy of Science (Shanghai, China). The four recombinant adenoviruses (Ad-MOCK and Ad-VT) were constructed and preserved in our laboratory.24
Female nude mice aged four to five weeks were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. The experimental animal protocols were approved by the Institutional Animal Care and Use Committee of the Changchun University of Chinese Medicine (approval number: 2021079). Animal welfare and experimental procedures were carried out strictly in accordance with the guide for the care and use of laboratory animals (National Research Council of USA) and the related ethical regulations of our university.
Measurement of Cytotoxic Synergy
The BC cells were cultured in 96-well plates (1 × 104 cells), and Ad-VT and cyclophosphamide (alone or in combination) were added. Before the assessment, the CCK-8 reagent was added to each well, and the plate was incubated for 2 h in a 5% CO2 incubator at 37 °C. The absorbance value of each well was measured at 450 nm using an Infinite 200 multifunctional microplate reader (Tecan Trading AG, Switzerland).
CalcuSyn software analysis (Biosoft v2.0) was used to calculate the combination index (CI) value. CI = 1, > 1, and < 1, indicate additive, antagonistic, and synergistic effects, respectively. The best combination dose was selected based on the CI index and inhibition rate of each combination gradient.
Cell Inhibition Assays
The inhibition of MCF-7, MDA-MB-231, and MCF-10A cell proliferation was detected using crystal violet staining and a CCK-8 assay. MCF-7, MDA-MB-231, and MCF-10A cells were cultured in 12-well plates (2 × 105 cells). The Ad-VT (40 MOI) + cyclophosphamide (400 µM) combination group, Ad-VT (40 MOI) group, cyclophosphamide (400 µM) group, Ad-MOCK (40 MOI) group, and blank control group were established. After 48 h of treatment, a 0.4% crystal violet solution was added to each well for 5 min. Then, the dye solution was removed, the wells were washed three times with phosphate-buffered saline (PBS), and imaged. Subsequently, the CCK-8 assay was used to assess the cell inhibition rate.
Measurement of Apoptosis Levels in Breast Cancer Cells
Hoechst staining and the annexin V assessment method were used to detect the effects of combining Ad-VT and cyclophosphamide on apoptosis in BC cells. The BC cells were cultured in 12-well plates (2 × 105 cells). The Ad-VT (40 MOI) + cyclophosphamide (400 µM) combination group, Ad-VT (40 MOI) group, cyclophosphamide (400 µM) group, Ad-MOCK (40 MOI) group, and blank control group were established. After 48 hours, the culture medium was removed, Hoechst (Life Technologies, USA) staining solution (diluted 1:1000) at 1 mL/well was added, and the cells were cultured in the dark with 5% CO2 at 37 °C for eight minutes. The working solution was removed, the cells were washed twice with PBS, and culture medium was added. The cells adhering to coverslips were observed under a fluorescence microscope and photographed.
For the annexin V experiment, BC cells were seeded into a 12-well plate (2 × 105 cells). The above five groups (Ad-VT (40 MOI) + cyclophosphamide (400 µM) combination group, Ad-VT (40 MOI) group, cyclophosphamide (400 µM) group, Ad-MOCK (40 MOI) group, and blank control group) were established. After 48 hours, the cells were treated with a FITC-coupled annexin-V apoptotic kit (Cell Quest Pro, Becton Dickinson), and apoptosis was detected using FACS flow cytometry.
JC-1 Staining
The BC cells were seeded into a 12-well plate (2 × 105 cells). The above five groups were established. After 48 hours, the culture medium was removed, a JC-1 staining solution (1 mL/well) was added, and the cells were cultured in the dark in 5% CO2 at 37 °C for 15 minutes. The working solution was removed, the cells were washed twice with PBS, and culture medium was added. The cells adhering to the coverslips were observed under a fluorescence microscope and photographed.
Additional BC cells were seeded into 96-well plates (1 × 104 cells), and the five groups described above were set up. After 48 hours, the medium was removed, 100 μL of JC-1 solution was added to each well, and the plate was incubated in the dark for 15 minutes. The absorbance value of each well was measured at 435 nm and 585 nm using a microplate reader.
Analysis of Caspase and Cytochrome c Activities
BC cells were seeded into a 12-well plate (2 × 105 cells). After incubation, the five groups described were established. After 48 h, the cells were collected and lysed. Subsequently, the total cellular protein was extracted, and caspase-3, 7, and 9 and cytochrome c activities were measured using a caspase activity assay kit.
Cell Migration and Invasion Assays
The Biocat method and the scratch test were used in these experiments. For the Biocat method, cells were seeded into a 12-well plate (1 × 105 cells) and cultured for 24 h, and the five groups described previously were established. After 48 hours, the cells were collected and added to the upper chamber of the Biocat, and the plate was incubated for 24 h. After incubation, the cells were fixed with methanol, stained with crystal violet, observed under a microscope, and images were taken.
For the scratch test, the cells were seeded into a 6-well plate (1 × 106 cells) and cultured for 24 h. After incubation, a scratch was made in the cell layer with a sterile micropipette tip, and the scratch width at 0 h was photographed under a microscope. The five experimental groups were established. After 48 hours, the width of each scratch was measured. The cell migration ratio was calculated as Cell migration ratio = (0 h scratch width – 24/48 h scratch width)/ 0 h scratch width.
Tumor Inhibition Assay Using the Nude Mouse Tumor-Bearing Model
MDA-MB-231-LUC cells (cell density 5 × 106/100μL) were injected subcutaneously into the left hind limb of BALB/C nude mice. After the tumor-bearing model was established, the mice were randomly divided into six groups based on the type of treatment used: 20 mg/kg and 50 mg/kg cyclophosphamide, Ad-VT (1×109 PFU), 20 mg/kg cyclophosphamide + Ad-VT (1×109 PFU), 50 mg/kg cyclophosphamide + Ad-VT (1×109 PFU). Before determining the tumor size, the nude mice were injected intraperitoneally with fluorescein (Promega Corporation, USA) at 200μL/mouse (15mg/mL). After five minutes, each nude mouse was injected intraperitoneally with 120 μL of 1% sodium pentobarbital for anesthesia. Photographs of the nude mice tumor sites were taken once a week for six weeks to record changes in tumor size, survival time, and fluorescence intensity using a small animal live imaging system (Merck KGaA, Darmstadt, Germany).24,28,31
Statistical Analysis
The data are presented as means and standard deviations (SD). Data from a minimum of three separate experiments were analyzed. The GraphPad Prism 6.0 program was used to perform statistical analysis, including analysis of variance (ANOVA) or unpaired double-tailed Student’s t-tests. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 were considered statistically significant.
Results
Analysis of the Synergistic Effect of Combining Ad-VT and Cyclophosphamide
The synergistic inhibition of the combination of Ad-VT and cyclophosphamide was determined using the CCK-8 assay (Figure 1A–D). This experiment used a combination of Ad-VT and cyclophosphamide to reduce the cytotoxicity of cyclophosphamide and improve the therapeutic effect. To screen for an optimal concentration, different concentrations of Ad-VT and cyclophosphamide were used to treat MCF-7 cells. Based on the CalcuSyn software calculation, we determined that the combinations groups, 600 µM + 60 MOI, 600 µM + 40 MOI, 400 µM + 40 MOI, 800 µM + 20 MOI, and 600 µM + 20 MOI, were less than 1, indicating these combinations exhibited a synergistic inhibitory effect (Figure 1A and B). Based on these results, we further narrowed the concentration range of cyclophosphamide to 200 µM, 400 µM, and 600 µM and Ad-VT to 20 MOI, 40 MOI, and 60 MOI.
We also performed a combination experiment in MDA-MB-231 cells to select the optimal combined concentration of Ad-VT and cyclophosphamide. The analysis of the treated MDA-MB-231 cells showed that a CI < 1 included the combinations, 400 µM + 40 MOI, 600 µM + 40 MOI, and 400 µM + 60 MOI. The combination group 400 µM + 40 MOI exhibited the lowest CI value and a favorable synergistic effect and was selected for subsequent use in the in vitro assays (Figure 1C and D).
The Combination of Ad-VT and Cyclophosphamide Inhibited BC Cell Proliferation
Breast cancer cells and normal breast epithelial cells were treated with Ad-VT and cyclophosphamide. After 48 hours, the cells were stained with crystal violet. The results indicated that Ad-VT inhibited MCF-7 and MDA-MB-231 cells but had no inhibitory effect on MCF-10A cells. However, cyclophosphamide had some toxic effects on MCF-10A cells. When cyclophosphamide was combined with Ad-VT, the cytotoxicity of cyclophosphamide on MCF-10A cells was significantly lower than on MCF-7 and MDA-MB-231 cells (Figure 2A).
In the CCK-8 assay, we observed that the use of Ad-VT alone inhibited the two kinds of breast cancer cells by approximately 40% but had no toxic effect on MCF-10A cells (Figure 2B). On the other hand, cyclophosphamide was distinctly cytotoxic for MCF-10A cells (P < 0.01). The combination of Ad-VT and cyclophosphamide significantly reduced the cytotoxicity caused by cyclophosphamide alone (P < 0.05). The inhibition rate of breast cancer cells following the combined treatment was higher than 60%, significantly higher than the Ad-VT or cyclophosphamide groups (P < 0.05). Ad-MOCK did not have any significant effect on the death of breast cancer cells (P > 0.05). These results indicated that the combined use of Ad-VT and cyclophosphamide had a synergistic effect and significantly reduced cytotoxicity in normal breast epithelial cells.
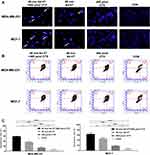
The Combination of Ad-VT and Cyclophosphamide Increased Apoptosis in BC Cells
Hoechst is a dye that freely enters cells and binds to nucleic acids to display blue fluorescence. The results revealed the presence of significant nuclear fragmentation and nuclear overstaining in cells treated with a combination of Ad-VT and cyclophosphamide compared to the control and Ad-MOCK groups (Figure 3A).
The quantitative experimental results for apoptosis indicated that the combined treatment, Ad-VT, and cyclophosphamide induced cell apoptosis, but to different degrees. In MDA-MB-231 cells, the apoptosis rates of cyclophosphamide, Ad-VT, and the combination treatment groups were 15.62%, 37.59%, and 51.24%, respectively. In MCF-7 cells, the apoptotic rates of cyclophosphamide, Ad-VT, and the combination therapy groups were 19.28%, 45.68%, and 62.76%, respectively. Among these, the apoptotic rate of BC cells in the combination treatment group was significantly higher than in the other treatment groups (P < 0.05) (Figure 3B and C).
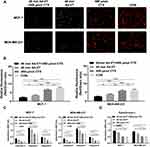
Endogenous Apoptosis Pathway Induction in BC Cells with the Combination of Ad-VT and Cyclophosphamide
The results of the JC-1 staining demonstrated that Ad-VT, cyclophosphamide, and the combination of Ad-VT and cyclophosphamide caused mitochondrial damage and decreased MMP, with no apparent change in MMP in the control cells (Figure 4A). After calculating the ratio of red to green fluorescence, it was observed that the red/green ratio in cells from the combined treatment group was significantly lower than the other groups (P < 0.01) (Figure 4B).
From the results of the caspase and cytochrome c activity assays, it was found that the activities of caspase-3, −7, −9 and cytochrome c in the cells in the combination treatment and the Ad-VT groups are significantly increased, while in the cyclophosphamide group these levels did not significantly change (Figure 4C and D). The caspase and cytochrome c activities of the combined group were higher than that of the single drug group (Figure 4C and D). These results suggest that the combination of Ad-VT and cyclophosphamide induces apoptosis in BC cells by activating endogenous apoptosis pathway.
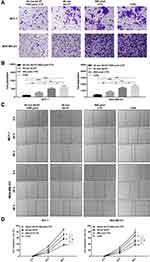
The Combination of Ad-VT and Cyclophosphamide Showed Increased Inhibition of BC Cell Migration and Invasion
The results of the scratch test showed that the combination of Ad-VT and cyclophosphamide, Ad-VT and cyclophosphamide significantly inhibits the healing ability of BC cells (p <0.01). Among them, combination of Ad-VT and cyclophosphamide had the most significant effect (p < 0.01) (Figure 5A and B).
The BioCat results showed that the ability of cell invasion in the combination of Ad-VT and cyclophosphamide, Ad-VT and cyclophosphamide groups was significantly lower than that in the control group (p < 0.001), indicating that all treatment groups can inhibit the invasion ability of BC cells. Among them, combination of Ad-VT and cyclophosphamide had the most significant effect (p < 0.05) (Figure 5C and D). Above results indicated that the inhibiting tumor metastasis effect of the combination group was stronger than that of the single drug groups.
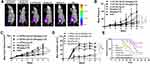
The Anti-Tumor Effect of the Combined Ad-VT and Cyclophosphamide Treatment in vivo
During the six-week monitoring of tumor bioluminescence intensity, it was found that the tumor bioluminescence intensity of the control group increased rapidly during the entire experimental period. In contrast, the rate of increase for the tumor bioluminescence intensity in other groups (20 mg/kg and 50 mg/kg cyclophosphamide, Ad-VT (1×109 PFU), 20 mg/kg cyclophosphamide + Ad-VT (1×109 PFU), 50 mg/kg cyclophosphamide + Ad-VT (1×109 PFU)) was slower. In addition, the difference in tumor volume between the treatment and control groups was statistically significant (P < 0.001). The inhibition rates observed for each treatment group were 11.2%, 19.7%, 38.5%, 55.4%, and 64.2%, respectively (Figure 6A–D).
After successful tumor production, the survival of the nude mice in each group was recorded. The survival rates in the 20 mg/kg cyclophosphamide + Ad-VT (1 × 109 PFU) and the 50 mg/kg cyclophosphamide + Ad-VT (1 × 109 PFU) groups were 70% and 80%, respectively, which were significantly higher than the control group (P < 0.001) (Figure 6E). These results indicated that Ad-VT combined with cyclophosphamide could significantly inhibit tumor growth in vivo and significantly improve the survival rate of the mice.
Discussion
Cancer is the second leading cause of death in developing countries, and its incidence and mortality are rapidly increasing. According to 2020 statistics, there were 19.29 million new cancer cases and 9.96 million deaths worldwide.1 Breast cancer is a common malignant tumor in women. At present, the primary treatment for breast cancer is a combination of surgery and chemotherapy. However, these mainstream therapies have serious side effects, are ineffective for metastatic patients, and have a post-therapeutic recurrence risk. One of the most critical causes of recurrence is chemoresistance development. Currently, the mechanisms of chemoresistance have not been clarified, and patients also exhibit severe toxic reactions during chemotherapy. Although targeted molecular therapy has made some advances in BC treatment through immunotherapy and other precision therapies,32 most of these studies are still in the clinical investigation stage due to small sample sizes and inconsistent results.33
Recently, the development of gene therapy has encouraged the emergence of numerous novel therapeutic technologies, such as oncolytic virus therapy. Oncolytic viruses can specifically infect, replicate in, and kill tumor cells. At present, Newcastle disease virus (NDV), herpes simplex virus-1 (HSV-1), reovirus, oncolytic adenovirus, and others are modified to become oncolytic viruses based on oncophilic properties. These viruses specifically recognize and infect tumor cells, eventually leading to cell swelling and destruction of the tumor cells.34 Among these viruses, adenovirus is widely used in anti-tumor research due to its small size and easy transformation. Many studies have demonstrated that modifying adenovirus to kill tumor cells more efficiently is a very effective strategy.
In our previous studies, we constructed a recombinant adenovirus with tumor-specific replication and killing ability.35 The recombinant adenovirus can replicate, proliferate, and express apoptin in tumor cells. As a pathogenic gene of the chicken anemia virus, apoptin can specifically cause apoptosis in tumor cells. The hTERT promoter is a tumor-specific promoter that is only active in tumor cells. Using these two genes, this oncolytic adenovirus can effectively improve the anti-tumor effect of t viruses.
Currently, the combination of oncolytic adenoviruses and chemotherapy drugs is one of the critical directions being taken in cancer treatment. This is mainly because combining oncolytic adenovirus and chemotherapy drugs can reduce the dose required for the chemotherapy drugs, reducing their toxic effects and improving their efficacy in killing tumor cells. By selecting the appropriate concentrations used in the combined application, the chemotherapy drugs do not interfere with the oncolytic effect of the oncolytic adenovirus. Moreover, their combination can produce a synergistic effect, and the ability to kill tumor cells is significantly higher than with chemotherapy or oncolytic adenovirus alone. Previous studies have shown that the anti-malignant melanoma drug cyclophosphamide alone could not reduce tumor growth in mouse models of melanoma, while a combination of low-dose cyclophosphamide and Ad5/3-Δ24‐GM‐CSF did result in complete tumor regression.36 The mechanism of action might be related to increased angiogenesis inhibition and a reduction in regulatory T cells.37,38
In this study, we analyzed the combined anti-breast cancer effects of Ad-VT and cyclophosphamide. Ad-VT and cyclophosphamide both have a significant inhibitory effect on BC cells. When Ad-VT was combined with cyclophosphamide, it had a significantly greater effect on BC cells, indicating that the combination of Ad-VT and cyclophosphamide had a considerable synergistic effect. Cyclophosphamide exhibited a significant toxic effect on MCF-10A cells. However, the toxicity of cyclophosphamide on MCF-10A cells was significantly reduced when combined with Ad-VT, indicating that the combination of two drugs could enhance the anti-tumor effect and reduce the toxicity of the drugs on normal cells.
Other studies have shown that Ad-shVEGF, an oncolytic adenovirus, could inhibit angiogenesis in vivo.39 Another study showed that in a mouse tumor model established using renal cancer cells, TOS-3LN, intra-tumoral injection of Axd Ad B-3, or the intra-peritoneal injection of gemcitabine (120mg/kg) increased cell death of renal carcinoma cells. However, the combination of gemcitabine and Axd Ad B-3 exhibited a greater ability to kill tumor cells, thus, significantly inhibiting tumor growth. The in vivo anti-tumor experiment in this study revealed that the effect of Ad-VT combined with cyclophosphamide was significantly better than the single drug group. These results are consistent with the previous experimental results.
However, this study has some limitations. Cyclophosphamide is an alkylating agent that can suppress the immune system. In this study, only immunodeficient mice were used for the in vivo anti-tumor experiments. Therefore, we will establish immune competent models in future studies to further refine the in vivo experimental studies.
Conclusions
The combination of Ad-VT and cyclophosphamide exhibited a synergistic effect, which improved the inhibition of BC cells and reduced the toxic effect of chemotherapy drugs on normal cells. In addition, we found that the combination of Ad-VT and cyclophosphamide inhibited the metastatic ability of BC cells. These results indicated that Ad-VT has excellent potential in treating breast cancer when used alone or in combination with cyclophosphamide.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Changchun University of Chinese Medicine (approval number: 2021079). Animal welfare and experimental procedures were carried out strictly in accordance with the guide for the care and use of laboratory animals (National Research Council of USA) and the related ethical regulations of our university.
Informed Consent
For this type of study, informed consent is not required.
Acknowledgments
The authors would like to express their gratitude to EditSprings for the expert linguistic services provided.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, data acquisition, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Science and Technology Research Project of Jilin Provincial Department of Education (Grant No. JJKH20220873KJ), the Important Biological Pathogen Vaccine Research Project (Grant No. 19SWAQ08), the Jilin Province Youth Scientific and Technological Talent Support Project (Grant No. QT202111) and the Major Science and Technology Project for Major Disease Prevention and Control in Jilin Province (Grant No. 20210303002SF).
Disclosure
The authors declare that they have no competing interests.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. English. doi:10.3322/caac.21660
2. Cerullo V, Diaconu I, Kangasniemi L, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol ther. 2011;19(9):1737–1746. doi:10.1038/mt.2011.113
3. Fulci G, Chiocca EA. Oncolytic viruses for the therapy of brain tumors and other solid malignancies: a review. Front Biosci. 2003;8:e346–60. doi:10.2741/976
4. Chiocca EA, Abbed KM, Tatter S, et al. A Phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. doi:10.1016/j.ymthe.2004.07.021
5. Kemeny N, Brown K, Covey A, et al. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum Gene Ther. 2006;17(12):1214–1224. doi:10.1089/hum.2006.17.1214
6. Kirn D. Replication-selective oncolytic adenoviruses: virotherapy aimed at genetic targets in cancer. Oncogene. 2000;19(56):6660–6669. English. doi:10.1038/sj.onc.1204094
7. Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–662. doi:10.1038/nrd4663
8. Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841–849. doi:10.1001/jamaoncol.2016.2064
9. Garza-Morales R, Gonzalez-Ramos R, Chiba A, et al. Temozolomide enhances triple-negative breast cancer virotherapy in vitro. Cancers. 2018;10(5):144. doi:10.3390/cancers10050144
10. Garofalo M, Iovine B, Kuryk L, et al. Oncolytic adenovirus loaded with L-carnosine as novel strategy to enhance the antitumor activity. Mol Cancer Ther. 2016;15(4):651–660. doi:10.1158/1535-7163.MCT-15-0559
11. Naik S, Galyon GD, Jenks NJ, et al. Comparative oncology evaluation of intravenous recombinant oncolytic vesicular stomatitis virus therapy in spontaneous canine cancer. Mol Cancer Ther. 2018;17(1):316–326. doi:10.1158/1535-7163.MCT-17-0432
12. Wan B, Pidduck W, Zhang L, et al. Patient-reported pain in patients with breast cancer who receive radiotherapy. Pain Manage Nurs. 2021;22(3):402–407. doi:10.1016/j.pmn.2020.12.007
13. Backendorf C, Visser AE, de Boer AG, et al. Apoptin: therapeutic potential of an early sensor of carcinogenic transformation. Annu Rev Pharmacol Toxicol. 2008;48:143–169. doi:10.1146/annurev.pharmtox.48.121806.154910
14. Noteborn MH, Todd D, Verschueren CA, et al. A single chicken anemia virus protein induces apoptosis. J Virol. 1994;68(1):346–351. doi:10.1128/jvi.68.1.346-351.1994
15. Yuasa N, Yoshida I, Taniguchi T. Isolation of a reticuloendotheliosis virus from chickens inoculated with Marek’s disease vaccine [Article]. Natl Inst Anim Health Q (Tokyo). 1976;16(4):141–151.
16. Noteborn MH, de Boer GF, van Roozelaar DJ, et al. Characterization of cloned chicken anemia virus DNA that contains all elements for the infectious replication cycle. J Virol. 1991;65(6):3131–3139. eng. doi:10.1128/jvi.65.6.3131-3139.1991
17. Malla W, Arora R, Khan R, Mahajan S, Tiwari A. Apoptin as a tumor-specific therapeutic agent: current perspective on mechanism of action and delivery systems. Front Cell Develop Biol. 2020;8:524. doi:10.3389/fcell.2020.00524
18. Rohn JL, Zhang YH, Aalbers RI, et al. A tumor-specific kinase activity regulates the viral death protein Apoptin. J Biol Chem. 2002;277(52):50820–50827. doi:10.1074/jbc.M208557200
19. Danen-Van Oorschot AA, Zhang YH, Leliveld SR, et al. Importance of nuclear localization of apoptin for tumor-specific induction of apoptosis. J Biol Chem. 2003;278(30):27729–27736. doi:10.1074/jbc.M303114200
20. Poon IK, Oro C, Dias MM, Zhang J, Jans DA. Apoptin nuclear accumulation is modulated by a CRM1-recognized nuclear export signal that is active in normal but not in tumor cells. Cancer Res. 2005;65(16):7059–7064. doi:10.1158/0008-5472.CAN-05-1370
21. Heilman DW, Teodoro JG, Green MR. Apoptin nucleocytoplasmic shuttling is required for cell type-specific localization, apoptosis, and recruitment of the anaphase-promoting complex/cyclosome to PML bodies. J Virol. 2006;80(15):7535–7545. doi:10.1128/JVI.02741-05
22. Tabori U, Ma J, Carter M, et al. Human telomere reverse transcriptase expression predicts progression and survival in pediatric intracranial ependymoma. J Clin Oncol. 2006;24(10):1522–1528. doi:10.1200/JCO.2005.04.2127
23. Patnaik MM, Kamath PS, Simonetto DA. Hepatic manifestations of telomere biology disorders. J Hepatol. 2018;69(3):736–743. doi:10.1016/j.jhep.2018.05.006
24. Xiao L, Yan L, Zhongmei W, et al. Potent anti-tumor effects of a dual specific oncolytic adenovirus expressing apoptin in vitro and in vivo. Mol Cancer. 2010;9(1):10.
25. Liu L, Wu W, Zhu G, et al. Therapeutic efficacy of an hTERT promoter-driven oncolytic adenovirus that expresses apoptin in gastric carcinoma. Int J Mol Med. 2012;30(4):747–754. doi:10.3892/ijmm.2012.1077
26. Qi Y, Guo H, Hu N, et al. Preclinical pharmacology and toxicology study of Ad-hTERT-E1a-Apoptin, a novel dual cancer-specific oncolytic adenovirus. Toxicol Appl Pharmacol. 2014;280(2):362–369. doi:10.1016/j.taap.2014.08.008
27. Yang G, Meng X, Sun L, et al. Antitumor effects of a dual cancer-specific oncolytic adenovirus on colorectal cancer in vitro and in vivo. Exp Ther Med. 2015;9(2):327–334. doi:10.3892/etm.2014.2086
28. Zhang M, Wang J, Li C, et al. Potent growth-inhibitory effect of a dual cancer-specific oncolytic adenovirus expressing apoptin on prostate carcinoma. Int J Oncol. 2013;42(3):1052–1060.
29. Liu X, Yang Z, Li Y, et al. Chemovirotherapy of lung squamous cell carcinoma by combining oncolytic adenovirus with gemcitabine. Front Oncol. 2020;10:229. doi:10.3389/fonc.2020.00229
30. Li T, Fang J, Chu J, et al. In vivo and in vitro inhibition of SCLC by combining dual cancer-specific recombinant adenovirus with Etoposide. J Cancer Res Clin Oncol. 2022;148(5):1073–1085. doi:10.1007/s00432-021-03899-7
31. Sun LL, Jin NY, Xiao L. [Anti-tumor effects of apoptin gene on human laryngeal carcinoma Hep-2]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;42(2):148–150. Chinese.
32. Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121(5):664–672. doi:10.1002/cncr.29098
33. Ng KK, Vauthey JN, Pawlik TM, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12(5):364–373. doi:10.1245/ASO.2005.06.004
34. Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2(4):295–300. doi:10.1158/2326-6066.CIR-14-0015
35. Xiao L, Yan L, Zhongmei W, et al. Potent anti-tumor effects of a dual specific oncolytic adenovirus expressing apoptin in vitro and in vivo. Mol Cancer. 2010;9. doi:10.1186/1476-4598-9-10
36. Hirvinen M, Rajecki M, Kapanen M, et al. Immunological effects of a tumor necrosis factor alpha-armed oncolytic adenovirus. Hum Gene Ther. 2015;26(3):134–144. doi:10.1089/hum.2014.069
37. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9(5):361–371. doi:10.1038/nrc2628
38. Yoo JY, Kim JH, Kwon YG, et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther. 2007;15(2):295–302. doi:10.1038/sj.mt.6300023
39. Wang H, Satoh M, Chen GP, Li DC, Hamada H, Arai Y. E1A, E1B double-restricted adenovirus enhances the cytotoxicity and antitumor activity of gemcitabine to renal cell carcinoma. Chin Med J. 2011;124(7):1082–1087.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.