Back to Journals » International Journal of Nanomedicine » Volume 14
Recent expansions of novel strategies towards the drug targeting into the brain
Authors Alexander A, Agrawal M, Uddin A, Siddique S , Shehata AM , Shaker MA , Ata Ur Rahman S , Abdul MIM , Shaker MA
Received 2 April 2019
Accepted for publication 13 June 2019
Published 30 July 2019 Volume 2019:14 Pages 5895—5909
DOI https://doi.org/10.2147/IJN.S210876
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Amit Alexander,1 Mukta Agrawal,1 Ajaz Uddin,1 Sabahuddin Siddique,2 Ahmed M Shehata,3,4 Mahmoud A Shaker,5,6 Syed Ata Ur Rahman,7 Mohi Iqbal M Abdul,3 Mohamed A Shaker6,7
1Department of Pharmaceutics, Rungta College of Pharmaceutical Sciences and Research, Bhilai, Chhattisgarh, India; 2Patel College of Pharmacy, Madhyanchal Professional University, Bhopal, Madhya Pradesh, India; 3Department of Pharmacology and Toxicology, College of Pharmacy, Taibah University, Al-Madinah Al-Munawarah, Kingdom of Saudi Arabia; 4Department of Pharmacology and Toxicology, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt; 5Department of Pharmaceutics, College of Pharmacy, King Khalid University, Abha, Kingdom of Saudi Arabia; 6Pharmaceutics Department, Faculty of Pharmacy, Helwan University, Cairo, Egypt; 7Pharmaceutics and Pharmaceutical Technology Department, College of Pharmacy, Taibah University, Al-Madinah Al-Munawarah, Kingdom of Saudi Arabia
Abstract: The treatment of central nervous system (CNS) disorders always remains a challenge for the researchers. The presence of various physiological barriers, primarily the blood–brain barrier (BBB) limits the accessibility of the brain and hinders the efficacy of various drug therapies. Hence, drug targeting to the brain, particularly to the diseased cells by circumventing the physiological barriers is essential to develop a promising therapy for the treatment of brain disorders. Presently, the investigations emphasize the role of different nanocarrier systems or surface modified target specific novel carrier system to improve the efficiency and reduce the side effects of the brain therapeutics. Such approaches supposed to circumvent the BBB or have the ability to cross the barrier function and thus increases the drug concentration in the brain. Although the efficacy of novel carrier system depends upon various physiological factors like active efflux transport, protein corona of the brain, stability, and toxicity of the nanocarrier, physicochemical properties, patient-related factors and many more. Hence, to develop a promising carrier system, it is essential to understand the physiology of the brain and BBB and also the other associated factors. Along with this, some alternative route like direct nose-to-brain drug delivery can also offer a better means to access the brain without exposure of the BBB. In this review, we have discussed the role of various physiological barriers including the BBB and blood-cerebrospinal fluid barrier (BCSFB) on the drug therapy and the mechanism of drug transport across the BBB. Further, we discussed different novel strategies for brain targeting of drug including, polymeric nanoparticles, lipidic nanoparticles, inorganic nanoparticles, liposomes, nanogels, nanoemulsions, dendrimers, quantum dots, etc. along with the intranasal drug delivery to the brain. We have also illustrated various factors affecting the drug targeting efficiency of the developed novel carrier system.
Keywords: brain, drug targeting, nanocarrier, BBB, liposome, intranasal drug delivery
Introduction
The brain is one of the most complex and vital organs covers around 2.0% of the total body weight (1.2–1.4 kg).1 It is the control center of the nervous system which receives the signals from the sensory organs and regulates the respective response through motor neurons. It regulates almost all the body activities like muscular movement, physiological secretion from the glands, hormone secretion, controlling the body temperature, breathing, physical growth, and many more. It senses the environment and surrounding stimulus; make decisions; process, controls and integrate all the information; develop thoughts, feelings, and plans; and store the memories of events throughout the life.2 The Brain is shielded by various physiological barriers like blood–brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB) which regulates the entry of foreign particles to the brain, protect it from the toxins and other harmful stimuli and maintain the homeostasis of the brain.3
Any variation in the structure and function of the brain and its barriers functions due to physical changes, environmental factors, toxins, infection, mutation, aging, etc. may result in various neurological disorders. In the present scenario, the treatment of brain disorders is a foremost challenge in front of medical science as we still not have any promising therapy to treat the brain disorders by circumventing the BBB.4 Therefore, researchers focused on the development of novel strategies like nanoparticles, inclusion complex, liposome, dendrimers, colloidal carriers, etc. to target the drug to the brain.5 Such carrier system specifically the nanocarriers possess smaller particle size, amphiphilic behavior, high drug loading, controlled and sustained release, protect the drug from the surrounding environments, reduces the dosing frequency, undesirable effects and offers a patient-friendly dosage form. On the other hand, poor drug loading ability (with some nanoparticles), poor stability, dose dumping, unpredictable interaction between excipients or with body components, accumulation due to smaller size and surface charge, sometimes unexpected release behavior, higher toxicity, etc. limits its applicability.6 Also, the difference in the preclinical and clinical responses, lack of uniform strategies for the development and sometimes unpredictable physiological responses of the human body. This is the reason why very few nanocarrier based formulations are approved for commercial use till date.7 In this review, we summarized barriers to brain drug delivery, drug transport mechanism; different novel approaches used for drug targeting to the brain and factors affecting brain targeting.
The barriers to a brain targeting
The Human brain is protected from any external stimuli, pathogens, toxins, foreign materials and separated from the peripheral system via various physiological barriers. These barriers maintain the homeostasis of the brain and regulate the passage of essential nutrients, ions, proteins, and metabolites inside and outside the brain.8 The main physiological barriers of the brain are discussed below.
BBB
Paul Ehrlich firstly discovered the BBB in the year 1885 during his study. It is the essential barrier structure which separates the brain from the general circulation.9 The BBB protects the brain from a noxious stimulus, toxins, infectious particles and maintains the homeostasis of the brain.3 It consists of different types of cells like brain capillary endothelial cells (BCECs), astrocytes, pericytes, and nerve cells. BCECs are the primary component of BBB which is responsible for selective permeability to small lipophilic molecules. These cells are joint together by a tight junction which further prevents the paracellular drug transport across the BBB. The tight junction is also responsible for the high transendothelial electrical resistance (TEER) between the brain and blood and thus restricts the passive diffusion of external compounds.10,11 The pericytes and astrocytes support the BCECs and assists to maintain the structure and function of BBB.4 The BBB is a highly selective semipermeable membrane which allows the entry of only small, low molecular weight, non-polar compounds (<400 Da) to the brain.12 Although the BBB is decorated with some special transport proteins, receptors or other mechanisms like efflux transporters, ion mediated channel, etc. to facilitate the passage of many essential components and metabolites to the brain.13 A better understanding of the physiology of BBB and nature and function of transport mechanisms assist the development of a promising carrier system in delivering the drug to the brain.
BCSFB
It is a barrier between the blood circulation and cerebrospinal fluid (CSF), prevents the entry of drug, toxin, microbes or any material to the CSF. It is composed of arachnoidal and choroid epithelial cells which separate the subarachnoidal CSF and ventricular CSF respectively, from the systemic circulation. The primary component of BCSFB is choroid plexus, made of choroidal epithelial cells.14 The choroid plexus act as a physical, immunological and enzymatic barrier which assists the drug transport, metabolism and signaling functions. The epithelial cells at the choroid plexus are joint together via gap-junction which limits the permeability of the BSCFB. The gap junctions are less stiff than the tight junctions and hence more permeable for the drug and other substances.15
Transport across BBB
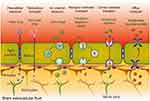
The BBB is a highly selective semipermeable membrane, supposed to restrict the entry of almost all the drugs, toxins and other foreign molecules to the brain. Although, there are some provisions to assist the transport of essential components to the brain. The polar or hydrophilic compounds can cross the BBB via a paracellular pathway through a normal diffusion process. While, the small lipophilic substances like alcohol, steroids, etc. are transported through the transcellular path. Along with this, some active transport mechanisms like carrier-mediated transport, receptor-mediated transport, adsorptive transcytosis, and efflux transport can also assist the drug permeation through the BBB.13,16 Different transport mechanism across the BBB is graphically explained in Figure 1.
Passive diffusion
Passive diffusion is the ubiquitous transport mechanism by which most of the essential nutrients including amino acid (AA), neurotransmitters, hormones, etc. and small lipophilic drugs entered into the brain from the systemic circulation. Passive diffusion involves the transfer of drug and endogenous molecules from the blood to the brain under a concentration gradient. It depends on the size and physicochemical properties of the drug.17 The drug initially dissolved into the lipid bilayer of the brain microcapillary endothelial cell and then releases inside the brain. This mean of transport is suitable only for lipophilic, small size, low molecular weight, neutral compound to cross the BBB.18
Receptor-mediated transport (RMT)
It is also known as clathrin-dependent endocytosis which is highly specific and involves the internalization of the ligand-receptor complex in the endocytic vesicle. It is a kind of active transport or energy mediated drug transport mechanism. Once a particular ligand entered into the blood circulation, it binds explicitly with the specific receptor. Then the ligand-receptor complex entered into the endothelial cytoplasm through receptor-mediated endocytosis, and finally, the exocytosis releases the ligand bound compound to the abluminal side.18 The ligand could be a natural or artificial compound mostly an antibody or peptide which have ability to specifically interact with their particular receptors at BBB. Transferrin receptor, insulin receptor, LRP1 (low-density lipoprotein receptor 1) and LRP2 (low-density lipoprotein receptor 2), etc. are most abundant receptor proteins present at BCECs surface and assist the transfer of their specific ligand or ligand bound carrier/drug.19–21
Adsorptive mediated transcytosis (AMT)
Adsorptive mediated transcytosis is an active transport mechanism in which the polycationic ligand (mostly protein and peptides) electrostatically binds with the micro-anionic moieties at the luminal surface of the brain endothelial cells. The electrostatic interaction between the positively charged ligand (like albumin, bovine serum albumin, etc.) and the negatively charged glycoprotein at brain endothelial cells, is the primary principle of AMT. Various transporters involved in AMT to the brain are GLUT1 (Glucose transporter 1), MCT1 (monocarboxylate transporter 1), EAAT (excitatory amino acid transporter), LAT1 (L-amino acid transporter 1), organic cations and cationic AA (amino acid). Melphalan, a nitrogen mustard alkylating agent, crosses the BBB via LAT1 transporter and chlorambucil via GLUT1 transporter.3,18
Carrier-mediated transcytosis
The transport of essential endogenous substances like vitamin, AA, glucose and some neuropeptides to the brain is mediated by some specific carrier molecules present at the BBB. Such transportation phenomenon is known as carrier-mediated transport. This transport mechanism can also be used for drug targeting to the brain. The drug molecules are chemically modified so that it resembles the endogenous compounds and can be quickly picked up by the carrier molecules and further transported to the brain by crossing the cellular barrier. For example, dopamine, an anti-Parkinsonian drug is converted into levodopa, which is carried out by the neutral AA transporter and get easily entered into the brain.22
Cell-mediated transport
Macrophages, neutrophils, monocytes, etc. are an essential part of defense mechanism or inflammatory responses which get activated during physiological dysfunctioning. These cells have a tendency to accumulate at the injured site in order to protect the injuries and kill the infection. This phenomenon can also be used to deliver the bioactives to a particular site in the brain. They act as “Trojan Horses” and assist the drug transport across the BBB.23,24 Among the monocytes, macrophages, and neutrophils, the monocytes offer a better carrier to transport bioactive materials. Tong et al (2016) investigated the ability of monocytes to carry superparamagnetic iron oxide nanoparticle to the inflamed area of the brain. The study shows good permeation behavior.25,26 Some other similar studies also demonstrated the application of monocytes for drug cargo to the brain. Although, cell-mediated transport has major disadvantages like early release of the freight, failure to reach the particular site of action and poor drug loading ability.27
Efflux transport
Efflux transport is an active transport or energy-dependent transport mechanism at BBB, responsible for the outward movement of various metabolites, toxic substances, neurotransmitters and antibiotics.28 Efflux transporter confines the exposure of brain tissue to many therapeutic agents even though from the lipophilic smaller molecules which possess high permeability to the BCECs.29 It comprises of many surface proteins present at cell surface or BBB which are known as efflux transporters. The ABC transporter or ATP-binding cassette transporter is recognized as the protein superfamilies present in almost all the living organisms.30 The P-glycoprotein (P-gp) is the key element of ABC efflux transporter which actively regulates the outflux of many lipophilic drugs across the BBB.31 It is membrane-bound protein, greatly expressed over the luminal membrane of BCECs while in a lesser extent on the brain parenchyma, nerve cells, and glial cells. It is considered as the most important ABC efflux transporter in the brain or CNS.32 The efflux transporter including P-gp and other ABC-transporters prevents the accumulation of drug and toxins into the brain and expel out the drugs from the CNS. It affects the pharmacokinetic efficacy of many drugs including anti-cancer agents, and other CNS acting molecules.33 Hence, some approaches focus on evading the efflux transporter or utilizes the P-gp inhibitor in order to improve the therapeutic effect of the drug. In addition, it also modifies the drug distribution in the brain.34 Thus, the proper understanding of the physiological function of efflux transporter is essential for enhanced drug delivery to the brain.35
Novel strategies for brain targeting
Various novel strategies adapted for effective brain targeting of the bioactives including different nanocarrier system and intranasal drug delivery are discussed below:
Nanocarrier system for brain targeting
The nano-sized particles ranging from 1 to 100 nm facilitates the drug transport across the BBB due to the smaller size and surface functionalization with the target-specific ligands.36–38 An average human body cell is approximately 10–100 µm in size.39 Thus, the nano-sized materials can easily be absorbed by the cells and deliver the drug inside.40 On the basis of the preparation method, component used, drug loading and release behavior different types of nanocarriers are available. Among these, some of the important nanocarriers are discussed below and shown in Figure 2.
Liposome
Liposomes are small lipophilic vesicles, primarily consists of one or more concentric phospholipid bilayer or sometimes made with the cholesterol or combination of different natural lipids (phosphatidylcholine, egg yolk phosphatidylcholine, soy lecithin, soybean phosphatidylcholine, etc.) or synthetic lipid (dipalmitoylphosphatidylcholine, etc.) with an aqueous core.41–43 The basic properties of the liposomes like fluidity, rigidity, size and surface behavior may vary with the selection of lipid component and method of preparation. It was first discovered by a British scientist Alec Bangham in 1961. Initially, it was called as “Banghasomes” or “multilamellar sematic mesophase.” The term “Liposome” was given by Weissmann, after the lysosome, because of their resemblance.44 The size of the liposome may range from 30 nm to 100 µm. Depending upon the size and number of concentric lipid bilayer the liposome can be divided into (1) Multilamellar vesicle, (2) Oligo lamellar vesicle and (3) Unilamellar vesicle (Large and small unilamellar).45 The liposome can entrap both the hydrophilic and lipophilic compounds in the aqueous core and lipid bilayer, respectively. Because of this unique ability, it is extensively used as a carrier system for a wide variety of drugs.45,46
Liposome offers site-specific delivery, protects the drug from enzymatic degradation, reduce the adverse effect and represents a biodegradable and biocompatible delivery system which increases both the research and commercial interest for novel drug delivery.47–49 On the other hand, the low encapsulation efficiency and poor stability limit its applicability to some extent.50 Various investigations, both in the laboratory and commercial scale are under process, utilizes liposome as a potential carrier system for direct nose-to-brain delivery of the drug. Sometimes, liposomes are surface modified with the specific ligand or carrier molecules like polyethylene glycol, monoclonal antibodies, transferrin, lactoferrin, glutathione, etc. to assist the drug targeting to the brain.51 For example; H102 peptide-loaded liposome was developed by Zheng and co-workers 2015 for intranasal delivery to the brain. The brain targeting efficacy of liposome was assessed by estimating the in vivo pharmacokinetic behavior of the drug carrier system.52 Similarly, Asmari et al in 2016, developed donepezil loaded liposomes and investigated its brain targeting efficiency by comparing the drug concentration in both the rat brain and plasma.53 In addition, the surface modified liposome shows higher brain targeting potency. Xiao et al (2019) synthesized ascorbic acid thiamine disulfide modified liposome with “lock-in” function to achieve effective brain targeting of docetaxel via GLUT1 and sodium ion-dependent vit. C transporter. The study shows 3.24 times higher brain uptake of the drug.54 In another study, Li et al (2019) utilize liposome and polymeric nanoparticles for combined delivery of atorvastatin and curcumin to treat atherosclerosis.55 Subsequently, Kuo et al developed phosphatidic acid, cardiolipin and TAT (transactivator of transcription) peptide modified liposome to target the nerve growth factor, rosmarinic acid, quercetin and curcumin to the hippocampal nerve region in order to treat tau-hyperphosphorylation based neurodegeneration. The TAT peptide improves the BBB permeability while the phosphatidic acid and cardiolipin assist the activity against tau hyperphosphorylation.56 Such studies demonstrated the applicability of liposome for targeting the drug to the brain. Though, all the investigations are performed on animal models, including rat, rodent, and rabbit, etc. The clinical investigations on human brain/human volunteer are still limited due to lack of desired success rate in preliminary stages and availability of well-established experimental protocol as well as resources.
Polymeric nanoparticle
These are nano-sized particles, range from 1 to 100 nm, surrounding an interfacial layer composed of some ionic, organic and inorganic molecules.57–61 Based on the structure and drug entrapment behavior, the nanoparticles are mainly of two types; (1) nanosphere and (2) nanocapsule. Nanospheres consist of a polymeric matrix in which the drug molecules are entrapped while the nanocapsule comprises of a void or inner core surrounded by a polymeric shell and the drug is encapsulated into the central void.37,62 The nanoparticles intended for brain targeting, composed of biodegradable polymers like polylactic acid, polylactic-co-glycolic acid,63 chitosan,64 polycaprolactone, polyacrylamide, poly(lysine) and poly (alkyl cyanoacrylate).65,66 The size, composition, and structure of nanoparticle significantly affect the transport mechanism of the nanoparticle to the brain via the nasal route. Such as, polysorbate coated nanoparticle enters into the brain via the capillary wall through the low-density lipoprotein (LDL) receptor.67 Owing to the smaller size, it can easily penetrate the cellular layers and target the drug to the specific site.68,69 The PLA coated nanoparticle shows enhanced drug transport to the brain via the intranasal route. Polysorbate-80 coated nanoparticle facilitates the receptor-mediated endocytosis by selectively binds with plasma proteins like apolipoprotein E (ApoE) and Apolipoprotein B (ApoB).70–72 Similarly, the cationic protein (like albumin and chitosan) modified nanoparticle also covalently bounds to the ApoE and access the brain through absorption mediated transcytosis. Similarly, various ligands are used for targeting the drug-loaded nanocarrier to the brain. Together with the drug targeting ability nanoparticle also promote the drug permeation through nasal mucosa, increases the brain bioavailability of the drug, protects the drug enzymatic degradation and increases the drug retention time in the nasal cavity (chitosan nanoparticle).73,74 Kuo et al (2019) targeted the carmustine, etoposide and doxorubicin to the glioblastoma cells of the human brain by encapsulating the drug into folic acid and wheat germ agglutinin conjugated methoxy PEG-PCL nanoparticle. The wheat germ agglutinin and folic acid assist the targeting efficiency of the system while mPEG and PCL control the drug release behavior.75 Likewise, Li et al (2018) also used the PEG-PLGA nanoparticle for brain targeting of shikonin to treat glioma. To improve the targeting efficiency, the nanoparticle is decorated with lactoferrin at the surface. The lactoferrin modified PEG-PLGA nanoparticle demonstrated higher drug targeting to the brain.76 Further, chitosan coated nanoparticle can also be used as a promising carrier system for brain drug delivery. Recently, Fernandes et al (2018) worked on the development of AA conjugated chitosan nanoparticle for effective targeting of a dipeptidyl peptidase-4 enzyme inhibitor, saxagliptin. The study indicated 3.42 folds higher AUC in the brain than the plain drug.77 In addition, Rukmangathen et al (2018) applied chitosan nanoparticle to improve the therapeutic efficiency of selegiline, a potent anti-Parkinson’s agent. It also shows a similar result, ie, the higher pharmacokinetic profile of the drug in the brain.78 All these reports assure the potency of polymeric nanoparticles for brain targeting of many drug compounds.
Inorganic nanoparticles
These are the nanoparticles made of different inorganic materials like metal, metal oxides, silica, carbon, etc., which are commonly used for the diagnostic purpose.79 The inorganic nanoparticles are uniform sized, stable nanoparticles which form a monodisperse suspension in the body fluid and can be surface functionalized to promote the brain targeting. The mesoporous silica nanoparticle offers greater surface area, high pore volume, excellent biocompatibility and ease of surface functionalization. Ku et al (2010) developed fluorescein modified magnetic silica nanoparticles and covalently conjugated it with the second generation polyamidoamine (PAMAM) dendrimer through 3-(triethoxysilyl) propyl isocyanate (ICP) to produce PAMAM-fluorescein-magnetic silica nanoparticle (PFMSN). Further, this PFMSN was treated with (methoxy polyethylene glycol)-5000 (mPEG) to get PEGylated PFMSN. The study shows that the PEGylated PFMSN has the ability to cross the BBB via transcytosis, diffused into the cerebral parenchyma and distributed throughout the neurons. While the non-PEGylated nanoparticles can not cross the BBB.80 On a similar note, other studies also demonstrated that the PEGylation contributed to the BBB permeability of different inorganic nanoparticles like silica nanoparticles, carbon nanotubes, etc.81 Similarly, Ren and coauthors (2012) also demonstrated the role of PEG functionalization on the brain targeting of the oxidized multiwalled carbon nanotube. The oxidized multiwalled carbon nanotube was surface modified with PEG and angipep-2. This dual targeting nanoparticulate system initially crossed the BBB and then interact with the tumor cells.82 In other studies, the efficiencies of some more targeting molecules were studied, and it was found that the lactoferrin more prominently improves the BBB permeation of nanocarrier than the PEG coating.83 Also, iron oxide nanoparticles, gold nanoparticles, mesoporous silica nanoparticle, and some other inorganic nanoparticles are proved as a promising carrier to deliver the bioactives across the BBB.84 Interestingly, Tominaka et al combine the gold nanoparticle and magnetic nanoparticle to develop a multi-modal imaging probe as a powerful diagnostic agent for brain disorders. They used the magnetic nanoparticle as core material while the gold nanoparticle as the outer shell and established their theranostic application.85 Additionally, Zhao et al (2019) utilized modified gold nanoparticle as novel SPECT (Single Photon Emission Computed Tomography) imaging tool and radionuclides therapy of glioma. They synthesized polyethyleneimine loaded chlorotoxin peptide and PEG-modified gold nanoparticle. The results illustrated that it serves as a potential diagnostic and therapeutic tool for glioma.86 On other hand, Fahmy et al targeted the thymoquinone to the brain by encapsulating in the mesoporous silica nanoparticle and observed its antioxidant potency. They have studied the in vivo efficiency of thymoquinone loaded silica nanoparticle and brain distribution pattern. The study demonstrated selective targeting and better distribution of drug when loaded in mesoporous silica nanoparticles.87 However, the brain targeting ability of inorganic nanoparticles is hindered by its toxicity, poor drug release profile and non-biodegradability.88
Nanogel
The nanogels are primarily the nanosized hydrogel or can be defined as chemically or physically crosslinked 3 Dimensional network of polymers which swells in water or aqueous fluid.89,90 The higher water content of the hydrogel imparts excellent biocompatibility and facilitates the drug diffusion (both the drug loading and release) from the swollen network of the polymer.91,92 These properties make the nanogel a potential candidate for brain drug targeting. Studies reported that the cationic nanogel promotes the internalization of nanocarrier in the cells as compared to the neutral nanogels. Chen et al (2017) developed doxorubicin loaded pH-responsive PVA nanogel to target the human glioblastoma or tumor cells. The nanogel consist of disulfide and surface modified with cycloRGD-peptide. The study shows, the drug-carrier system was inactive in normal physiological condition and releases the drug to the tumor site due to change in pH; hence, it effectively targets the drug. The surface modified nanogel also lessens the associated adverse effect.93 In the same way, Warren et al (2015) designed biodegradable amphiphilic cationic nanogel as a carrier system for brain delivery of triphosphorylated nucleoside reverse transcriptase inhibitor to treat HIV. The nanogel was made of cholesterol-ε-polylysine and supposed to reduce the unwanted side peripheral side effect and neurotoxicity of the bioactive.94 Earlier in 2012, Azadi et al prepared and optimized surface modified methotrexate loaded nanogel for the treatment of brain tumor. The nanogel consist of sodium tripolyphosphate and chitosan and surface modified with polysorbate 80 which impart site specificity. Although the study was only limited to the in vitro examination, no further studies are performed.95 Seok et al (2008) prepared β-cyclodextrin and poly(β-aminoester) based polysaccharide nanoparticles for brain targeting of doxorubicin and insulin. This cationic nanogel significantly improves the BBB permeability of insulin when tested on the in vitro BBB model.96,97 The studies elucidated that nanogel could represent a potential carrier system for brain targeting of bioactives. However, during a literature survey, we found that in comparison to the other novel carriers nanogel is less explored for brain delivery.
Nanoemulsion
The nanoemulsions are referred to as nanosized, heterogeneous dispersion of water-in-oil or oil-in-water stabilized through a suitable emulsifier.98 The nanoemulsions are suitable for the delivery of both the hydrophilic and lipophilic drugs. The surface functionalization with suitable ligand facilitates the permeation of nanoemulsion via RMT. The nanoemulsions are commonly made of vegetable or animal oils like peanut oil, flaxseed oil, sunflower oil, fish oil, hemp oil, wheat germ oil, egg phosphatidylcholine, etc. which makes it highly biocompatible with the biological membranes. Also, the size of nanoemulsion is very small, <200 nm which makes it a promising carrier system for brain targeting of drugs.99 Although, the stability issues limit its application.89 In recent scientific investigations, nanoemulsions are frequently used for direct nose-to-brain delivery of drugs and secondly it is intended for parenteral route. In 2018, Shobo et al formulated pretomanid loaded nanoemulsion for intranasal administration to enhance the brain permeation of the drug.100 Likewise, Ahmad and coworkers, developed quercetin loaded mucoadhesive intranasal nanoemulsion to treat cerebral ischemia and studied the targeting efficiency as well as the therapeutic efficiency using the rat model.101 Further, the same group of authors investigated the competence of nanoemulsion for brain targeting of therapeutics. They prepared safranal, an anti-oxidant, loaded mucoadhesive nanoemulsion for intranasal administration to treat cerebral ischemia.102 In addition, Abdou et al (2017) encapsulated the zolmitriptan, an anti-migraine agent, to the mucoadhesive nanoemulsion and delivered it via intranasal route. They assessed the brain targeting efficiency of nanoemulsion and observed the mucoadhesive intranasal nanoemulsion significantly enhances the drug permeability, AUC and bioavailability in the brain.103 On the other hand, Dordevic et al (2015), developed risperidone loaded lecithine nanoemulsion for drug delivery to the brain via parenteral route. The nanoemulsion made of sodium oleate (aqueous phase), lecithin (lipid phase), polysorbate 80 (emulsifier) and poloxamer 188 (co-emulsifier). The polysorbate 80 based nanoemulsion increased the brain bioavailability up to 7.4 folds than the other formulations.104 Similarly, Tan et al (2015) also used parenteral nanoemulsion for brain targeting of carbamazepine to treat seizure and evaluated its pharmacokinetic efficiency. The study shows higher pharmacokinetic profile and lower side effects of the drug when delivered as nanoemulsion than the free drug solution which assures the potency of carrier system.105 From the available research work, it can be concluded that mucoadhesive nanoemulsion mostly intended for parenteral and intranasal administration to the brain was extensively studied and found as promising carrier system which reduces the associated adverse effect, improves the therapeutic potency and offers a non-invasive patient-friendly technique to access the brain.106
Dendrimers
Dendrimers are highly branched, monodispersed, symmetric polymeric macromolecules with some reactive groups on the surface.107,108 The dendrimer is a 3 Dimensional shaped spheroidal carrier system, composed of repetitively branched molecules. The core is suitable for drug loading while the surface with a number of reactive ends allows the multifunctionality and closely packed periferi to improve the drug loading ability. The nanosized dendrimers represent an attractive drug carrier system for brain targeting.109,110 Presently, studies focused on the development of surface modified dendrimer with the BBB or tumor cell specific ligand to improve its brain targeting efficiency.111 Lu et al (2018) synthesized arsenic trioxide loaded RGD-PEG-modified PAMAM for targeting brain glioblastoma cells. The surface modification with PEG minimized the cytotoxicity to BCECs as compared to the unmodified PAMAM dendrimer. The carrier system prolonged the drug release and considerably increased the pharmacokinetic profile and therapeutic efficiency of the drug.112 In the same sequence, Gothwal et al (2018) developed rivastigmine loaded lactoferrin modified PAMAM dendrimer to treat the neurodegenerative disorder and boost the memory function of the brain. The drug-loaded lactoferrin modified dendrimer demonstrated 9.8 fold lower cytotoxicity and 8 folds higher brain uptake thus improved the bioavailability of the drug. It also shows significant improvement in locomotor activity and memory of the rat brain.113 Li and coworkers (2012) developed a fourth generation, transferrin modified PAMAM dendrimers for brain delivery of tamoxifen. The study demonstrated considerably higher drug loading and enhanced BBB permeation.114 The studies confirm the proficiencies of dendrimer for brain targeting of drugs.
Quantum dots
Quantum dots are colloidal nanocrystalline semiconductor materials, consists of metalloid crystal core and nonreactive metallic shell which covers the crystalline core.115,116 The long-term photostability, high brightness, size-tunable narrow emission spectra make it a promising diagnostic tool.117 It also offers a great surface area and can encapsulate a wide variety of therapeutic and diagnostic agents. Thus, it can also be used as a promising carrier system for brain targeting. The bioactive agents can be loaded into the core of the quantum dots while the surface can be functionalized with the targeting ligands to facilitates the brain targeting.118 However, just like inorganic nanoparticles the higher toxicity profile, non-biodegradability, and poor drug release profile limit its application.89 Qiao et al (2018) explored the application of D-glucose and L-aspartic acid based carbon dots as a diagnostic and therapeutic tool to identify and target brain tumor. The study suggested the unique design and properties of carbon dots offers a potential theranostic tool.119 Tang et al (2017) constructed a novel PEGylated quantum dot nanoprobe conjugated with aptamer 32 for fluorescent imaging of brain tumor. It possesses the ability to specifically binds with the glioma cells and thus could be used as a promising tool for diagnosis, investigation and surgical intervention of brain tumor.120 Similarly, Yang et al (2017) also used quantum dots as a promising diagnostic tool. They Cd-Se-ZnS quantum dots, incorporated into pH-triggered polymeric micelle and used as fluorescent imaging nanoprobe to distinguish cerebral ischemia affected region in the brain.121 Thus, it can be said that the quantum dots displayed astonishing potency as a diagnostic agent for brain disorders.
Nose-to-brain delivery system
In the past few years, the intranasal route appears as an alternative and effective approach for drug delivery to the brain. It is claimed to deliver the drug directly to the brain without entering into the systemic circulation.122,123 The nasal cavity is divided into three different regions, (1) vestibular region, (2) respiratory region and (3) olfactory region. The drug or any dosage form instilled into the nasal cavity can primarily absorb through the respiratory area and enters into the systemic circulation. This region also consists of some trigeminal neurons; hence some of the drugs reached directly to the brain via trigeminal nerves. Also, the drug reached/or instilled in the posterior region, ie, the olfactory region, enter directly to the brain via olfactory and trigeminal neurons.122,124 The drug entered into the systemic circulation further needs to cross the BBB while the drug entered through intraneural pathway follows cellular transport mechanism. From the olfactory region, the drug primarily enters into the olfactory bulb via trigeminal and olfactory neurons, followed by absorption into lamina propria and then entered in the CSF. It further, reaches to different brain region from the CSF.9,125 On the other hand, the intranasal route also has some limitations which reduce its efficiency. Firstly, the volume of the nasal cavity is very small which only allows a lower volume of drug to be instilled. Secondly, the shorter drug retention time again reduces the amount of drug available for absorption into the brain or systemic circulation. Further, mucociliary clearance and enzymatic degradation also reduce bioavailability.122 Thus, various novel drug delivery strategies are under investigation which coverup such limitations and improves the efficiency of the intranasal route. Nigam et al (2019) utilized PLGA nanoparticle for direct nose-to-brain delivery of lamotrigine. The study shows the intranasal PLGA-nanoparticle significantly improved the pharmacokinetic behavior of the drug and also the brain targeting efficiency.126 Similarly, Musumeci et al (2018) formulated oxcarbazepine loaded PLGA nanoparticle to treat epileptic seizure and deliver it through the intranasal route. The strategy demonstrated improved brain targeting and higher drug concentration of drug in the brain.127 Similarly, Chu et al utilized surface modified PLGA nanoparticle for intranasal administration of temozolomide to treat a malignant brain tumor. The nanocarrier was functionalized with a tyrosine kinase antibody, EPHA3 (ephrin type-A receptor 3) which effectively target the drug-loaded nanocarrier to the glioblastoma in the brain, The results show 1.3 fold prolonged release and significantly higher brain concentration.128 However, all the studies are performed on the animal models, ie, still in the preclinical stage, which suggested the need of a lot of further clinical studies on human volunteers to establish its applicability in real patients.
Factors considered during the design of the brain targeting system
The drug targeting efficiency of the nanocarrier is affected by various physical, chemical properties of the carrier system and physiological responses of the human body. The factors affecting the targeting ability of nanocarriers are discussed below
Neurotoxicity of the nanoparticle
The human brain is highly protected from the acquaintance of toxins, infectious substances or any other harmful foreign materials by the BBB.129 The novel brain targeting strategies not only increase the drug concentration in the brain but also improve the risk of neurotoxicity by raising the exposure of the normal brain to the nanoparticles and other chemicals. The BBB targeting system broadly and unselectively improves the concentration of nanoparticles and drugs throughout the brain which may lead to severe side effects. Thus, the study of neurotoxicity gains significant attention in recent years. In this aspect, the dual brain targeting which directs the drug to the particular diseased region of the brain may be helpful.9
Premature drug release
The novel drug delivery system especially the dual-targeting system, claims to target the drugs to the diseased cells of the brain and improves the therapeutic efficacy of the drug. Such systems are modified with two targeting ligands or one ligand and an active molecule with targeting efficiency like curcumin, which has ability to particularly target the amyloid plaque. In the majority of cases, one of the ligands assists the transport across the BBB while others target the particular disease site in the brain. Along with higher therapeutic efficacy,41,130 it may also be disseminated to the other normal tissues of the body and results in unwanted side effect. In addition, some of the drugs get released into the systemic circulation during the normal circulatory period. All these factors reduce the final concentration of drug into the target site. Hence, an ideal targeted system should hold the drug inside the carrier until it reaches the desired site and releases it quickly once reaches to the specific region. In this sequence, the pH-triggered system, enzyme responsive system, dual targeted system, use of cross-linkers, etc. offers effective drug targeting.131
Homogeneity of the delivery system
The brain targeting efficacy of the delivery system depends on various properties of the drug carrier system including particle size, size distribution, surface behavior, ligand density, nature of ligand, lipophilicity or hydrophilicity of the nanocarrier, surface charge, etc. Hence, proper optimization of the nanocarrier is essential. The lack of homogeneity may reduce the targeting efficiency of the system. For example; particle size is a very important parameter for brain drug delivery as the BBB only allows the permeation of smaller size particles. It also affects the in vivo distribution of the nanocarrier system. Similarly, the surface charge also plays an important role in drug permeation across the BBB. Thus, an effective brain targeted system should need to maintain the homogeneity of the system.132
The effect of the protein corona
A protein corona is referred to as a layer of protein adsorbed or bound to the surface of nanoparticle in the biological system. Depending upon the time and physiological conditions, the protein gets exchanged with other serum proteins. Once the nanocarriers are entered into the systemic circulation, a protein corona is formed around by serum protein.133 It delayed the circulation time and distribution of the nanoparticles and affected the drug release pattern.134 Sometimes, it also hinders the targeting ability of the system by covering the targeting ligand and thus, prevents the interaction between the ligand and target receptor. For example; the targeting ability or reaction between Tf and Tf-receptor was mired if kept in a culture medium containing the serum protein.135 Some other studies show similar results on the effect of protein corona. Regrettably, most of the studies for brain targeting of the drug does not concern the effect of protein corona on the targeting efficiency of the formulation.
Off-target potency of the delivery system
The basic concept of drug targeting is the interaction of the ligand with their specific receptor or transporter molecules. These carriers or receptors are supposed expressed over the diseased cells or BBB. But in reality, most of these are also present on the normal cells of the body which distract the targeting ligand and reduces the targeting ability of the nanocarriers. Moreover, it also results in the adverse effect of the drug.136 For example, the Tf receptors are overexpressed on the BBB and the tumor cell surface but, it is also present on the normal brain cells. If the Tf-modified nanoparticles are administered, it binds with the Tf-receptors on the BBB, normal brain cells and tumor cells. Further, releases the drug to the normal cells also and produce undesirable side effects.137
Conclusion and prospects
Drug delivery to the brain by evading the BBB and development of a potential therapy with reduced peripheral toxicity is a foremost challenge for the scientist working in neurology. In the past few decades, enormous work has been done in this aspect, and the targeted drug delivery system appears as a promising approach. The research scientists utilize different transporters, receptors, adsorption mediated transcytosis, cell-mediated endocytosis, active efflux pump, etc. for the transport of drugs across the physiological barriers. Different novel drug carrier system primarily the nanoparticulate carriers like liposome, nanoparticles, dendrimers, nanoemulsions, nanogels, quantum dots, etc. found useful in brain targeting. Also, the direct nose-to-brain drug delivery appears as a potential and alternative approach for effective brain drug delivery. Despite many successful investigations, still, not one of these strategies are commercialized to date which may be due to the clinical data fails to recapitulate the preclinical success rate. The efficacy of the developed novel targeted systems is hindered by various physiological factors, the toxicity profile of the system, drug loading, and drug release behavior, stability, and biocompatibility of the system.
Acknowledgments
The authors want to acknowledge Rungta College of Pharmaceutical Sciences and Research, India for providing the necessary facilities for the compilation of the work. We also want to show our gratitude to Patel College of Pharmacy, India for providing the necessary literature. The authors want to extend the gratitude to the Deanship of Scientific Research and their helpful team of employees at Helwan University, Cairo, Egypt for their support.
Author contributions
Conceptualization, Dr. Amit Alexander; Literature collection, Dr. Ajazuddin; Writing, Mukta Agrawal; Formal analysis, Dr. Ahmed M Shehata and Dr. Mahmoud A Shaker; Supervision, Dr. Amit Alexander and Dr. Syed Ata Ur Rahman; Review and Editing, Dr. Sabahuddin Siddique and Dr. Amit Alexander; and Funding acquisition, Dr. Mohi Iqbal M Abdul and Dr. Mohamed A. Shaker. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Parent AC. M.B. Carpenter’s Human Neuroanatomy. Michigan: Williams & Wilkins; 1995.
2. Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62(8):847–855. doi:10.1016/j.biopsych.2007.03.001
3. Patel MM, Patel BM. Crossing the blood-brain barrier: recent advances in drug delivery to the brain. CNS Drugs. 2017;31(2):109–133. doi:10.1007/s40263-016-0405-9
4. Nagpal K, Singh SK, Mishra DN. Drug targeting to brain: a systematic approach to study the factors, parameters and approaches for prediction of permeability of drugs across BBB. Expert Opin Drug Deliv. 2013;10(7):927–955. doi:10.1517/17425247.2013.762354
5. Lu CT, Zhao YZ, Wong HL, Cai J, Peng L, Tian XQ. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int J Nanomedicine. 2014;9(1):2241–2257. doi:10.2147/IJN.S61288
6. Odiba A, Ottah V, Ottah C, et al. Therapeutic nanomedicine surmounts the limitations of pharmacotherapy. Open Med. 2017;12:271–287. doi:10.1515/med-2017-0041
7. Agrahari V, Agrahari V, Mitra AK. Nanocarrier fabrication and macromolecule drug delivery: challenges and opportunities. Ther Deliv. 2016;7(4):257–278.
8. Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discovery. 2016;15(4):275–292.
9. Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharmaceutica Sinica B. 2016;6(4):268–286.
10. Reeve A, Simcox E, Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor?. Ageing Res Rev. 2014;14:19–30. doi:10.1016/j.arr.2014.01.004
11. Neuwelt E, Abbott NJ, Abrey L, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7(1):84–96. doi:10.1016/S1474-4422(07)70326-5
12. Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3(2):90–105, 151. doi:10.1124/mi.3.2.90
13. Chen Y, Liu L. Modern methods for delivery of drugs across the blood–brain barrier. Adv Drug Deliv Rev. 2012;64(7):640–665. doi:10.1016/j.addr.2011.11.010
14. Pardridge WM. Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS. 2011;8(1):7. doi:10.1186/2045-8118-8-7
15. de Boer AG, Gaillard PJ. Drug targeting to the brain. Annu Rev Pharmacol Toxicol. 2007;47:323–355. doi:10.1146/annurev.pharmtox.47.120505.105237
16. Terasaki T, Tsuji A. Drug delivery to the brain utilizing blood-brain barrier transport systems. J Controlled Release. 1994;29(1):163–169. doi:10.1016/0168-3659(94)90132-5
17. Fischer H, Gottschlich R, Seelig A. Blood-brain barrier permeation: molecular parameters governing passive diffusion. J Membr Biol. 1998;165(3):201–211.
18. Garg T, Bhandari S, Rath G, Goyal AK. Current strategies for targeted delivery of bio-active drug molecules in the treatment of brain tumor. J Drug Target. 2015;23(10):865–887. doi:10.3109/1061186X.2015.1029930
19. Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res. 2007;24(9):1759–1771. doi:10.1007/s11095-007-9379-0
20. Wang YY, Lui PC, Li JY. Receptor-mediated therapeutic transport across the blood-brain barrier. Immunotherapy. 2009;1(6):983–993. doi:10.2217/imt.09.75
21. Lajoie JM, Shusta EV. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu Rev Pharmacol Toxicol. 2015;55:613–631. doi:10.1146/annurev-pharmtox-010814-124852
22. Mena I, Cotzias GC. Protein intake and treatment of Parkinson’s disease with levodopa. N Engl J Med. 1975;292(4):181–184. doi:10.1056/NEJM197501232920404
23. Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18(9):998–1000. doi:10.1096/fj.04-1517fje
24. Priller J, Flugel A, Wehner T, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7(12):1356–1361. doi:10.1038/nm1201-1356
25. Tong HI, Kang W, Davy PM, et al. Monocyte Trafficking, Engraftment, and Delivery of Nanoparticles and an Exogenous Gene into the Acutely Inflamed Brain Tissue - Evaluations on Monocyte-Based Delivery System for the Central Nervous System. PLoS One. 2016;11(4):e0154022. doi:10.1371/journal.pone.0154022
26. Arora W. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–3471. doi:10.2147/IJN.S30320
27. Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert Opin Drug Deliv. 2011;8(4):415–433. doi:10.1517/17425247.2011.559457
28. Bay DC, Turner RJ. Small Multidrug Resistance Efflux Pumps. In: Li X-Z, Elkins CA, Zgurskaya HI, editors. Efflux-Mediated Antimicrobial Resistance in Bacteria: Mechanisms, Regulation and Clinical Implications. Cham: Springer International Publishing; 2016:45–71.
29. Golden PL, Pollack GM. Blood-brain barrier efflux transport. J Pharm Sci. 2003;92(9):1739–1753. doi:10.1002/jps.10424
30. Qosa H, Miller DS, Pasinelli P, Trotti D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015;1628(Pt B):298–316. doi:10.1016/j.brainres.2015.07.005
31. Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2(1):86–98. doi:10.1602/neurorx.2.1.86
32. Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60(2):196–209. doi:10.1124/pr.107.07109
33. Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol Med Today. 1996;2(3):106–113.
34. Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97(7):3473–3478. doi:10.1073/pnas.050585397
35. Demeule M, Regina A, Jodoin J, et al. Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascul Pharmacol. 2002;38(6):339–348.
36. Alexander A, Saraf S, Saraf S, et al. Amalgamation of stem cells with nanotechnology: A unique therapeutic approach. Curr Stem Cell Res Ther. 2018;14(2):83–92.
37. Agrawal M, Saraf S, Saraf S, et al. Recent advancements in the field of nanotechnology for the delivery of anti-Alzheimer drug in the brain region. Expert Opin Drug Deliv. 2018;15(6):589–617. doi:10.1080/17425247.2018.1471058
38. Khare S, Ajazuddin AA, Amit. N. Biomedical applications of nanobiotechnology for drug design, delivery and diagnostics. Res J Pharm Technol. 2014;7(8):915–925.
39. Jane B. Reece, Martha R. Taylor, Eric J. Simon, Jean L. Dickey.Campbell Biology—Concepts and Connections. North Carolina: Pearson Education; 2009.
40. Alexander A, Saraf S, Saraf S. A comparative study of chitosan and poloxamer based thermosensitive hydrogel for the delivery of PEGylated melphalan conjugates. Drug Dev Ind Pharm. 2015;41(12):1954–1961. doi:10.3109/03639045.2015.1011167
41. Agrawal M, Tripathi DK, Saraf S, et al. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J Controlled Release. 2017;260:61–77. doi:10.1016/j.jconrel.2017.05.019
42. Pedro Ramos-Cabrer FC. Liposomes and nanotechnology in drug development: focus on neurological targets. Int J Nanomedicine. 2012;8(1):951–960.
43. Salade L, Wauthoz N, Deleu M, et al. Development of coated liposomes loaded with ghrelin for nose-to-brain delivery for the treatment of cachexia. Int J Nanomedicine. 2017;13:8531–8543.
44. Szebeni J, Baranyi L, Savay S, et al. Role of complement activation in hypersensitivity reactions to doxil and hynic PEG liposomes: experimental and clinical studies. J Liposome Res. 2002;12(1–2):165–172. doi:10.1081/LPR-120004790
45. Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. doi:10.1186/1556-276X-8-102
46. Débora B, Vieira LFG. Getting into the brain: liposome-based strategies for effective drug delivery across the blood–brain barrier. Int J Nanomedicine. 2016;11:5381–5414. doi:10.2147/IJN.S117210
47. Hofheinz RD, Gnad-Vogt SU, Beyer U, Hochhaus A. Liposomal encapsulated anti-cancer drugs. Anticancer Drugs. 2005;16(7):691–707.
48. Barbara Kneidl MP, Winter G, Lindner LH, Hossann M. Thermosensitive liposomal drug delivery systems: state of the art review. Int J Nanomedicine. 2014;9(1):4387–4398. doi:10.2147/IJN.S49297
49. Callum Ross MT, Fullwood N, Allsop D. Liposome delivery systems for the treatment of Alzheimer’s disease. Int J Nanomedicine. 2018;13:8507–8522. doi:10.2147/IJN.S183117
50. Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. doi:10.2147/IJN.S68861
51. Ross C, Taylor M, Fullwood N, Allsop D. Liposome delivery systems for the treatment of Alzheimer’s disease. Int J Nanomedicine. 2018;13:8507–8522. doi:10.2147/IJN.S183117
52. Zheng X, Shao X, Zhang C, et al. Intranasal H102 peptide-loaded liposomes for brain delivery to treat Alzheimer’s disease. Pharm Res. 2015;32(12):3837–3849. doi:10.1007/s11095-015-1744-9
53. Al Asmari AK, Ullah Z, Tariq M, Fatani A. Preparation, characterization, and in vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des Devel Ther. 2016;10:205–215. doi:10.2147/DDDT.S93937
54. Xiao W, Fu Q, Zhao Y, et al. Ascorbic acid-modified brain-specific liposomes drug delivery system with “lock-in” function. Chem Phys Lipids. 2019. doi:10.1016/j.chemphyslip.2019.01.005
55. Li X, Xiao H, Lin C, et al. Synergistic effects of liposomes encapsulating atorvastatin calcium and curcumin and targeting dysfunctional endothelial cells in reducing atherosclerosis. Int J Nanomedicine. 2019;14:649–665. doi:10.2147/IJN.S189819
56. Kuo YC, Chen CL, Rajesh R. Optimized liposomes with transactivator of transcription peptide and anti-apoptotic drugs to target hippocampal neurons and prevent tau-hyperphosphorylated neurodegeneration. Acta Biomater. 2019;87:207–222. doi:10.1016/j.actbio.2019.01.065
57. Batista CA, Larson RG, Kotov NA. Nonadditivity of nanoparticle interactions. Science (New York, NY). 2015;350(6257):1242477. doi:10.1126/science.1242477
58. Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (London, England). 2016;11(6):673–692. doi:10.2217/nnm.16.5
59. Schmalz G, Hickel R, van Landuyt KL, Reichl FX. Nanoparticles in dentistry. Dental Mater. 2017;33(11):1298–1314. doi:10.1016/j.dental.2017.08.193
60. Dwivedi GR, Singh DP, Sharma A, Darokar MP, Srivastava SK. Nano particles: emerging warheads against bacterial superbugs. Curr Top Med Chem. 2016;16(18):1963–1975.
61. Abrahamse H, Kruger CA, Kadanyo S, Mishra A. Nanoparticles for advanced photodynamic therapy of cancer. Photomed Laser Surg. 2017;35(11):581–588.
62. Portioli C, Bovi M, Benati D, et al. Novel functionalization strategies of polymeric nanoparticles as carriers for brain medications. J Biomed Mater Res Part A. 2017;105(3):847–858.
63. Alexander A, Ajazuddin KJ, Saraf S, Saraf S. Poly(ethylene glycol)-poly(lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications. J Controlled Release. 2013;172(3):715–729.
64. Alexander A, Ajazuddin KJ, Saraf S, Saraf S. Formulation and evaluation of chitosan-based long-acting injectable hydrogel for PEGylated melphalan conjugate. J Pharm Pharmacol. 2014;66(9):1240–1250.
65. Alexander A, Ajazuddin PRJ, Saraf S, Saraf S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J Controlled Release. 2016;241:110–124.
66. Maria João Gomes J, Bruno S. Nanoparticle-based drug delivery to improve the efficacy of antiretroviral therapy in the central nervous system. Int J Nanomedicine. 2014;9(1):1757–1769.
67. Song H, Wei M, Zhang N, et al. Enhanced permeability of blood–brain barrier and targeting function of brain via borneol-modified chemically solid lipid nanoparticle. Int J Nanomedicine. 2018;13:1869–1879.
68. Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Controlled Release. 2004;100(1):5–28.
69. Alexander A, Ajazuddin M, Swarna M, Sharma M, Tripathi D. Polymers and permeation enhancers: specialized components of mucoadhesives. Stamford J Pharm Sci. 2011;4(1):91–95.
70. Gelperina S, Maksimenko O, Khalansky A, et al. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: influence of the formulation parameters. Eur J Pharm Biopharm. 2010;74(2):157–163.
71. Alexander A, Dwivedi S, Giri TK, et al. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Controlled Release. 2012;164(1):26–40.
72. Bruno Fonseca-Santos MPDG, Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomedicine. 2015;10(1):4981–5003.
73. Khan AR, Liu M, Khan MW, Zhai G. Progress in brain targeting drug delivery system by nasal route. J Controlled Release. 2017;268:364–389.
74. Ramanathan S, Archunan G, Sivakumar M, et al. Theranostic applications of nanoparticles in neurodegenerative disorders. Int J Nanomedicine. 2018;13:5561–5576.
75. Kuo YC, Chang YH, Rajesh R. Targeted delivery of etoposide, carmustine and doxorubicin to human glioblastoma cells using methoxy poly(ethylene glycol)poly(epsiloncaprolactone) nanoparticles conjugated with wheat germ agglutinin and folic acid. Mater Sci Eng C Mater Biol Appl. 2019;96:114–128.
76. Li H, Tong Y, Bai L, et al. Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int J Biol Macromol. 2018;107(Pt A):204–211.
77. Fernandes J, Ghate MV, Basu Mallik S, Lewis SA. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int J Pharm. 2018;547(1–2):563–571.
78. Rukmangathen R, Yallamalli IM, Yalavarthi PR. Biopharmaceutical potential of selegiline loaded chitosan nanoparticles in the management of Parkinson’s disease. Curr Drug Discov Technol. Epub April 18, 2018. doi:10.2174/1570163815666180418144019
79. Yang H. Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm Res. 2010;27(9):1759–1771.
80. Ku S, Yan F, Wang Y, Sun Y, Yang N, Ye L. The blood-brain barrier penetration and distribution of PEGylated fluorescein-doped magnetic silica nanoparticles in rat brain. Biochem Biophys Res Commun. 2010;394(4):871–876.
81. Teleanu DM, Chircov C, Grumezescu AM, Volceanov A. Blood-brain delivery methods using nanotechnology. Pharmaceutics. 2018;10(4):269.
82. Ren J, Shen S, Wang D, et al. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33(11):3324–3333.
83. Qiao R, Jia Q, Huwel S, et al. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano. 2012;6(4):3304–3310.
84. Frigell J, Garcia I, Gomez-Vallejo V, Llop J, Penades S. 68Ga-labeled gold glyconanoparticles for exploring blood-brain barrier permeability: preparation, biodistribution studies, and improved brain uptake via neuropeptide conjugation. J Am Chem Soc. 2014;136(1):449–457.
85. Tomitaka A, Ota S, Nishimoto K, Arami H, Takemura Y, Nair M. Dynamic magnetic characterization and magnetic particle imaging enhancement of magnetic-gold core-shell nanoparticles. Nanoscale. 2019;11:6489–6496.
86. Zhao L, Li Y, Zhu J, et al. Chlorotoxin peptide-functionalized polyethylenimine-entrapped gold nanoparticles for glioma SPECT/CT imaging and radionuclide therapy. J Nanobiotechnology. 2019;17(1):30.
87. Fahmy HM, Fathy MM, Abd-Elbadia RA, Elshemey WM. Targeting of Thymoquinone-loaded mesoporous silica nanoparticles to different brain areas: in vivo study. Life Sci. 2019;222:94–102.
88. Yang Z, Zhang Y, Yang Y, et al. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomedicine. 2010;6(3):427–441.
89. Li X, Tsibouklis J, Weng T, et al. Nano carriers for drug transport across the blood-brain barrier. J Drug Target. 2017;25(1):17–28.
90. Alexander A, Ajazuddin KJ, Saraf S, Saraf S. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur J Pharm Biopharm. 2014;88(3):575–585.
91. Li XCY. Study on synthesis and chloramphenicol release of poly (2-hydroxyethylmethacrylate-co-acrylamide) hydrogels. Chin J Chem Eng. 2008;16:640–645.
92. Weng T, Guo J, Li X, et al. Synthesis, chloramphenicol uptake, and in vitro release of poly(AMPS–TEA-Co-AAm) gels with affinity for both water and alcohols. Int J Polym Mater Polym Biomater. 2014;63(2):73–79.
93. Chen W, Zou Y, Zhong Z, Haag R. Cyclo(RGD)-decorated reduction-responsive nanogels mediate targeted chemotherapy of integrin overexpressing human glioblastoma in vivo. Small. 2017;13(6):1–9.
94. Warren G, Makarov E, Lu Y, et al. Amphiphilic cationic nanogels as brain-targeted carriers for activated nucleoside reverse transcriptase inhibitors. J J Neuroimmune Pharmacol. 2015;10(1):88–101.
95. Azadi A, Hamidi M, Khoshayand MR, Amini M, Rouini MR. Preparation and optimization of surface-treated methotrexate-loaded nanogels intended for brain delivery. Carbohydr Polym. 2012;90(1):462–471.
96. Seok GELL. Invention of polysaccharide-based nanoparticles for enhancing drug permeability across the blood brain barrier. NSTI-Nanotech. 2008;2:379–381.
97. Li X, Cui Y. Study on synthesis and chloramphenicol release of poly(2-hydroxyethylmethacrylate-co-acrylamide) Hydrogels. Chin J Chem Eng. 2008;16(4):640–645.
98. Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6(3):928–939.
99. Ganta S, Deshpande D, Korde A, Amiji M. A review of multifunctional nanoemulsion systems to overcome oral and CNS drug delivery barriers. Mol Membr Biol. 2010;27(7):260–273.
100. Shobo A, Pamreddy A, Kruger HG, et al. Enhanced brain penetration of pretomanid by intranasal administration of an oil-in-water nanoemulsion. Nanomedicine (London, England). 2018;13(9):997–1008. doi:10.2217/nnm-2017-0365
101. Ahmad N, Ahmad R, Naqvi AA, et al. Intranasal delivery of quercetin-loaded mucoadhesive nanoemulsion for treatment of cerebral ischaemia. Artif Cells Nanomed Biotechnol. 2018;46(4):717–729. doi:10.1080/21691401.2017.1337024
102. Ahmad N, Ahmad R, Abbas Naqvi A, et al. The effect of safranal loaded mucoadhesive nanoemulsion on oxidative stress markers in cerebral ischemia. Artif Cells Nanomed Biotechnol. 2017;45(4):775–787. doi:10.1080/21691401.2016.1228659
103. Abdou EM, Kandil SM, Miniawy H. Brain targeting efficiency of antimigrain drug loaded mucoadhesive intranasal nanoemulsion. Int J Pharm. 2017;529(1–2):667–677. doi:10.1016/j.ijpharm.2017.07.030
104. Dordevic SM, Cekic ND, Savic MM, et al. Parenteral nanoemulsions as promising carriers for brain delivery of risperidone: design, characterization and in vivo pharmacokinetic evaluation. Int J Pharm. 2015;493(1–2):40–54. doi:10.1016/j.ijpharm.2015.07.007
105. Tan SL, Stanslas J, Basri M, et al. Nanoemulsion-based parenteral drug delivery system of carbamazepine: preparation, characterization, stability evaluation and blood-brain pharmacokinetics. Curr Drug Deliv. 2015;12(6):795–804.
106. Bonferoni MC, Rossi S. Nanoemulsions for “Nose-to-Brain” Drug Delivery. Pharmaceutics. 2019;11(2):84–101.
107. Yan H, Wang J, Yi P, et al. Imaging brain tumor by dendrimer-based optical/paramagnetic nanoprobe across the blood-brain barrier. Chem Commun (Cambridge, England). 2011;47(28):8130–8132. doi:10.1039/c1cc12007g
108. He H, Li Y, Jia XR, et al. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials. 2011;32(2):478–487. doi:10.1016/j.biomaterials.2010.09.002
109. Dhanikula RS, Argaw A, Bouchard JF, Hildgen P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: enhanced efficacy and intratumoral transport capability. Mol Pharm. 2008;5(1):105–116. doi:10.1021/mp700086j
110. Verma C, Janghel A, Deo S, et al. Comprehensive advancement on nanomedicinesalong with its various biomedical applications. Res J Pharm Technol. 2015;8(7):945. doi:10.5958/0974-360X.2015.00159.6
111. Lu Y, Han S, Zheng H, et al. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int J Nanomedicine. 2018;13:5937–5952. doi:10.2147/IJN.S175418
112. Lu Y, Han S, Zheng H, et al. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int J Nanomedicine. 2018;13:5937–5952. doi:10.2147/IJN.S175418
113. Gothwal A, Nakhate KT, Alexander A, Ajazuddin GU. Boosted memory and improved brain bioavailability of rivastigmine: targeting effort to the brain using covalently tethered lower generation PAMAM dendrimers with lactoferrin. Mol Pharm. 2018;15(10):4538–4549. doi:10.1021/acs.molpharmaceut.8b00537
114. Li Y, He H, Jia X, Lu WL, Lou J, Wei Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials. 2012;33(15):3899–3908. doi:10.1016/j.biomaterials.2012.02.004
115. Ghaderi S, Ramesh B, Seifalian AM. Fluorescence nanoparticles “quantum dots” as drug delivery system and their toxicity: a review. J Drug Target. 2011;19(7):475–486. doi:10.3109/1061186X.2010.526227
116. Shafq Al-Azzawi DM, Guildford AL, Phillips G, Santin M. Dendrimeric poly(Epsilon-Lysine) delivery systems for the enhanced permeability of flurbiprofen across the blood-brain barrier in Alzheimer’s disease. Int J Mol Sci. 2018;19(10):3224. doi:10.3390/ijms19103224
117. Gao J, Chen K, Xie R, et al. In vivo tumor-targeted fluorescence imaging using near-infrared non-cadmium quantum dots. Bioconjug Chem. 2010;21(4):604–609. doi:10.1021/bc900323v
118. Gao X, Chen J, Chen J, Wu B, Chen H, Jiang X. Quantum dots bearing lectin-functionalized nanoparticles as a platform for in vivo brain imaging. Bioconjug Chem. 2008;19(11):2189–2195. doi:10.1021/bc8002698
119. Qiao L, Sun T, Zheng X, Zheng M, Xie Z. Exploring the optimal ratio of d-glucose/l-aspartic acid for targeting carbon dots toward brain tumor cells. Mater Sci Eng C Mater Biol Appl. 2018;85:1–6. doi:10.1016/j.msec.2017.12.011
120. Tang J, Huang N, Zhang X, et al. Aptamer-conjugated PEGylated quantum dots targeting epidermal growth factor receptor variant III for fluorescence imaging of glioma. Int J Nanomedicine. 2017;12:3899–3911. doi:10.2147/IJN.S133166
121. Yang HY, Fu Y, Jang MS, et al. CdSe@ZnS/ZnS quantum dots loaded in polymeric micelles as a pH-triggerable targeting fluorescence imaging probe for detecting cerebral ischemic area. Colloids Surf B Biointerfaces. 2017;155:497–506. doi:10.1016/j.colsurfb.2017.04.054
122. Agrawal M, Saraf S, Saraf S, et al. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Controlled Release. 2018;281:139–177. doi:10.1016/j.jconrel.2018.05.011
123. Alam S, Khan ZI, Mustafa G, et al. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study. Int J Nanomedicine. 2012;7:5705–5718. doi:10.2147/IJN.S35329
124. Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi:10.1016/j.lfs.2017.12.025
125. Alexander A, Saraf S. Nose-to-brain drug delivery approach: A key to easily accessing the brain for the treatment of Alzheimer’s disease. Neural Regener Res. 2018;13(12):2102. doi:10.4103/1673-5374.233432
126. Nigam K, Kaur A, Tyagi A, et al. Nose-to-brain delivery of lamotrigine-loaded PLGA nanoparticles. Drug Deliv Transl Res. 2019. doi:10.1007/s13346-019-00622-5
127. Musumeci T, Serapide MF, Pellitteri R, et al. Oxcarbazepine free or loaded PLGA nanoparticles as effective intranasal approach to control epileptic seizures in rodents. Eur J Pharm Biopharm. 2018;133:309–320. doi:10.1016/j.ejpb.2018.11.002
128. Chu L, Wang A, Ni L, et al. Nose-to-brain delivery of temozolomide-loaded PLGA nanoparticles functionalized with anti-EPHA3 for glioblastoma targeting. Drug Deliv. 2018;25(1):1634–1641.
129. Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev. 2012;64(7):686–700. doi:10.1016/j.addr.2011.10.007
130. Kuo YC, Chou PR. Neuroprotection against degeneration of sk-N-mc cells using neuron growth factor-encapsulated liposomes with surface cereport and transferrin. J Pharm Sci. 2014;103(8):2484–2497. doi:10.1002/jps.24081
131. Su Z, Xing L, Chen Y, et al. Lactoferrin-modified poly(ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas. Mol Pharm. 2014;11(6):1823–1834. doi:10.1021/mp500238m
132. Karim R, Palazzo C, Evrard B, Piel G. Nanocarriers for the treatment of glioblastoma multiforme: current state-of-the-art. J Controlled Release. 2016;227:23–37. doi:10.1016/j.jconrel.2016.02.026
133. Nel AE, Madler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–557. doi:10.1038/nmat2442
134. Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428–437. doi:10.1016/j.addr.2009.03.009
135. Salvati A, Pitek AS, Monopoli MP, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8(2):137–143. doi:10.1038/nnano.2012.237
136. Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31(10):1111–1137. doi:10.1016/S1357-2725(99)00070-9
137. Xie R, Dong L, Du Y, et al. In vivo metabolic labeling of sialoglycans in the mouse brain by using a liposome-assisted bioorthogonal reporter strategy. Proc Natl Acad Sci U S A. 2016;113(19):5173–5178. doi:10.1073/pnas.1516524113
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.