Back to Journals » Journal of Inflammation Research » Volume 13
Recent Updates on Anti-Inflammatory and Antimicrobial Effects of Furan Natural Derivatives
Authors Alizadeh M, Jalal M, Hamed K, Saber A , Kheirouri S , Pourteymour Fard Tabrizi F, Kamari N
Received 13 May 2020
Accepted for publication 5 August 2020
Published 19 August 2020 Volume 2020:13 Pages 451—463
DOI https://doi.org/10.2147/JIR.S262132
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Mohammad Alizadeh,1,* Moludi Jalal,2 Khodaei Hamed,3 Amir Saber,2,* Sorayya Kheirouri,1 Fatemeh Pourteymour Fard Tabrizi,3 Negin Kamari2
1Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran; 2Department of Nutritional Sciences, School of Nutritional Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran; 3Department of Biochemistry and Diet Therapy, Faculty of Nutrition, Tabriz University of Medical Sciences, Tabriz, Iran
*These authors contributed equally to this work
Correspondence: Moludi Jalal
Department of Nutrition, School of Nutritional Sciences and Food Technologies, Kermanshah University of Medical Sciences, Isar Sq., Across from Farabi Hospital, Kermanshah, Iran
Tel +98-83 37102009
Fax +98-83 37102002
Email [email protected]
Khodaei Hamed
Department of Biochemistry and Diet Therapy, Faculty of Nutrition, Tabriz University of Medical Sciences, Daneshgah Street, Tabriz, Iran
Email [email protected]
Abstract: The furan nucleus is found in a large number of biologically active materials. In recent years, many natural furan derivatives were isolated and their biological effects were investigated. In this review, we focused on the anti-inflammatory and antimicrobial effects of some natural furans and discussed their effects on the immune system. Our investigation revealed that furan natural derivatives have effective antioxidant activities and exert regulatory effects on various cellular activities by modifying some signaling pathways such as MAPK (mitogen-activated Protein Kinase) and PPAR-ɣ (peroxisome proliferator-activated receptor gamma). The antimicrobial activity of these natural compounds was performed through selective inhibition of microbial growth and modification of enzymes. Further studies are needed for isolation and detection of different furan derivatives from natural compounds and investigation of their precise mechanisms for revealing health beneficial effects of these compounds.
Keywords: anti-inflammatory, antimicrobial, furans, benzofurans, furan natural derivatives
Introduction
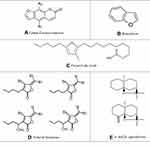
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings is also referred to as furans. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature.1,2 Furan derivative is an imperative class of heterocyclic compound that has important biological properties.3–5 Furan is rapidly and extensively absorbed from the intestine and the lung. It can pass through biological membranes, and enter various organs. Compounds comprising the furan or tetrahydrofuran ring are biologically active and are existent in a number of pharmaceutical products. Furfurylamine is an intermediate in the diuretic, furosemide. 5-(Di methyl amine methyl) furfuryl alcohol is an intermediate in the preparation of ranitidine, which is used for treating peptic ulcers. 2-Acetylfuran, prepared from acetic anhydride and furan is an intermediate in the synthesis of cefuroxime, a penicillin derivative. 2-Furoic acid is prepared by the oxidation of furfural. Both furoic acid and furoyl chloride are used as pharmaceutical intermediates. The compounds and its derivatives are naturally occurring in many foods.6,7 In recent years, many furan derivatives are isolated from natural compounds such as plants,8 fruits,9 oils10 and marine foods.11 Numerous investigations showed that these furan derivatives possess beneficial effects on human health.12,13 Some previous investigations based on cumulative data drawn from animal models suggested that furans are mainly carcinogenic agents and possibly can be carcinogenic to humans.14 The furan ring system is the basic skeleton of numerous compounds possessing cardiovascular activities.15 These compounds are widely employed as antibacterial, antiviral, anti-inflammatory, anti-fungal, anti-tumor, anti-hyperglycemic, analgesic, anti-convulsant, etc.16–19 More recently, an increasing body of evidence indicated that furans may possess some desirable immune-regulatory effects. Several Furans natural derivatives have been introduced with favorable biological functions including antioxidant activity, anti-proliferative and antiviral activity.7,20 Moreover, furan, benzofurans, furan fatty acids, agarofurans and furanocoumarins derivatives constitute a major group of furan natural derivatives that exhibited extensive pharmacological activities such as anti-inflammatory and antimicrobial effects (Figure 1). Despite these findings, there are so many doubts about the actual therapeutic potential of these compounds due to lack of knowledge about their complex effects on all features of human health. Therefore, in this review, we focused on the mechanisms of anti-inflammatory and antimicrobial activities of some natural furan derivatives (Table 1).
 |
Table 1 Suggested Pathways for Anti-Inflammatory Activity of Some Natural Furans |
Methods
A literature search of the PubMed and Embase databases was conducted for the terms “natural furans”, “furan natural derivatives”, “anti-inflammatory” and “antimicrobial”. Searches were limited to English-language articles published prior to or on January 29, 2019. Results of any relevant articles were manually identified by the authors for review and duplicate articles were excluded.
Natural Benzofurans
Benzofuran is a colorless liquid with an aromatic odor, boiling point (101.3 kPa) 171.4°C, density (20°C) 1.0948 g/cm3, melting point (−28.9°C) and can be synthesized by dehydrogenating and cyclization of 2-ethylphenol.19 It is a heterocyclic organic compound with two benzene rings fused to a central furan ring (Figure 1B). Natural derivatives of benzofuran can be produced in some trees21 and plants.20
Anti-Inflammatory Properties of Natural Benzofurans
Some investigations showed that benzofuran natural derivatives can affect immune responses in some cell lines. In this way, Jih-Jung Chen et al isolated a new dibenzofuran, lucidafuran and some known compounds from the stems of a small endemic deciduous tree and investigated their anti-inflammatory effects. The anti-inflammatory effects of the isolated compounds were evaluated on fMet-Leu-Phe (fMLP)-induced O2 generation by human neutrophils. They indicated that lucidafuran and another dibenzofuran derivative eriobofuran inhibited O2 generation of human neutrophil.22
Another study by Chu-Hung Lin et al confirmed the inhibitory effects of some dibenzofuran natural derivatives on human O2 generation. Four dibenzofuran compounds from roots of Rhaphiolepis indica, including 2-hydroxy-3,4,6-trimethoxydibenzofuran, 2-hydroxy-3,4,9-trimethoxydibenzofuran, 2-hydroxy-3,4,6,9-tetramethoxydibenzofuran, and 1,2-methylenedioxy-3,4,6-trimethoxydibenzofuran were shown to efficiently suppress O2 production by human neutrophils.23 Natural derivatives of benzofuran such as 2-arylbenzo [b] furan, egonol, XH-14, and ailanthoidol were studied by Hwang et al for possible anti-inflammatory effects in lipopolysaccharide (LPS)-stimulated RAW 264–7 macrophages. It was found that these compounds significantly inhibited the production of inflammatory mediators, nitric oxide, without showing any significant cytotoxicity. Furthermore, ailanthoidol was the most anti-inflammatory compound with a unique possibility to inhibit nitric oxide (NO) production at 10 µM.24
Furthermore, Park et al investigated the anti-inflammatory effects of XH-14, isolated from Salvia miltiorrhiza, and revealed that this compound can inhibit LPS-induced NO production in a dose-dependent manner most probably via a reduction in iNOS protein expression.25 They also noticed that PGE2 was decreased after the exposure to XH-14. Previous studies showed that pro-inflammatory mediators such as mRNA expression of iNOS, COX-2, IL-1β, and IL-6 were significantly decreased by exposure to 5 and 20 µM concentrations of XH-14. This has been widely adopted that pro-inflammatory prostanoids such as PGE2 play a key role in guiding and governing various aspects of the inflammatory responses. This property has been linked to activities of inducible COX-2. Therefore, the production of PGE2 is often taken as an indicator of COX-2 activity and its inhibition as an index of the ability of COX-2 selective inhibitors.26–28 Many natural neolignans have displayed significant potential as a novel anti-inflammatory compound.29 XH-14, which is also known as Danshen can bind the A1 adenosine receptor with relatively high affinity and inhibit adipocyte differentiation and induction of the adipokines in adipocytes.30 XH-14 also might be attributed to its inhibiting effect on PGE2 production through blocking COX-2 gene expression as well as the activity of COX-2.25 Furthermore, synthesized derivatives tend to have significant anti-inflammatory activity and shall prove as structural templates in the design and development of new anti-inflammatory drugs.31
Moreover, benzofuran can exert regulatory effects on various cellular activities, potentially by modifying signaling pathways such as MAPK and PPAR-ɣ.32 As well, Park et al studied MAPKs pathways by evaluating the phosphorylation of JNK, p38, and ERK. The phosphorylation of these proteins was inhibited after treatment with different doses of XH-14 through stimulation with LPS.25 Induction of PPAR-ɣ and C/EBPα as transcription factors that regulate adipocyte marker genes was inhibited by XH-14 derivative and suggested as therapeutic applications against obesity-related metabolic and inflammatory diseases.30
In general, compounds with benzofuran skeleton, such as ailanthoidol, XH-14, and egonol are natural products that are known as characteristic members of the benzofuran lignan family. Benzofuran lignans mostly contain a 2-phenyl-7-methoxy-benzofuran skeleton that may possess a substantial role in anti-inflammatory properties of natural benzofurans.33 The exact mechanism by which benzofuran exhibits anti-inflammatory actions is not clear yet. It seems that it can inhibit the production of PGE2 and at the same time can decrease lipoxygenase activity.29
Another anti-inflammatory property of benzofuran is the ability to inhibit NO production as a free radical that is involved in various inflammatory responses.25,34 Therefore, it has been proposed that NO scavenging by benzofuran plays an important role in the regulation of inflammatory pathways.
Antimicrobial Properties of Natural Benzofurans
Anti-fungal activity of natural dibenzofurans is responsible for another possible effect of these compounds on the human immune system. In an investigation that was conducted by Qu et al they isolated dibenzofuran bis (bibenzyl) from the liverwort Estrella angusta. The anti-fungal activity was investigated by evaluating minimal inhibitory quantity (MIQ) and minimal inhibitory concentration (MIC) of these compounds on Candida albicans and results showed that dibenzofuran bis (bibenzyl) might be an anti-fungal compound with MIC values ranging from (16 µg/mL) to (512 µg/mL).35 It has been stated that benzofurans, accompanied by anti-microbial effects, can inhibit the fungal activity and previous in-vivo studies indicated anti-fungal activity of natural dibenzofuran compounds such as Entomosporium eriobotryae,36 Venturia inaequalis37 and Chondrostereum purpureum38 in some hosts pathogenic fungus. This finding may offer new insights about potential therapies from a fungal disease, but their effects on human pathogens need more investigations.
Natural Furan Fatty Acids
Furan fatty acids (F-acids) are a class of heterocyclic fatty acids which have a furan moiety in the central part of the molecule (Figure 1C). F-acids with methyl or dimethyl substituents on the furan ring are known as valuable minor fatty acids in foods and have been found in the lipids of various fishes,39 plants such as grasses, wheat, potatoes8 and soya bean oils.40,41
Anti-Inflammatory Properties of Natural Furan Fatty Acids
Numerous studies have confirmed the anti-inflammatory effects of F-acids, which isolated from the planet and animal sources. In this way, Wakimoto et al showed the anti-inflammatory effects of F-acids, isolated from the green-lipped mussel, on an arthritis-induced model of rats. This in-vivo study indicated that swelling of the paw in F-acids groups was lower than in the control, suggesting that anti-inflammatory effects of these compounds are more potent than of or that of eicosapentaenoic acid.42 Naturally occurring F-acids are potent hydroxyl radical scavengers and serve as an antioxidant in many biological systems. Competition of F-acids with DMPO (5,5-Dimethyl-1-pyrroline N-oxide) for hydroxyl radical (HO•) scavenging activity resulted in a reduction of DMPO-OH adduct signal and F-acids were found to react rapidly with HO• at approximately a diffusion-controlled rate.43 Besides, Okada and colleagues examined hydroperoxide-decomposer and a chain-breaking antioxidant activity of F-acids and concluded that antioxidant properties of these compounds could not be related to the decomposition of hydroperoxides. For investigating a chain-breaking antioxidant activity, they evaluated the reduction of DPPH (2, 2-diphenyl-1-picrylhydrazyl) and reaction of F-acids toward the peroxyl radicals generated from a radical initiator, AAPH (2, 2ʹ-Azobis (2-amidinopropane) dihydrochloride). Generation of peroxyl radical from linoleic acid oxidation was decreased by scavenging activity of F-acids.44
It seems that the extraction of F-acids from natural resources is one of the most important steps of these studies. Factors such as instability and exposure time of fatty acids in the environment may decompose these compounds and influence the results of such studies. Therefore, the selection of the exact method for extraction of F-acids is a determining factor for the evaluation of the anti-inflammatory effects of these compounds. As well, Teixeira et al treated rat brain astroglioma cells with F-acids and concurrently with hydrogen peroxide as oxidative stress inducer. They showed that cell leakage or mitochondrial dysfunction of astroglioma cells was decreased after the exposure with F-acids and radical scavenging activity of these compounds sufficiently protected astroglioma cells from oxidative stress-induced by the hydrogen peroxide. It is proposed that oxidative stress is rescued by F-acids species scavenging radicals elicited by lipid peroxidation within the cell membrane. Oxidative processes outside the cell membrane, such as protein carbonylation, are not affected by the F-acids species.45
It appears that anti-inflammatory properties of natural furan fatty acids are closely linked to the high antioxidant properties of these compounds. It is known that F-acid’s antioxidant activity is mainly due to the electron transferability of the furan ring to peroxyl radical or addition of peroxyl radical to the ring.44 However, our review indicates that there are still many gaps and inconsistencies in revealing underlying mechanisms involved in the anti-inflammatory properties of these compounds which warrants further investigations.
Antimicrobial activity of furan fatty acid, 7, 10-epoxyoctadeca-7, 9-dienoic acid against methicillin-resistant Staphylococcus aureus (MRSA) has been reported in previous studies. Dasagrandhi et al demonstrated that anti-staphylococcal activity of 7.10-EODA and its consequences on cell physiology in disc diffusion and conclude that the utilization of furan fatty acids as novel anti-MRSA agents.46 A similar conclusion was reached by Knechtle et al, who demonstrated that the furan fatty acid is an inhibitor of Trichophyton infection.47
Natural Furanocoumarins
Furanocoumarins (FCs), characterized by a furan ring fused with coumarin, are known as secondary organic chemical components.48 Furanocoumarins (Figure 1A) are divided into two subgroups, including linear furanocoumarins (Psoralen, Imperatorin, Isooxypeucedanin, Pabulenol, etc.)49 and angular furanocoumarins (Angelicin, Isobergapten, Sphondin, Pimpinellin, etc.).50 These chemicals are widely distributed in fruits (lemon peel 75 μg g-1),48 vegetables (parsnip 26.2 μg g-1, parsleys 1.4 μg g-1),51 celery (49.8 μg g-1)52 (DI, grapefruit juices and many other plants.53
Anti-Inflammatory Properties of Natural Furanocoumarins
The anti-inflammatory effects of these compounds were investigated through different in-vivo and in-vitro studies. Amponsah et al investigated the anti-inflammatory and antioxidant activity of furanocoumarins from the stem bark of Moraceae. Carrageenan-induced foot edema, a model of inflammation in the chick, was used to investigate the anti-inflammatory effects of these compounds. Two furanocoumarins, bergapten, and oxypeucedanin hydrate exhibited significant anti-inflammatory activities in a dose-dependent manner with ED50 values of 01.6±0.003 mg/kg and 126.4±0.011 mg/kg, respectively.54 This finding was consistent with a previous study which claimed that bergapten, isolated from Angelica pubescens, has both anti-inflammatory and analgesic activities in mice.55,56 Interestingly, the inhibitory effect of furanocoumarins from Kaffir lime on the production of iNOS and COX-2 was reported in previous studies.57
Several natural compounds with a coumarinic moiety have been reported to elicit many biological activities.48 Coumarin Ring consists of fused benzene and α-pyrone rings. These structures enable the compounds to be carried out by proteins through microvessels into tissue spaces.18 This may explain how these compounds can inhibit tissue swelling and edema as reported by Fylaktakidou et al. These effects might be explained by the findings due to lysosomal enzymes by macrophage activation and proteolysis.58 In addition, other studies indicated that the anti-inflammatory effects of many natural compounds such as luteolin, and anthocyanidin are due to a hydroxyl group presented in their structure.59 Also, the antioxidant activity of furanocoumarins was examined by examining the DPPH scavenging activity of these compounds. There was a high antioxidant activity of oxypeucedanin hydrate as compared to bergapten which was attributed to the presence of hydroxyl groups on the 5-oxyprenyl side chain.54 In line with these findings, Lan Piao et al concluded that structural specificity might be the main factor in demonstrating the antioxidative activity of furanocoumarins. Hydroxyl group’s position might result in different antioxidative activities of individual coumarin components on AAPH-induced cell damage. Further investigations showed that other furanocoumarins such as 9-hydroxy-4-methoxypsoralen and 4-Hydroxy-9-(3-methyl-2-buten-1-yl) −7H-furo [3, 2-g] [1] benzopyran-7-one are potent antioxidants.60 However, more studies are needed to reveal their exact mechanisms.
Moreover, it is shown that linear furanocoumarins possess a protective ability in rat myocardium against lipid peroxidation. A particular furanocoumarin named as marmesinin significantly reduced the level of lipid peroxides in both the heart and serum in rats, which could be due to the fact that marmesinin blocks the formation of lipid peroxides from fatty acids. The reduction of lipid peroxides by marmesinin is most likely mediated via inhibition of cyclooxygenase and lipoxygenase activity on arachidonic acid.61
Inhibition of prostaglandin E2 (PGE2) syntheses by furanocoumarins is another clue for the anti-inflammatory property of these compounds. Also, Seung Ban et al investigated the effect of five furanocoumarins, isolated from Angelica dahurica, on lipopolysaccharide (LPS)-induced PGE2 production in rat peritoneal macrophages. They suggested that furanocoumarins suppressed PGE2 production via inhibition of COX-2 and mPEGS expression that this suppression might be the same as that for the inhibition of iNOS expression by similar transcription factors. Phospholipase A2 that catalyzes the hydrolysis of the Sn-2 position of membrane glycerophospholipids to liberate arachidonic acid (AA) might have no role in suppression of PGE2 production.62 In addition, an in-vivo study by Jhong Huang et al demonstrated that imperatorin has an inhibitory effect on the serum PGE2 level of mice. They revealed that imperatorin decreased Carr-induced iNOS and COX-2 expressions in paw edema.63
Activities of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), and Glutathione peroxidase (GPx) might be a feature of the anti-inflammatory mechanism of furanocoumarins as the activity of these enzymes increased significantly after treating with furanocoumarins. The inhibition of iNOS and COX-2 expressions has been proposed as a potential therapy for different inflammatory disorders. Large amounts of iNOS could lead to inflammation. In many inflammatory diseases, excessive iNOS expression has been observed.64 So, it would be interesting to improve potent and selective inhibitors of iNOS and COX-2 expressions for possible therapeutic use (Figure 2).
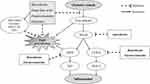 |
Figure 2 Possible pathways that suggested for anti-inflammatory effects of furan natural derivatives. |
Antimicrobial Properties of Natural Furanocoumarins
Furanocoumarins are a kind of organic compounds produced by plants which have diverse effects on many organisms.65 Furanocoumarins, isolated from the Heracleum maximum root extract were identified as anti-mycobacterial compounds. Besides, 6-isopentenyloxyisobergapten was more active than other furanocoumarins by MICs of (167μM) and IC50s of (27 μM) against Mycobacterium tuberculosis that it may be related to isoprenyl group attached to this compound. The antimicrobial activity of 8-geranyloxy psoralen, a furanocoumarin isolated from Prangos uloptera roots, was examined against Staphylococcus epidermidis, Bacillus subtilis, Escherichia coli, Candida glabrata, Candida krusei and Candida kefyr. As well, 8-geranyloxy psoralen showed the most antibacterial activity against Staphylococcus epidermidis by MICs of (100 mgmL-1) and anti-fungal effects against Candida krusei and Candida kefyr, with MICs of (300 and 100 mgmL-1), respectively.66 Razavi et al indicated that furanocoumarins are more effective on gram-positive bacteria.67 Moreover, furocoumarins inhibited auto-inducer (AI) signaling and biofilm formation in bacteria. As well, Girennavar et al isolated furocoumarins from grapefruit and investigated the effect of these natural compounds on different bacterial strains. The results indicated that furocoumarins of grapefruit juice were potent inhibitors of both N-acylhomoserine lactone (AI-1) and molecule, autoinducer-2 (AI-2) activities, while interfere with bacterial growth was not observed.68 Despite many studies exhibited the antimicrobial activity of natural furanocoumarins, recognition of the precise mechanism of these compounds against microbial activity needs more investigations (Figure 3).69–71
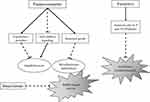 |
Figure 3 Possible pathways that suggested for antimicrobial effects of furan natural derivatives. |
Natural Agarofurans
Agarofurans (Figure 1E) are other furan derivatives that can be isolated from plants,72 trees,73 fruits74 and seeds.75 Many biological activities of these compounds are investigated and revealed new insight into the study about their effect on human health regarding their cytotoxic,76 immunosuppressive,77 antitumor activities,78 and possible anti-HIV properties.79
Anti-Inflammatory Properties of Natural Agarofurans
Arciniegas et al isolated two esterified polyhydroxyagarofurans of Mortonia greggii gray and investigated their anti-inflammatory activity in carrageenan and 12-O-tetradecanoylphorbol-13-acetate induced models of inflammation. They indicated that agarofuran derivative, 6-acetoxy-1, 9-dibenzoyloxy-4-hydroxydihydro- β-agarofuran, inhibited carrageenan-induced paw edema and 1, 2, 6, 14-tetraacetoxy-9- benzoyloxydihydro-β-agarofuran inhibited TPA-induced ear edema in rats. Also, NO production in LPS (lipopolysaccharide)-activated macrophages were suppressed by these two agarofuran derivatives and this activity could be related to effects on iNOS synthesis.74 Many studies have indicated that the activity of anti-inflammatory compounds could be eliminated by the deletion of the hydroxyl group.80 Thus, it seems that the hydroxyl group in various agarofurans may induce anti-inflammatory properties. Zi Jin et al investigated the anti-inflammatory effect of β-agarofuran isolated from Celastrus orbiculatus and determined that these compounds inhibited LPS-induced NF-κB activation and decreased NO production in murine macrophage RAW264.7 cells.81 Using agarofuran compounds as anti-inflammatory agents, which selectively downregulate pro-inflammatory genes, maybe another aspect of their activity. Carroll et al isolated agarofurans from seeds of Celastrus subspicata and investigated the expression of pro-inflammatory genes associated with the glucocorticoid receptor-ligand complex. They suggested that these compounds can downregulate pro-inflammatory genes and also can be mildly cytotoxic.82 Taken all together, anti-inflammatory properties of agarofurans are dependent on two factors; 1) the metal-chelating potential, which is strongly dependent on the arrangement of the hydroxyl group around the molecule and 2) the presence of hydrogen-/electron-donating substituents which reduce free radicals.
Natural Furanones
Furanone is a heterocyclic organic compound that classified as an unsaturated lactone.83 Natural halogenated furanones (Figure 1D) isolated from sea organisms84 and also other natural furanone derivative compounds found in a number of fruits.85 2,5-Dimethyl-4-hydroxy-3-[2H] furanone (DMHF) or furanone derivatives or furanone compounds was the first furanone that isolated and identified in pineapple.86 The biological activity of these compounds has recently been identified and medicinal chemistry synthesized their derivatives as effective compounds.87
Anti-Inflammatory Properties of Natural Furanones
Furanones are potent superoxide anion scavengers and lipid peroxidation inhibitors.87 The antioxidative activity of one of the furanone derivative, 4-hydroxy-3 (2H)-furanones, was investigated on the onset of cataract in spontaneous cataract rats. This compound that has been isolated from natural sources such as pineapple and strawberry inhibited spontaneous cataract formation. Reduction of ferric ion and lipid peroxidation were measured for investigating the antioxidative activity of furanone derivatives, and the results showed that although the antioxidative activity of these furanone derivatives was significantly lower than vitamin C, the inhibition of cataract formation was related to the suppression of lipid peroxide.88 Moreover, Henning et al evaluated the antioxidant activity of 2, 5-Dimethyl-4-hydroxy-3-[2H] furanone (DMHF) in-vitro to determine its potential contribution to the health benefits of strawberries. They suggested that DMHF may contribute considerably to the total antioxidant activity of strawberries.89 Another study indicated 4-Hydroxy-2 (or 5) -ethyl-5 (or 2) -methyl-3 (2H)-furanone can protect the iron in human erythrocyte membranes from ion-induced oxidative modification and can be considered as a potential antioxidant agent.90 In addition, some furanones which extracted by acetone from the mushroom showed effective antioxidant and anti-inflammatory activities.91 Oxidative stress has been investigated during inflammation and elevated levels of malonaldehyde, as a lipid peroxidation marker, were found in a remote localized inflammation.92,93 Consequently, scavengers of superoxide and hydroxyl radicals were effective as anti-inflammatory agents.94 The results indicate that several states of reduced oxygen and lipid peroxides are involved in the inflammatory response, and natural Furanones are potent superoxide anion scavengers which in turn reduce chronic inflammation. Besides phenol moieties that exist in all families of plants are well known for their antioxidant properties. Numerous investigations have already shown that these natural compounds have diverse effects on human health, including ameliorating of oxidative stress and subsequent diseases. Investigations of mechanisms have revealed that phenol moieties may scavenge free radicals and ROS such as superoxide anion and suppress the production of TNF-α, as a pro-inflammatory cytokine, LOXs, iNOS, chemokines, and other inflammatory molecules (Figure 2). Also, these molecules can inhibit NF-κB and MAPKs pathways.24,95 Moreover, two aromatic rings on the furanone skeleton enhance the lipophilicity of these compounds which enhance its antioxidative and anti-inflammatory effects. Taken all together, it is expected that the presence of enol and phenol moieties in furanones could induce free radical scavenger activity and related effects.87 Using the total extract of foods to evaluate the antioxidative or anti-inflammatory effects is the most important weakness of the studies, because in this situation, the identification of the precise mechanism effect of a component may be impossible due to the synergistic or antagonistic effects of different components in combination with together. Therefore, it may be necessary to separate each compound from the total extract and examine those compounds separately.
Antimicrobial Properties of Natural Furanones
In an investigation by Sung et al, the antimicrobial effects of DMHF on various human pathogens, including antibiotic-resistant bacteria were confirmed. This compound showed the antibacterial activity against gram-positive and gram-negative bacterial strains which performed through an energy-dependent manner and arrested the cell cycle at the S and G2/M phase.96 In another study, Ren et al studied the antimicrobial activity of halogenated furanones, one of the furanones derivative that isolated from the marine red algae Delisea pulchra, revealed that these compounds inhibited swarming and biofilm formation of E. coli without affecting its growth.97 Furanone also inhibited quorum sensing mediated behaviors by interfering with various N-Acylhomoserine lactone (AHL) signal compounds of different acyl chain lengths. This result indicated that furanone can be an effective quorum-sensing (QS) inhibiting against many different bacterial genera that produce different types of AHL signal molecules.98 These compounds suppressed swarming motility by targeting the quorum-sensing receptor. Also, furanones compete with N-butanoyl-L-homoserine lactone (BHL) in their binding position to the receptor, disrupt the autoinducer-2 (AI-2) biosynthetic pathway99 and modify and inactivate S-ribosylhomocysteinelyase (LuxS), the enzyme which produces AI-2.100 A similar conclusion was reached by Chepkiruia et al who show that moderate antimicrobial activity against various test organisms of new furan.101
Knowledge Gaps and Future Directions
To illuminate uncharted areas of the effects of furan derivatives on inflammation and oxidative stress biomarkers, future investigation should focus on the effect of furan derivatives as potent inhibitory activities against lipoxygenases (LOXs) which plays crucial roles in inflammatory diseases. So, it is proposed that the effects of furan derivatives on other activities involved in LOXs pathways will be examined in future studies.
Conclusion
The study of furan natural derivatives is tough and complex due to the heterogeneity of the different molecular structures and the inadequacy of investigation about related mechanisms. The reviewed furan moiety has shown a wide spectrum of biological activities. Various substituted furan is having significant antimicrobial and anti-inflammatory activity. In recent years, many furan derivatives are isolated from a variety of natural organisms such as plants, fruits, vegetables, marine organisms and oils, and their biological effects are intensively investigated.1,7 Many studies have revealed that furan natural derivatives inhibited inflammatory and microbial activity due to their special structure. These furans can exhibit anti-inflammatory effects through different mechanisms, including suppression of O2, NO and PGE2 production, regulation of mRNA expression of inflammatory mediators, antioxidant activity through hydroxyl and DPPH-free radicals scavenging activity and reduction of lipid peroxides.24,44,54 Investigations have shown that the presence of furan and other aromatic rings in the structure of natural furans strongly affects their biological activity.44,77 Further, enol and phenol moieties together with the hydroxyl group may help to clarify their anti-inflammatory activity.87,102 The antimicrobial activity of these natural compounds indicated through selective inhibition of microbial growth, suppression of swarming motility and modification of some enzymes. It is evident that different structures of furan derivatives is important for the evaluation of their different biological activities. Hence, it is concluded that anti-inflammatory and antimicrobial effects of furan natural derivatives from different structures warrants further studies to elucidate underlying mechanisms. Further investigations are needed to isolate, detect, and examine the health beneficial effects of different furan derivatives from natural compounds.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
References
1. Kottke RH. Furan derivatives. In: Kirk‐Othmer Encyclopedia of Chemical Technology. 2000.
2. Moran WJ, Rodríguez A. Metal-catalyzed furan synthesis. A review. Org Prep Proced Int. 2012;44(2):103–130. doi:10.1080/00304948.2012.657558
3. Viola G, Vedaldi D, dall’Acqua F, et al. Synthesis, cytotoxicity, and apoptosis induction in human tumor cells by geiparvarin analogues. Chem Biodivers. 2004;1(9):1265–1280. doi:10.1002/cbdv.200490089
4. Lukevits É, Demicheva L. Biological activity of furan derivatives (review). Chem Heterocycl Compd. 1993;29(3):243–267. doi:10.1007/BF00531499
5. Loğoğlu E, Yilmaz M, Katircioğlu H, et al. Synthesis and biological activity studies of furan derivatives. Med Chem Res. 2010;19(5):490–497. doi:10.1007/s00044-009-9206-8
6. Ghadimi S, Alizadeh M, Tarighat Esfanjani A, et al. Assessment of dietary exposure to 5-hydroxymethylfurfural from traditional iranian flat breads. Ital J Food Sci. 2014;26(2):169–175.
7. Rahimzadeh N, Alizadeh M, Ghaemmaghami Hezaveh SJ. Estimated bioaccessibility to 5-hydroxymethylfurfural from frequently consumed dried fruits in Iran. J Chem Health Risks. 2018;4(3). doi:10.22034/jchr.2018.544071
8. Hannemann K, Puchta V, Simon E, et al. The common occurrence of furan fatty acids in plants. Lipids. 1989;24(4):296–298. doi:10.1007/bf02535166
9. Kim DK, Kwak JH. A furan derivative from Cornus officinalis. Arch Pharm Res. 1998;21(6):787–789. doi:10.1007/BF02976779
10. Boselli E, Grob K, Lercker G. Determination of furan fatty acids in extra virgin olive oil. J Agric Food Chem. 2000;48(7):2868–2873. doi:10.1021/jf990857j
11. Ishii K, Okajima H, Koyamatsu T, et al. The composition of furan fatty acids in the crayfish. Lipids. 1988;23(7):694–700. doi:10.1007/bf02535671
12. Spiteller G. Furan fatty acids: occurrence, synthesis, and reactions. Are furan fatty acids responsible for the cardioprotective effects of a fish diet? Lipids. 2005;40(8):755–771. doi:10.1007/s11745-005-1438-5
13. Mousavi R, Alizadeh M, Saleh-Ghadimi S. Consumption of 5-hydroxymethylfurfural-rich dried fruits is associated with reduction in urinary excretion of 8-hydroxy-2′-deoxyguanosine: a randomized clinical trial. Eur Food Res Technol. 2016;242(5):677–684. doi:10.1007/s00217-015-2575-y
14. Hayes R. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 63. Springer; 1996.
15. Arshad L, Jantan I, Bukhari SN, et al. Immunosuppressive effects of natural α,β-unsaturated carbonyl-based compounds, and their analogs and derivatives, on immune cells: a review. Front Pharmacol. 2017;8:22. doi:10.3389/fphar.2017.00022
16. Koca M, Servi S, Kirilmis C, et al. Synthesis and antimicrobial activity of some novel derivatives of benzofuran: part 1. Synthesis and antimicrobial activity of (benzofuran-2-yl)(3-phenyl-3-methylcyclobutyl) ketoxime derivatives. Eur J Med Chem. 2005;40(12):1351–1358. doi:10.1016/j.ejmech.2005.07.004
17. Kirilmis C, Ahmedzade M, Servi S, et al. Synthesis and antimicrobial activity of some novel derivatives of benzofuran: part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives. Eur J Med Chem. 2008;43(2):300–308. doi:10.1016/j.ejmech.2007.03.023
18. Kirsch G, Abdelwahab AB, Chaimbault P. Natural and synthetic coumarins with effects on inflammation. Molecules. 2016;21(10):1322. doi:10.3390/molecules21101322
19. Chand K, Rajeshwari HA, Hiremathad A, et al. A review on antioxidant potential of bioactive heterocycle benzofuran: natural and synthetic derivatives. Pharmacol Rep. 2017;69(2):281–295. doi:10.1016/j.pharep.2016.11.007
20. Yan FL, Wang AX, Jia ZJ. Benzofuran derivatives from ligularia stenocephala. J Chin Chem Soc. 2004;51(4):863–868. doi:10.1002/jccs.200400130
21. Sun SG, Chen RY, Yu DQ. Structures of two new benzofuran derivatives from the bark of mulberry tree (Morus macroura Miq.). J Asian Nat Prod Res. 2001;3(4):253–259. doi:10.1080/10286020108040364
22. Chen JJ, Luo YT, Liao CH, et al. A new dibenzofuran and further constituents from the stems of pourthiaea lucida with inhibitory activity on superoxide generation by neutrophils. Chem Biodivers. 2009;6(5):774–778. doi:10.1002/cbdv.200800118
23. Lin CH, Chang HS, Liao CH, et al. Anti-inflammatory biphenyls and dibenzofurans from rhaphiolepis indica. J Nat Prod. 2010;73(10):1628–1631. doi:10.1021/np100200s
24. Hwang J-W, Choi D-H, Jeon J-H, et al. Facile preparation of 2-arylbenzo[b]furan molecules and their anti-inflammatory effects. Bull Korean Chem Soc. 2010;31(4):965–970. doi:10.5012/bkcs.2010.31.04.965
25. Park GM, Jun JG, Kim JK. XH-14, a novel danshen methoxybenzo[b]furan derivative, exhibits anti-inflammatory properties in lipopolysaccharide-treated RAW 264.7 cells. J Inflamm (Lond). 2013;10(1):1. doi:10.1186/1476-9255-10-1
26. Giuliano F, Warner TD. Origins of prostaglandin E2: involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. J Pharmacol Exp Ther. 2002;303(3):1001–1006. doi:10.1124/jpet.102.041244
27. Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol. 2013;35(2):123–137. doi:10.1007/s00281-012-0342-8
28. Morteau O. Prostaglandins and inflammation: the cyclooxygenase controversy. Arch Immunol Ther Exp (Warsz). 2000;48(6):473–480.
29. Shih HC, Kuo PC, Wu SJ, et al. Anti-inflammatory neolignans from the roots of Magnolia officinalis. Bioorg Med Chem. 2016;24(7):1439–1445. doi:10.1016/j.bmc.2016.01.049
30. Sung H-Y, Jun J-G, Kang S-W, et al. Novel danshen methoxybenzo [b] furan derivative antagonizing adipogenic differentiation and production of inflammatory adipokines. Chem Biol Interact. 2010;188(3):457–466. doi:10.1016/j.cbi.2010.09.017
31. Gandhi D, Agarwal DK, Kalal P, et al. Synthesis, characterization and evaluation of novel benzothiazole clubbed chromene derivatives for their anti-inflammatory potential. Phosphorus Sulfur Silicon Relat Elem. 2018;193(12):840–847. doi:10.1080/10426507.2018.1514502
32. Xu X, Kwon OK, Shin IS, et al. Novel benzofuran derivative DK-1014 attenuates lung inflammation via blocking of MAPK/AP-1 and AKT/mTOR signaling in vitro and in vivo. Sci Rep. 2019;9(1):862. doi:10.1038/s41598-018-36925-9
33. Kao CL, Chern JW. A novel strategy for the synthesis of benzofuran skeleton neolignans: application to ailanthoidol, XH-14, and obovaten. J Org Chem. 2002;67(19):6772–6787. doi:10.1021/jo0258960
34. Keay BA, Dibble PW. Furans and their benzo derivatives: applications. Compr Heterocycl Chem II. 1996;2:395–436.
35. Qu J, Xie C, Guo H, et al. Antifungal dibenzofuran bis(bibenzyl)s from the liverwort Asterella angusta. Phytochemistry. 2007;68(13):1767–1774. doi:10.1016/j.phytochem.2007.04.036
36. Miyakado M, Nakayama I, Inoue A, et al. Insecticidal activities of phenoxy analogues of dihydropipercide. J Pestic Sci. 1985;10(1):25–30. doi:10.1584/jpestics.10.25
37. Hrazdina G, Borejsza-Wysocki W, Lester C. Phytoalexin production in an apple cultivar resistant to venturia inaequalis. Phytopathology. 1997;87(8):868–876. doi:10.1094/phyto.1997.87.8.868
38. Kemp MS, Burden RS. Isolation and structure determination of γ-pyrufuran, a third induced antifungal dibenzofuran from the wood of pyrus communis L. infected with chondrostereum purpureum(Pers. ex Fr.) pouzar. J Chin Chem Soc. 1984;1441–1443. doi:10.1039/P19840001441
39. Glass RL, Krick TP, Olson DL, et al. The occurrence and distribution of furan fatty acids in spawning male freshwater fish. Lipids. 1977;12(10):828–836. doi:10.1007/bf02533272
40. Guth H, Grosch W. Detection of furanoid fatty acids in soya-bean oil – cause for the light-induced off-flavour. Lipid/Fett. 1991;93(7):249–255. doi:10.1002/lipi.19910930703
41. Vetter W, Wendlinger C. Furan fatty acids – valuable minor fatty acids in food. Lipid Technol. 2013;25(1):7–10. doi:10.1002/lite.201300247
42. Wakimoto T, Kondo H, Nii H, et al. Furan fatty acid as an anti-inflammatory component from the green-lipped mussel perna canaliculus. Proc Natl Acad Sci U S A. 2011;108(42):17533–17537. doi:10.1073/pnas.1110577108
43. Okada Y, Kaneko M, Okajima H. Hydroxyl radical scavenging activity of naturally occurring furan fatty acids. Biol Pharm Bull. 1996;19(12):1607–1610. doi:10.1248/bpb.19.1607
44. Okada YOH, Konishi H, Terauchi M, Ishii K, Liu IM, Watanabe H. Antioxidant effect of naturally occurring furan fatty acids on oxidation of linoleic acid in aqueous dispersion. J Am Oil Chem Soc. 1990;67(11):858–862. doi:10.1007/BF02540506
45. Teixeira A, Cox RC, Egmond MR. Furan fatty acids efficiently rescue brain cells from cell death induced by oxidative stress. Food Funct. 2013;4(8):1209–1215. doi:10.1039/c3fo60094g
46. Dasagrandhi C, Ellamar JB, Kim YS, et al. Antimicrobial activity of a novel furan fatty acid, 7,10-epoxyoctadeca-7,9-dienoic acid against methicillin-resistant Staphylococcus aureus. Food Sci Biotechnol. 2016;25(6):1671–1675. doi:10.1007/s10068-016-0257-6
47. Knechtle P, Diefenbacher M, Greve KB, et al. The natural diyne-furan fatty acid EV-086 is an inhibitor of fungal delta-9 fatty acid desaturation with efficacy in a model of skin dermatophytosis. Antimicrob Agents Chemother. 2014;58(1):455–466. doi:10.1128/aac.01443-13
48. Hung W-L, Suh JH, Wang Y. Chemistry and health effects of furanocoumarins in grapefruit. J Food Drug Anal. 2017;25(1):71–83. doi:10.1016/j.jfda.2016.11.008
49. Shalaby NM, Abd-Alla HI, Aly HF, et al. Preliminary in vitro and in vivo evaluation of antidiabetic activity of ducrosia anethifolia boiss. and its linear furanocoumarins. Biomed Res Int. 2014;2014:480545. doi:10.1155/2014/480545
50. Sardari S, Mori Y, Horita K, et al. Synthesis and antifungal activity of coumarins and angular furanocoumarins. Bioorg Med Chem. 1999;7(9):1933–1940. doi:10.1016/s0968-0896(99)00138-8
51. Peroutka R, Schulzová V, Botek P, et al. Analysis of furanocoumarins in vegetables (Apiaceae) and citrus fruits (Rutaceae). J Sci Food Agric. 2007;87(11):2152–2163. doi:10.1002/jsfa.2979
52. Diawara MM, Trumble JT, Quiros CF, et al. Implications of distribution of linear furanocoumarins within celery. J Agric Food Chem. 1995;43(3):723–727. doi:10.1021/jf00051a030
53. Sakamaki N, Nakazato M, Matsumoto H, et al. Contents of furanocoumarins in grapefruit juice and health foods. Shokuhin Eiseigaku Zasshi. 2008;49(4):326–331. doi:10.3358/shokueishi.49.326
54. Amponsah IK, Fleischer TC, Dickson RA, et al. Evaluation of anti-inflammatory and antioxidant activity of furanocoumarins and sterolin from the stem bark of ficus exasperata vahl (moraceae). J Sci Innov Res. 2013;2:880–887.
55. Chen YF, Tsai HY, Wu TS. Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med. 1995;61(1):2–8. doi:10.1055/s-2006-957987
56. Pearce AN, Chia EW, Berridge MV, et al. E/Z-rubrolide O, an anti-inflammatory halogenated furanone from the New Zealand ascidian synoicum n. sp. J Nat Prod. 2007;70(1):111–113. doi:10.1021/np060188l
57. Kidarn S, Saenjum C, Hongwiset D, et al. Furanocoumarins from kaffir lime and their inhibitory effects on inflammatory mediator production. Cogent Chem. 2018;4(1):1529259. doi:10.1080/23312009.2018.1529259
58. Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, et al. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr Pharm Des. 2004;10(30):3813–3833. doi:10.2174/1381612043382710
59. Ueda H, Yamazaki C, Yamazaki M. A hydroxyl group of flavonoids affects oral anti-inflammatory activity and inhibition of systemic tumor necrosis factor-alpha production. Biosci Biotechnol Biochem. 2004;68(1):119–125. doi:10.1271/bbb.68.119
60. Piao XL, Park IH, Baek SH, et al. Antioxidative activity of furanocoumarins isolated from Angelicae dahuricae. J Ethnopharmacol. 2004;93(2–3):243–246. doi:10.1016/j.jep.2004.03.054
61. Vimal V, Devaki T. Linear furanocoumarin protects rat myocardium against lipid peroxidation and membrane damage during experimental myocardial injury. Biomed Pharmacother. 2004;58(6–7):393–400. doi:10.1016/j.biopha.2003.12.007
62. Ban HS, Lim SS, Suzuki K, et al. Inhibitory effects of furanocoumarins isolated from the roots of Angelica dahurica on prostaglandin E2 production. Planta Med. 2003;69(5):408–412. doi:10.1055/s-2003-39702
63. Huang GJ, Deng JS, Liao JC, et al. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory activity of imperatorin from Glehnia littoralis. J Agric Food Chem. 2012;60(7):1673–1681. doi:10.1021/jf204297e
64. Jean YH, Chen WF, Duh CY, et al. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from formosan soft coral lemnalia cervicorni. Eur J Pharmacol. 2008;578(2–3):323–331. doi:10.1016/j.ejphar.2007.08.048
65. Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E isoforms as modulators of lung inflammation. Nutrients. 2013;5(11):4347–4363. doi:10.3390/nu5114347
66. O’Neill T, Johnson JA, Webster D, et al. The Canadian medicinal plant heracleum maximum contains antimycobacterial diynes and furanocoumarins. J Ethnopharmacol. 2013;147(1):232–237. doi:10.1016/j.jep.2013.03.009
67. Razavi SM, Zahri S, Nazemiyeh H, et al. A furanocoumarin from prangos uloptera roots, biological effects. Nat Prod Res. 2009;23(16):1522–1527. doi:10.1080/14786410802691909
68. Girennavar B, Cepeda ML, Soni KA, et al. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int J Food Microbiol. 2008;125(2):204–208. doi:10.1016/j.ijfoodmicro.2008.03.028
69. Bisignano G, Sanogo R, Marino A, et al. Antimicrobial activity of mitracarpus scaber extract and isolated constituents. Lett Appl Microbiol. 2000;30(2):105–108. doi:10.1046/j.1472-765x.2000.00692.x
70. Wangchuk P, Pyne SG, Keller PA, et al. Phenylpropanoids and furanocoumarins as antibacterial and antimalarial constituents of the Bhutanese medicinal plant pleurospermum amabile. Nat Prod Commun. 2014;9(7):957–960.
71. Gowri PMHK, Kishore H, Manjusha O, Biswas S, Murty US. Microbial transformation of (+)-heraclenin by aspergillus niger and evaluation of its antiplasmodial and antimicrobial activities. Curr Sci. 2011;100(11):1706–1711.
72. Wibowo M, Levrier C, Sadowski MC, et al. Bioactive dihydro-β-agarofuran sesquiterpenoids from the Australian rainforest plant maytenus bilocularis. J Nat Prod. 2016;79(5):1445–1453. doi:10.1021/acs.jnatprod.6b00190
73. Cavalli JF, Tomi F, Bernardini AF, et al. Combined analysis of the essential oil of chenopodium ambrosioides by GC, GC-MS and 13C-NMR spectroscopy: quantitative determination of ascaridole, a heat-sensitive compound. Phytochem Anal. 2004;15(5):275. doi:10.1002/pca.761
74. Xu J, Guo YQ, Li X, et al. Cytotoxic beta-dihydroagarofuran sesquiterpenoids from the fruits of Celastrus orbiculatus. Z Naturforsch C J Biosci. 2008;63(7–8):515–518. doi:10.1515/znc-2008-7-808
75. Céspedes CL, Alarcón J, Aranda E, et al. Insect growth regulator and insecticidal activity of beta-dihydroagarofurans from Maytenus spp. (Celastraceae). Z Naturforsch C J Biosci. 2001;56(7–8):603–613. doi:10.1515/znc-2001-7-821
76. Kuo YH, King ML, Chen CF, et al. Two new macrolide sesquiterpene pyridine alkaloids from Maytenus emarginata: emarginatine G and the cytotoxic emarginatine F. J Nat Prod. 1994;57(2):263–269. doi:10.1021/np50104a011
77. Wang X, Gao W, Yao Z, et al. Immunosuppressive sesquiterpenes from Tripterygium wilfordii. Chem Pharm Bull (Tokyo). 2005;53(6):607–610. doi:10.1248/cpb.53.607
78. González AG, Tincusi BM, Bazzocchi IL, et al. Anti-tumor promoting effects of sesquiterpenes from Maytenus cuzcoina (Celastraceae). Bioorg Med Chem. 2000;8(7):1773–1778. doi:10.1016/s0968-0896(00)00107-3
79. Duan H, Takaishi Y, Bando M, et al. Novel sesquiterpene esters with alkaloid and monoterpene and related compounds from tripterygium hypoglaucum: a new class of potent anti-HIV agents. Tetrahedron Lett. 1999;40(15):2969–2972. doi:10.1016/S0040-4039(99)00339-1
80. Nowakowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem. 2007;42(2):125–137. doi:10.1016/j.ejmech.2006.09.019
81. Jin HZ, Hwang BY, Kim HS, et al. Antiinflammatory constituents of celastrus orbiculatus inhibit the NF-κB activation and NO production. J Nat Prod. 2002;65(1):89–91. doi:10.1021/np010428r
82. Carroll AR, Davis RA, Addepalli R, et al. Cytotoxic agarofurans from the seeds of the Australian rainforest vine Celastrus subspicata. Phytochem Lett. 2009;2(4):163–165. doi:10.1016/j.phytol.2009.05.002
83. Colin Slaughter J. The naturally occurring furanones: formation and function from pheromone to food. Biol Rev Camb Philos Soc. 1999;74(3):259–276. doi:10.1017/s0006323199005332
84. Dembitsky VM, Tolstikov GA. Natural halogenated furanones, higher terpenes and steroids. Sustain Dev. 2003;11:697–703.
85. Roscher R, Koch H, Herderich M, et al. Identification of 2,5-dimethyl-4-hydroxy-3[2H]-furanone beta-D-glucuronide as the major metabolite of a strawberry flavour constituent in humans. Food Chem Toxicol. 1997;35(8):777–782. doi:10.1016/s0278-6915(97)00055-0
86. Rodin JO, Himel CM, Silverstein RM, et al. Volatile flavor and aroma components of pineapple. l. isolation and tentative identification of 2,5-dimethyl-4-hydroxy-3(2H)-furanone. J Food Sci. 1965;30(2):280–285. doi:10.1111/j.1365-2621.1965.tb00302.x
87. Weber V, Coudert P, Rubat C, et al. Novel 4,5-diaryl-3-hydroxy-2(5H)-furanones as anti-oxidants and anti-inflammatory agents. Bioorg Med Chem. 2002;10(6):1647–1658. doi:10.1016/s0968-0896(02)00053-6
88. Sasaki T, Yamakoshi J, Saito M, et al. Antioxidative activities of 4-hydroxy-3(2H)-furanones and their anti-cataract effect on spontaneous cataract rat (ICR/f). Biosci Biotechnol Biochem. 1998;62(10):1865–1869. doi:10.1271/bbb.62.1865
89. Henning SM, Seeram NP, Zhang Y, et al. Strawberry consumption is associated with increased antioxidant capacity in serum. J Med Food. 2010;13(1):116–122. doi:10.1089/jmf.2009.0048
90. Ando K, Ogawa K, Li X, et al. Inhibition of iron ion-induced oxidative damage of erythrocyte membranes and low density lipoprotein by a maillard product, 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone (HEMF). Biol Pharm Bull. 2000;23(6):689–694. doi:10.1248/bpb.23.689
91. Kamiyama MHM, Umano K, Kondo K, Otsuka Y, Shibamoto T. Antioxidant/anti-inflammatory activities and chemical composition of extracts from the mushroom Trametes versicolor. Int J Nutr Food Sci. 2013;2(2):85–91. doi:10.11648/j.ijnfs.20130202.19
92. Bragt PC, Bonta IL. Oxidant stress during inflammation: anti-inflammatory effects of antioxidants. Agents Actions. 1980;10(6):536–539. doi:10.1007/BF02024159
93. Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43(8):1109–1120. doi:10.1016/j.freeradbiomed.2007.07.012
94. Grimble RF. Nutritional antioxidants and the modulation of inflammation: theory and practice. New Horiz. 1994;2(2):175–185.
95. Sergent T, Piront N, Meurice J, et al. Anti-inflammatory effects of dietary phenolic compounds in an in vitro model of inflamed human intestinal epithelium. Chem Biol Interact. 2010;188(3):659–667. doi:10.1016/j.cbi.2010.08.007
96. Sung WS, Jung HJ, Park K, et al. 2,5-dimethyl-4-hydroxy-3(2H)-furanone (DMHF); antimicrobial compound with cell cycle arrest in nosocomial pathogens. Life Sci. 2007;80(6):586–591. doi:10.1016/j.lfs.2006.10.008
97. Ren D, Bedzyk LA, Ye RW, et al. Differential gene expression shows natural brominated furanones interfere with the autoinducer-2 bacterial signaling system of Escherichia coli. Biotechnol Bioeng. 2004;88(5):630–642. doi:10.1002/bit.20259
98. Ponnusamy K, Paul D, Sam Kim Y, et al. 2(5H)-Furanone: a prospective strategy for biofouling-control in membrane biofilm bacteria by quorum sensing inhibition. Braz J Microbiol. 2010;41(1):227–234. doi:10.1590/s1517-838220100001000032
99. Ni N, Li M, Wang J, et al. Inhibitors and antagonists of bacterial quorum sensing. Med Res Rev. 2009;29(1):65–124. doi:10.1002/med.20145
100. Zang T, Lee BW, Cannon LM, et al. A naturally occurring brominated furanone covalently modifies and inactivates LuxS. Bioorg Med Chem Lett. 2009;19(21):6200–6204. doi:10.1016/j.bmcl.2009.08.095
101. Chepkirui C, Cheng T, Matasyoh J, et al. An unprecedented spiro [furan-2,1ʹ-indene]-3-one derivative and other nematicidal and antimicrobial metabolites from Sanghuangporus sp. (Hymenochaetaceae, Basidiomycota) collected in Kenya. Phytochem Lett. 2018;25:141–146. doi:10.1016/j.phytol.2018.04.022
102. Feng JY, Liu ZQ. Phenolic and enolic hydroxyl groups in curcumin: which plays the major role in scavenging radicals? J Agric Food Chem. 2009;57(22):11041–11046. doi:10.1021/jf902244g
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.