Back to Journals » OncoTargets and Therapy » Volume 10
Recombinant human endostatin in combination with CHOP regimen for peripheral T cell lymphoma
Authors Zhang Q, Cao J, Xue K, Liu X, Ji D, Guo Y, Hong X
Received 12 July 2016
Accepted for publication 14 September 2016
Published 22 December 2016 Volume 2017:10 Pages 145—151
DOI https://doi.org/10.2147/OTT.S117007
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Qunling Zhang,1,2 Junning Cao,1,2 Kai Xue,1,2 Xiaojian Liu,1,2 Dongmei Ji,1,2 Ye Guo,1,2 Xiaonan Hong1,2
1Department of Medical Oncology, Fudan University Shanghai Cancer Center, 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, People’s Republic of China
Abstract: Peripheral T cell lymphoma (PTCL) has a poor prognosis. Overexpression of vascular endothelial growth factor (VEGF) might contribute to the poor prognosis of PTCL and could be the target of novel therapy. The efficacy and safety of recombinant human endostatin (Endostar) in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (ECHOP) have been explored in 15 PTCL patients. The objective response rate was 80%, with 53.3% patients having achieved complete response (CR) rate. The CR rate was 100% (3/3) in angioimmunoblastic T cell lymphoma (AITL) patients compared to only 36.4% (4/11) in PTCL not otherwise specified (PTCL-NOS) patients. With a median follow-up of 69 months, the 5-year progression-free survival and overall survival (OS) were 53% and 60%, respectively. The 5-year OS was 100% in AITL but was only 45% in PTCL-NOS. Seven out of 11 patients showed overexpression of VEGFR2 in their tumor vessels and had a better efficacy than those with low expression of VEGFR2. Grade 3 or 4 neutropenia is the most common toxicity observed. ECHOP was safe and might display potential benefit in AITL patients.
Keywords: peripheral T cell lymphoma, recombinant human endostatin, VEGFR2, safety, efficacy, prognosis
Introduction
Peripheral T cell lymphomas (PTCL) are highly heterogeneous diseases with several distinct and provisional entities. Of these, PTCL not otherwise specified (PTCL-NOS), angioimmunoblastic T cell lymphoma (AITL) and anaplastic large cell lymphoma (ALCL) that is ALK positive or ALK negative are the most common aggressive PTCL subtypes. PTCL accounts for 10%–15% of non-Hodgkin’s lymphoma (NHL) and the prevalence varies geographically. The incidence of PTCL is higher in East Asia than in Western countries.1,2
The optimal treatment for patients with aggressive PTCL remains uncertain. CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) regimen is still the first-line treatment choice. The prognosis of PTCL is poor and the 5-year overall survival is approximately 38.5%.3 Incorporating novel targeted agents into the therapeutic regimens are encouraged to improve the outcome of patients with PTCL.
Angiogenesis plays an important role in tumor development and progression. The vascular endothelial growth factor (VEGF) is one of the most potent inducers of angiogenesis by stimulating endothelial cell proliferation.4 VEGF and its receptors are frequently expressed in NHL and strongly expressed in PTCLs, especially in AITL.5,6 Higher levels of VEGF expression also have been reported to be associated with resistance to chemotherapy and poor prognosis.7,8 Integrating anti-angiogenesis therapy with CHOP regimen may improve the survival of PTCL patients.
Endostatin, a fragment of collagen XVIII, is an endogenous inhibitor of angiogenesis. It suppresses angiogenesis through multiple pathways: by suppressing cell cycle control and anti-apoptosis genes expression,9 by blocking pro-angiogenic gene expression controlled by c-Jun N terminal kinase,10 by inhibiting the signaling pathways of Ras and Raf kinases and decreasing ERK-1 and p38 activity,11 and by blocking the VEGF downstream targets by direct interaction with vascular endothelial growth factor receptor 2 (VEGFR2) in endothelial cells.12,13 It was also identified that endostatin could inhibit tumor endothelial cell proliferation and tumor growth.14 A phase III study has shown that recombinant human endostatin (Endostar) in combination with NP (vinorelbine plus cisplatin) regimen significantly improved the response rate and the median time to tumor progression compared with NP alone in advanced non-small-cell lung cancer patients.15 Recombinant human endostatin has been approved by the China Food and Drug Administration for advanced lung cancer. Studies have also revealed the synergistic effects of recombinant human endostatin when combined with chemotherapy for advanced breast cancer, gastric cancer, colorectal cancer and metastatic melanoma.16–20 However, clinical evaluation of recombinant human endostatin for PTCL has not been reported.
The purpose of this study was to determine the efficacy and safety of recombinant human endostatin in combination with CHOP regimen (ECHOP) for PTCL patients (ClinicalTrials.gov; Identifier: NCT00974324). The study and the study protocol were approved by the institutional review board of Fudan University Shanghai Cancer Center.
Materials and methods
Eligibility and ineligibility
Newly diagnosed PTCL patients, aged 18–75 years old, were eligible for this study, excluding patients with ALK-positive ALCL, natural killer/T cell lymphoma, primary cutaneous T-cell lymphomas (mycosis fungoides and Sézary syndrome) and primary cutaneous ALCL. All patients had Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2 and adequate hepatic, renal and hematologic functions. Patients had at least one measurable target lesion. Patients with left ventricular ejection fraction less than 50%, which was evaluated by echocardiogram at baseline, were excluded. Patients with a history of severe heart disease, uncontrolled hemorrhage or infection were also excluded. Formalin-fixed, paraffin-embedded tissue sections were collected if available. All patients signed an informed consent.
Treatment and response evaluation
Patients received 6–8 cycles of ECHOP. Recombinant human endostatin (supplied by Shandong Simcere-Medgenn Bio-Pharmaceutical Co. Ltd) was administrated with 7.5 mg/m2 on days 2–15 intravenously, every 3 weeks. CHOP (cyclophosphamide 750 mg/m2 dl; doxorubicin 50 mg/m2 dl; vincristine 1.4 mg/m2 [max. 2 mg] dl; prednisone 100 mg orally daily on days 1–5) was administered every 3 weeks.
Response evaluation was performed by computed tomography scan for every 2 cycles. Patients received additional 2–4 cycles when they achieved complete response (CR) or partial response (PR) after 4 cycles of ECHOP. Response criteria were based on the International Workshop to Standardize Criteria for non-Hodgkin’s lymphomas.21 After the completion of therapy, patients were followed up every 3 months for the first 2 years, then every 6 months for 3 years.
Immunohistochemistry and score for VEGFR2
Immunohistochemical staining for VEGFR2 (Cell Signaling Technology, Beverly, MA, USA) in tumor vessels was performed on formalin-fixed, paraffin-embedded tissue sections using a standard Envision technique procedure (Dako). Negative control was performed by replacing the primary antibody with isotype IgG.
Protein expression levels were scored semi-quantitatively based on staining intensity (SI) and distribution using the immunoreactive score (IRS). Briefly, IRS = SI × PP (percentage of positive cells). SI was determined as 0=negative; 1= weak; 2= moderate and 3= strong. PP was defined as follows: 1, <25%; 2, 25%–50%; 3, 50%–75% and 4, 75%–100% positive cells. IRS >4 was referred to as overexpression.
Statistical analysis
This study was a single-center, one-arm and prospective Simon 2-stage phase-II clinical trial.22 The primary endpoints were objective response rate (ORR) and safety and the secondary endpoints were progression-free survival (PFS) and overall survival (OS). Previous studies have shown a conservative ORR of 60% for PTCL patients who received CHOP regimen. To achieve an improvement of response rate from 60% to 75% with the addition of recombinant human endostatin, 66 patients were required to providing 80% power at an overall 5% significance level (2-sided, with an alpha level of 0.05). In Simon’s first stage, 15 patients were enrolled. If the response rate is 75% or higher, then the second stage has to enroll additionally 79 patients (to ensure that 66 patients are available for evaluation). If the response rate is less than 75% in the first stage, then the study has to be stopped. The statistical analysis was performed using SPSS version 13.0 (IBM, Chicago, IL, USA). Median durations of PFS and OS were calculated using the Kaplan–Meier method, and comparisons between cohorts were made using log-rank tests. All statistical tests were 2-tailed, with significance defined as P<0.05.
Results
A total of 15 patients were enrolled between August 2009 and May 2010. The data cutoff for this analysis was September 30, 2015. Central pathological review was performed in all patients. Among the 15 patients, 11 were confirmed to be PTCL-NOS patients, 3 to be AITL patients and 1 to be ALCL, ALK-negative patient. Eleven patients had International Prognostic Index (IPI) score of 0–1. Detailed patient characteristics are listed in Table 1.
Of the 15 patients, 12 patients (80%) responded to ECHOP, among whom 53.3% patients (8/15) achieved CR and 26.7% patients (4/15) achieved PR. One patient was evaluated with stable disease (SD) after 2 cycles and 2 patients had progression disease (PD) during treatment. One patient with PR after 4 cycles withdrew from the study and received salvage therapy. When the response rate was analyzed by histological subtypes, all the 3 patients with AITL showed CR, whereas in patients with PTCL-NOS only 4 of the 11 (36.4%) showed CR. As the CR rate was low in PTCL-NOS (the most common subtype) patients, stage-2 recruitment was halted, even though the criteria to continue stage 2 were met.
With a median follow-up of 69 (10–75) months, 7 disease progression events were observed. Two patients progressed at the time of chemotherapy, 2 patients progressed during salvage therapy and 3 patients relapsed during the follow-up period. The estimated 3- and 5-year PFS rates were 60% and 53%, respectively. Six patients died, all due to disease progression. The estimated 3- and 5-year cumulative OS were 67% and 60%, respectively (Figure 1A and B).The 5-year PFS and OS for the 11 PTCL-NOS patients were 36% and 45%, respectively (Figure 1C and D). It was noteworthy that all of the 3 AITL patients were alive without any disease progression.
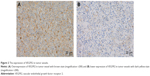
The expression of VEGFR2 in tumor vessels (Figure 2) was detected in 11 patients (9 PTCL-NOS and 2 AITL patients) whose paraffin sections were available. As shown in Table 2, 7 patients showed VEGFR2 overexpression with the highest overexpression found in 2 AITL patients. Among these 7 patients, 5 patients achieved CR and 2 achieved PR with 5-year PFS and OS of 71% and 86%, respectively. In contrast, 2 of the 4 patients with VEGFR2 low expression developed progression disease and the 5-year PFS and OS were both 25% (Figure 1E and F).
The adverse events are listed in Table 3. Hematological toxicity was the most common adverse event. Grade 3 or 4 neutropenia occurred in 86.7% (13/15) patients and febrile neutropenia in 26.7% (4/15) patients. Two patients presented second primary tumors: one diagnosed as rectal cancer 6 months after the completion of chemotherapy and another diagnosed as gray zone lymphoma with features intermediate between diffused large-B-cell lymphoma and classic Hodgkin’s lymphoma 4 years after the completion of chemotherapy. No patient showed heart failure of any grade defined as per the New York Heart Association (NYHA) Functional Classification. No hypertension, proteinuria or hemorrhage was observed in this study.
  | Table 3 Toxicity (n=15) |
Discussion
Currently the survival of PTCL is still dismal when treated with conventional anthracycline-based chemotherapy. The overexpression of VEGF and its receptors in PTCLs compared with B-cell lymphoma promotes the incorporation of anti-angiogenesis drugs into traditional regimens to improve the outcome. The feasibility of ECHOP was explored for PTCL in the present study. The ORR of the 15 patients enrolled in this study was 80% with a CR rate of 53.3%. With a median follow-up of 69 months, the 5-year PFS and OS were 53% and 60%, respectively. Our result is slightly better than the results obtained when the patients were treated with CHOP-like regimens in terms of 5-year OS, which was 35%–48% in early prospective studies.23,24 In ECOG 2404 study, bevacizumab, an anti-VEGF monoclonal antibody, when combined with CHOP regimen (ACHOP) showed an overall response rate of 90% in PTCL patients. However, high response rate under ACHOP treatment failed to transform into favored PFS or OS the 3-year PFS was 16% and OS was 37%.25
A better OS rate observed in this study is mostly due to the favorable response and survival of AITL patients treated with ECHOP. All of the 3 AITL patients achieved CR and the 5-year PFS and OS were both 100%, whereas the 5-year OS is only 45% in PTCL-NOS patients. This is consistent with the ECOG 2404 study, in which the 1-year PFS and OS of AITL (57% and 88%) were favorable compared with PTCL-NOS patients (15% and 67%).
AITL is characterized by a prominent proliferation of endothelial vessels caused by overexpression of VEGF and its receptors in the vascular endothelial cells. This high vascularity might attribute to a worse prognosis under anthracycline-based chemotherapy,26,27 the 5-year OS of AITL being only 33%–41%.28–31 Combined anti-angiogenesis drug with conventional chemotherapy may improve the survival of AITL patients.
VEGFR2, a downstream receptor of VEGF, was overexpressed in AITL patients.32 In our study, 2 AITL patients had the strongest VEGFR2 expression in tumor vessels. VEGFR2 can be a target in AITL therapy and anti-VEGFR2 antibody or VEGFR2 inhibitors have already been used in clinical studies and practice. They had attenuated tumor angiogenesis and inhibited the tumor growth,33,34 and had a synergistic effect with chemotherapy.35,36 Recombinant human endostatin also can exert anti-angiogenic activity by directly interacting with VEGFR2 of endothelial cells.13 It might contribute to good efficacy when combined with anthracycline-based regimen in AITL with VEGFR2 overexpression. Two AITL patients in this study both achieved CR and had a longer survival and the PTCL patients with VEGFR2 overexpression had a favorable outcome as well. As only a small number of patients were included in our study, further investigation is encouraged for AITL subtype to confirm this hypothesis.
The most common toxicities of ECHOP found were hematological toxicities, which was comparable to that of CHOP alone. There were no hypertension, hemorrhage or thrombosis, common toxicities of anti-angiogenesis drugs,37 reported in our study. No hand and foot syndrome was observed, which was a common adverse event of small-molecule anti-angiogenesis drugs sunitinib and sorafenib.37,38
Cardiac toxicity was not reported in our study. However, bevacizumab combined with CHOP was associated with excess cardiac toxicity in ECOG 2404 study, which resulted in the discontinuation of treatment in some patients.25
Conclusion
In conclusion, recombinant human endostatin in combination with CHOP regimen did not increase the survival rate in PTCL-NOS patients, but displayed a potential benefit in AITL patients with VEGFR2 overexpression. ECHOP regimen was tolerable and showed similar safety profile compared with CHOP. Further investigations of recombinant human endostatin in combination of chemotherapy with CHOP or other regimens are suggested for AITL patients as first- or second-line therapy, along with VEGFR2 detection as a biomarker.
Acknowledgments
The authors thank the patients and their families for their dedication, the investigators and all clinical staffs for their support and Minhao Fan for his valuable assistance in immunohistochemical staining procedure.
Disclosure
The authors report no conflicts of interest in this work.
References
Armitage JO. The aggressive peripheral T-cell lymphomas: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012; 87(5):511–519. | ||
Park S, Ko YH. Peripheral T cell lymphoma in Asia. Int J Hematol. 2014;99(3):227–239. | ||
Abouyabis AN, Shenoy PJ, Sinha R, Flowers CR, Lechowicz MJ. A systematic review and meta-analysis of front-line anthracycline-based chemotherapy regimens for peripheral T-cell lymphoma. ISRN Hematol. 2011;2011:623924. | ||
Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9(3):211–220. | ||
Jørgensen JM, Sørensen FB, Bendix K, et al. Angiogenesis in non-Hodgkin’s lymphoma: clinico-pathological correlations and prognostic significance in specific subtypes. Leuk Lymphoma. 2007;48(3):584–595. | ||
Piccaluga PP, Agostinelli C, Califano A, et al. Gene expression analysis of angioimmunoblastic lymphoma indicates derivation from T follicular helper cells and vascular endothelial growth factor deregulation. Cancer Res. 2007;67(22):10703–10710. | ||
Zhang W, Wang L, Zhou D, Cui Q, Zhao D, Wu Y. Expression of tumor-associated macrophages and vascular endothelial growth factor correlates with poor prognosis of peripheral T-cell lymphoma, not otherwise specified. Leuk Lymphoma. 2011;52(1):46–52. | ||
Jørgensen JM, Sørensen FB, Bendix K, et al. Expression level, tissue distribution pattern, and prognostic impact of vascular endothelial growth factors VEGF and VEGF-C and their receptors Flt-1, KDR, and Flt-4 in different subtypes of non-Hodgkin lymphomas. Leuk Lymphoma. 2009;50(10):1647–1660. | ||
Shichiri M, Hirata Y. Antiangiogenesis signals by endostatin. FASEB J. 2001;15(6):10441053. | ||
Yin G, Liu W, An P, et al. Endostatin gene transfer inhibits joint angiogenesis and pannus formation in inflammatory arthritis. Mol Ther. 2002;5(5 Pt 1):547–554. | ||
Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc Natl Acad Sci U S A. 2003;100(8):4766–4771. | ||
Kim YM, Hwang S, Kim YM, et al. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J Biol Chem. 2002;277(31):27872–27879. | ||
Ling Y, Yang Y, Lu N, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun. 2007;361(1):79–84. | ||
O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–285. | ||
Sun Y, Wang J, Liu Y, et al. Results of phase III trial of rh-endostatin (YH-16) in advanced non-small cell lung cancer (NSCLC) patients [abstract]. J Clin Oncol. 2005;23(16S):7138. | ||
Chen J, Yao Q, Li D, et al. Neoadjuvant rh-endostatin, docetaxel and epirubicin for breast cancer: efficacy and safety in a prospective, randomized, phase II study. BMC Cancer. 2013;13:248. | ||
Xu R, Ma N, Wang F, et al. Results of a randomized and controlled clinical trial evaluating the efficacy and safety of combination therapy with Endostar and S-1 combined with oxaliplatin in advanced gastric cancer. Onco Targets Ther. 2013;6:925–929. | ||
Gao SR, Li LM, Xia HP, Wang GM, Xu HY, Wang AR. Clinical observation on recombinant human endostatin combined with chemotherapy for advanced gastrointestinal cancer. Asian Pac J Cancer Prev. 2015; 16(9):4037–4040. | ||
Li BL, Hu XL, Zhao XH, Sun HG, Zhou CY, Zhang Y. Endostar combined with irinotecan/calcium folinate/5-fluorouracil (FOLFIRI) for treating advanced colorectal cancer: a clinical study. J Chemother. 2015;27(5):301–306. | ||
Cui C, Mao L, Chi Z, et al. A phase II, randomized, double-blind, placebo-controlled multicenter trial of Endostar in patients with metastatic melanoma. Mol Ther. 2013;21(7):1456–1463. | ||
Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999; 17(4):1244. | ||
Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. | ||
Karakas T, Bergmann L, Stutte HJ, et al. Peripheral T-cell lymphomas respond well to vincristine, adriamycin, cyclophosphamide, prednisone and etoposide (VACPE) and have a similar outcome as high-grade B-cell lymphomas. Leuk Lymphoma. 1996;24(1–2):121–129. | ||
Gisselbrecht C, Gaulard P, Lepage E, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood. 1998; 92(1):76–82. | ||
Ganjoo K, Hong F, Horning SJ, et al. Bevacizumab and cyclophosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: an Eastern Cooperative Oncology Group study (E2404). Leuk Lymphoma. 2014;55(4):768–772. | ||
Zhao S, Zhang L, Zhang M, et al. Angioimmunoblastic T-cell lymphoma: the effect of initial treatment and microvascular density in 31 patients. Med Oncol. 2012;29(4):2311–2316. | ||
Zhao WL, Mourah S, Mounier N, et al. Vascular endothelial growth factor-A is expressed both on lymphoma cells and endothelial cells in angioimmunoblastic T-cell lymphoma and related to lymphoma progression. Lab Invest. 2004;84(11):1512–1519. | ||
Mourad N, Mounier N, Brière J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111(9):4463–4470. | ||
Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013; 31(2):240–246. | ||
Xu B, Liu P. No survival improvement for patients with angioimmunoblastic T-cell lymphoma over the past two decades: a population-based study of 1207 cases. PLoS One. 2014;9(3):e92585. | ||
Tokunaga T, Shimada K, Yamamoto K, et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: a multicenter cooperative study in Japan. Blood. 2012;119(12):2837–2843. | ||
Konstantinou K, Yamamoto K, Ishibashi F, et al. Angiogenic mediators of the angiopoietin system are highly expressed by CD10-positive lymphoma cells in angioimmunoblastic T-cell lymphoma. Br J Haematol. 2009;144(5):696–704. | ||
Xuan ZX, Li LN, Zhang Q, et al. Fully human VEGFR2 monoclonal antibody BC001 attenuates tumor angiogenesis and inhibits tumor growth. Int J Oncol. 2014;45(6):2411–2420. | ||
Li T, Liu X, Shen Q, et al. Salinomycin exerts anti-angiogenic and anti-tumorigenic activities by inhibiting vascular endothelial growth factor receptor 2-mediated angiogenesis. Oncotarget. 2016;7(18): 26580–26592. | ||
Shen G, Li Y, Du T, et al. SKLB1002, a novel inhibitor of VEGF receptor 2 signaling, induces vascular normalization to improve systemically administered chemotherapy efficacy. Neoplasma. 2012;59(5): 486–493. | ||
Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. | ||
Elice F, Rodeghiero F. Side effects of anti-angiogenic drugs. Thromb Res. 2012;129(Suppl 1):S50–S53. | ||
Maitland ML, Kasza KE, Karrison T, et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15(19):6250–6257. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.