Back to Journals » OncoTargets and Therapy » Volume 13
Recent Advances of Magnetic Nanomaterials in the Field of Oncology
Authors Li T, Yang C , Wei Z, Pei D, Jiang G
Received 21 December 2019
Accepted for publication 1 May 2020
Published 28 May 2020 Volume 2020:13 Pages 4825—4832
DOI https://doi.org/10.2147/OTT.S243256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Tianyang Li,1,* Chunsheng Yang,2,* Zhiping Wei,1 Dongsheng Pei,1 Guan Jiang1
1Department of Dermatology, Affiliated Hospital of Xuzhou Medical University, Xuzhou 221002, People’s Republic of China; 2Department of Dermatology, The Affiliated Huai’an Hospital of Xuzhou Medical University, The Second People’s Hospital of Huai’an, Huai’an 223002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guan Jiang Email [email protected]
Abstract: Nanomagnetic devices, such as nano-field effect transistor biosensors and radio frequency magnetic induction therapies, came into being with the development of medical nanomaterials. The application of nanomagnetic materials in the treatment of cancers is rapidly becoming increasingly important because of its ability to target therapy and diagnose early. In this review, an untechnical overview of the fundamental of magnetism in nanomaterials and an illustration of how these materials are applied are presented. The applications of nano-field effect transistor biosensors for the detection of tumor biomarker nanomaterials in the therapy and diagnosis of cancers and nanomagnetic materials are summarized in this paper. A systemic summary of the use of nanomagnetic materials and nano-filed effect transistor biosensors for the treatment and diagnosis of tumors is also provided in the review.
Keywords: nanomagnetic materials, nano-field effect transistor, magnetic nanorobot, oncology
Introduction
In recent years, the morbidity and mortality of tumors show an evident upward trend. Cancer has become the leading cause of death in China. According to statistics, approximately 75% of patients with malignant tumors need physical therapy at different stages of their treatment. Physical therapy not only cures early malignant tumors but also relieves late pains and symptoms and improves the quality of life of patients.1 Hyperthermia is a common method of tumor physiotherapy. With the development of medical nanomaterials, nanomagnetic and radio frequency (RF) magnetic induction therapies have emerged. Intratumoral in situ injection or targeted aggregation method is used to specifically distribute nano magnetocaloric or RF heating nanomaterials to tumor tissues and add alternation.2 Through magnetic or RF field, nanomaterials produce tropical tumor tissues to accelerate tumor hyperthermia.3 Magnetic robots that can break through blood flow resistance and penetrate into the depth of lesion by an external magnetic field have been recently developed to send drug-carrying nanoparticles. These robots are useful in delivering therapeutic drugs into target tissues.4 In addition, nano-field effect transistor biosensors are based on charge changes and can detect trace biological markers associated with the early stage of tumors. Nano-field effect transistor biosensors has been widely used in nucleic acids, proteins, and other medical testing because of its high sensitivity and selectivity, high analysis speed, markup absence, miniaturization, and easy operation. Under the technical conditions of the normal restriction of heat source and nano drug, the normal tissue structure and the heat dissipation performance are good.5 Increased blood flow and other physiological reactions occur in normal tissues as blood vessels dilate when the body is under hot conditions, and the increased blood flow can take away the heat to maintain normal tissue temperature.6 Therefore, normal tissues cannot heat up in the alternating magnetic or RF field.
Application of Magnetic Nanomaterials in Tumor-Targeted Heat Therapy
With the development of medical nanomaterials, the emergence of nanomagnetic induction and RF magnetic induction therapies uses intratumoral injection or targeted aggregation method to distribute nanomagnetic heat or RF heating nanomaterials to tumor tissues. The role of the additional metamorphosis of magnetic or RF field is in the nanomaterial heat-producing tumor tissue, precisely heating the tumor through heat therapy. Under the technical conditions of a normal organization using the dual restriction of heat source and nanodrug, coupled with the normal organization structure, perfect cooling performance is good.7 Therefore, normal tissues cannot heat up in the intersection albeit magnetic or RF field. When the tumor tissue containing nanothermal particles located in the intersectional magnetic or RF field heats up, the automatic tracking of the motion target area and the realization of 4D tumor-appropriate heat therapy can be achieved.8,9 The nanosurface is modified to enhance CT or MRI image, allow ingress to the position of nanoparticles in real time, and carry chemotherapy drugs that can be automatically released when heat is encountered in tumor areas. At the same time, precise chemotherapy is achieved.10
Among the many thermal therapy technologies (e.g., microwave, RF, and ultrasound), magnetic induction heat therapy based on magnetic nanomaterials is a new treatment developed in recent years. After entering the tumor tissue by using a magnetic medium through an additional cross-change magnetic field, a fever is sensed due to the Neel Glow and Brownian Glow effects, bringing the tumor tissue to a certain temperature (42 °C or higher) and inducing tumor apoptosis. A high temperature can increase the synthesis of shock protein and stimulate the formation of active immunity to achieve the effect of treating malignant tumors. Given the various advantages of magnetic induction heat therapy, such as unique target, minimally invasive, non-toxic side effects, apparent efficacy, the therapy gradually attracts the attention of local and international researchers.
At present, only a few subject groups at home and abroad have done relevant research on animal models in targeting magnetic induction thermal therapy induced by magnetic nanomaterials (Figure 1). Gherardi et al11 increased its magnetic properties by assembling magnetic iron oxide nanoparticles into magnetic nanoclusters measuring from 60 nm to 100 nm and further synthesizing folic acid molecules on their surfaces, making them the target. By injecting this magnetic nanocluster (48 smol /kg through tail veins) into the mice, Baker et al12 actively targeted the tumor site and placed it inmagnetic field after a concentration of 24 h (frequency = 230 kHz, power = 2.4 kW). The results revealed that after the cross-change in magnetic induction heat therapy, the surface temperature of the mouse tumor became 6 °C higher than that of other sites. After many attempts of heat therapy, the growth of tumors in mice was evidently inhibited. Ji et al13 injected PEG-based magnetic nanoparticles into the tail veins of mice, causing the researchers to target cumulative concentrations in the tumor of approximately 1.9 mg Fe/g. After a two-minute continuous process at high frequency and strong magnetic field (980 kHz, 38 kA/m), the surface temperature of the mouse tumor increased to 60 °C and caused heat ablation in the tumor tissue, causing the tumor to subside. Ji et al13 prepared a single-packet, PEG-coated fusion of magnetic manganese-zinc ferrite nanocrystals with nuclear shell structure. They repeatedly gave a certain dose of this magnetic nanocrystal to the mouse tail through intravenous injection. After blood circulation, magnetic particles passively targeted the concentration of mouse tumor tissue. Through the multiple heat therapy of the tumor body, the surface of the tumor tissue could reach approximately 43 °C, thus effectively inhibiting the growth of the tumor.
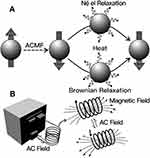 |
Figure 1 Diagrams of magnetic nanoparticles (A) Magnetic nanoparticles are heated under alternating magnetic field (ACMF); (B) ACMF installation. Reprinted with permission from Yoo D,Lee JH,Shin TH,Cheon J. Theranostic magnetic nanoparticles [J]. Acc. Chem. Res. 44(10):863–874. Copyright (2011) American Chemical Society.48 |
However, limited to the current technology, targeted magnetic induction thermal therapy still faces great difficulties, such as the comprehensive performance of magnetic nanomaterials is not ideal, tumor-target accumulation efficiency is low, and tumor area is difficult to achieve through effective temperature treatment. To enhance this kind of targeted magnetic induction heat therapy and make it widely used in biomedical and medical clinical fields, the performance of magnetic nanomaterials must be improved. The high performance of magnetic nanomaterials generally includes high magnetism, high magnetic thermal effect, biocompatibility, accurate tumor-targeting ability, and long-cycle capability of in-vivo transportation. Therefore, the methods of the controlled preparation and surface modification of high-performance magnetic nanostructures must be designed, developed, and optimized.14 The selection criteria of magnetic nanomaterials for thermal therapy, such as high performance, good biocompatibility, and active target magnetic nanomaterials, remain as key factors. Their appearance can efficiently achieve high heat production and reduce drug dose, thus decreasing the toxic side effects on the body and reducing the retention in the liver, spleen, and other organs. The targeting of magnetothermal therapy, achievement of intracellular hyperthermia, even heating of the tumor, and thorough and effective killing of tumor cells must be improved.15
Bulgarian researchers used magnetic materials to transmit heat to the tumor. They found that the specific absorption rate of tumor cells into destructive heat depends on the diameter and composition of nanoparticles.16 Traditional cancer treatments, such as pancreatic, brain, and liver tumors, are treated with chemotherapy, radiation, and surgery; but the survival rate is low. The researchers used an interlude magnetic field to activate magnetic nanoparticles, which are closely delivered to tumor cells. Heat therapy is effective if nanoparticles are well absorbed by tumor cells, rather than by cells in healthy tissues. The researchers also combined nanometer hydroxyapatite with magnetic nanometer iron tetroxide particles to produce a new spherical composite material, which can warm up to 45 °C in a short time under an external magnetic field. Therefore, the magnetothermal effect of this material can be used to treat tumors. Xu17 combined calcium phosphate bone cement with nano-iron tetroxide to prepare a magnetic calcium phosphate bone cement. The researchers injected the material into the mouse model of breast cancer under the guidance of ultrasound. The maximum temperature on the tumor surface could reach approximately 75 °C under the effect of an external magnetic field. The tumor on the body surface of the nude mice almost disappeared after 15 days. The effectiveness of reforestation depends on the specific absorption rate.18 The researchers studied several nanoparticles, such as iron oxide made of ferrite, with added small amounts of copper, nickel, manganese, and cobalt atoms.19 Magnetic heat therapy based on these particles, including mouse and cell cultures, uses two different heating methods.20 These methods are directly or indirectly coupled with the magnetic moments of magnetic particles through the magnetic field in terms of how they generate heat.21 Tumor absorption turns out to be largely dependent on the diameter of the nanoparticles only if the material is horizontally added high enough and the diameter does not exceed the set maximum size (cobalt adds up to 14 nm, copper 16 nm). Subsequently, absorption rate increases with the particle size. Nanoparticles that can be used in location and targeted therapy for tumors are summarized in Table 1.
 |
Table 1 Nanomagnetic Materials Used in Tumor Location and Treatment |
Magnetic Nanorobots Can Transport Nanodrugs Deep into Tumor Tissues
The international scientific team at the Massachusetts Institute of Technology has designed a miniature magnetic robot that can break through blood flow resistance and send drug-carrying nanoparticles to the depths of tumors or other lesions. Nanoparticle drugs possess many benefits in the treatment of diseases, such as tumors. However, barriers such as vulnerability to blood flow and difficulty in deep tissue, exist.
A recent study published in the American Journal of Scientific Progress shows that the 3D-printed robot is about the same size as cells with a structure similar to bacterial whiplash that drives the robot forward.22 The surface of the robot is coated with a layer of nickel-titanium alloy, which can be controlled by an external magnetic field to penetrate the lesion.
Researchers have designed a microfluidic system that simulates the vascular environment around the tumor. The artificial flagella of the robot begins to rotate into a 200 micron wide simulated vascular channel that carries fluid through the pores when an external magnetic field is applied it. Thus, the combined sizes of 200 nanopolystyrene particles are pushed into the target tissue at almost twice the depth of the immersion tissue, which is almost twice as deep as the robot without the help of a magnetic field.23
The team has also enabled the direct use of magnetic bacteria present in nature instead of magnetic robots to deliver anti-cancer drugs. The bacteria, which produce iron oxide, can quickly push nanoparticles to the target tissue when a rotating magnetic field is applied in a specific direction.24 The researchers explain that the nanoparticles used in the study are large enough to carry heavy loads, such as the “gene scissors” system CRISPR. Subsequently, animal experiments have been conducted.
Application of Nano-Field Effect Transistors in the Detection of Tumors
The rapid development of nanomaterial preparation technology can be observed in recent years. Many high-performance nanomaterials are used in medical testing, such as silicon nanowires, graphene, and disulfide. The continuous convergence of physics, chemistry, and biology has led to the continuous development of new biosensors based on different principles, such as fluorescent biosensors based on fluorescent changes, surface plasma resonance sensors based on mass changes, and nano-field effect transistor biosensors based on charge changes. These biosensors play an important role in bedside disease detection. Among them, nano-field effect transistor biosensor has been widely used in nucleic acids, proteins, and other medical testing because of its high sensitivity and selectivity, high analysis speed, no markup, miniaturization, and easy operation. These advantages are especially suitable for the early diagnosis of tumors.24 High sensitivity and selectivity can enable the sensor to detect trace markers associated with the early stage of tumors. Through analysis, miniaturization, and simple operation, the rapid detection of tumors can easily be achieved at an early stage.25
Structure and Working Principle of Nano-Field Effect Transistors
Nano-field effect transistor biosensor structure includes the source leakage bipolar and the conductive material between the poles. These conductive materials are silicon nanowire, graphene, disulfide, and other nanomaterials. The substrate material and silver chloride act as gates in nanomaterials for modifying various biological capture molecules to detect the target biomolecules. The detection mechanism of nano-field effect transistor biosensors mainly includes charge-doping and electrostatic effects.26 When a charged biomolecule is close to the surface of a nanomaterial, the biomolecule affects the charge density of the nanomaterial. The conductivity of the nano-field effect transistor or the offset of the Dirac dot (lowest conductivity) is also affected.27 In addition, the biomolecule seqr is detected by change softening electrical signals (Figure 2).
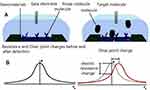 |
Figure 2 Map of the nanomaterial field-effect transistor (A) Structure of the nanomaterial field-effect transistor; (B) The Change of the function of the nanomaterial field-effect transistor before and after detection. Reprinted with permission from Stine R,Mulvaney SP,Robinson JT,Tamanaha CR,Sheehan PE. Fabrication, optimization, and use of graphene field effect sensors[J]. Anal Chem. 85(2):509–521. Copyright (2013) American Chemical Society.49 |
Application of Nano Field-Effect Transistors for the Identification of Tumor Maker
The early detection and treatment of tumors provide important help. However, problems regarding the early detection of tumors in nano field-effect transistors are present.28
- The detection of actual samples remains poor, and additional tests are conducted in a buffer solution.
- The limited functionalization of the surface of nanomaterials limits sensor sensitivity and specificity.
- The performance of homogeneity among nano field-effect transistors is difficult to guarantee.
To solve these problems, researchers must explore other nanomaterial functional methods and field-effect transistors to prepare large-scale cheap preparation methods. With the efforts of researchers, nano field-effect transistors play important roles in the early detection of tumors and in other medical testing fields.29 The application of field-effect transistor biosensors based on silicon nanowire, graphene,30 and molybdenum disulfide to tumor-related protein tumor markers is introduced. The superior electrical properties and large-scale and inexpensive preparation of nanomaterials provide great advantages for the construction of high-sensitive, selective, and inexpensive rapid-detection microsystems,31 especially in the early detection of tumors through nano field-effect transistor biosensors. Ultra-high sensitivity, excellent specificity,32 and anti-interference ability are important properties for the early diagnosis, early detection, and treatment of tumors.
Doughton et al33 used graphene-field-effect-tube biosensor to detect prostate specific antigen antichymotrypsin (PSA-ACT). When the PSA-ACT to be tested is added to the sensor detection area, the PSA antibody is modified on the surface of the reduced graphene. In addition, PSA-ACT can be captured by the PSA antibody. Considering that PSA-ACT has a charge, it can cause the Dirac point of the sensor transfer specific curve to shift. The higher the concentration of PSA-ACT, the faster the shift of the Dirac point. The larger the deviation, the antigen content can more likely be calculated according to the deviation of the Dirac point. The detection limit of the sensor is as low as the flying mole.34 The detection range spans six orders of magnitude. The sensor also has high sensitivity and specificity for PSA-ACT in serum samples.35 To improve the detection sensitivity of the sensor, Arriortua et al assembled nanoparticles and NP-encapsulated graphene into rGO-NPs to increase the surface area ratio and improve sensor sensitivity. Antibodies of human epidermal growth factor receptor-2 (HER2) and epidermal growth factor receptor (EGFR) were immobilized on rGO-NPs. The detection limits of HER2 and EGFR are respectively 1 pmol/L and 100 pmol/L and are highly specific.36
Badrigilan et al deposited platinum particles on the graphene surface. HER3 genetically engineered scFv on platinum particles were then modified to detect tumor marker HER3. Platinum particles can increase the body surface ratio, and the use of single-chain antibodies can solve the Debye length problem of the sensor.37 The sensor can detect 300 fg/mL HER3 at a minimum, and the detection range is 300 fg/mL–300 ng/mL, which has great advantages in bedside detection.38 Cardoso et al used G-FET to obtain the real-time detection of tumor marker CEA.39 When the concentration of the added CEA was high, the output current further changed, and CEA was quantitatively detected by the change of current.40,41
Zeng et al42 used polymethyl methacrylate as a flexible substrate and carboxylated multi-walled carbon nanotubes or reduced graphene oxide as channel materials to construct field-effect transistors. CA125 aptamers were also modified as capture probes on the conductive channel. The aptamer sensor can detect a minimum of 5.0 U/mL × 1010 U/mL CA125. The sensor has a good correlation with the results of traditional enzyme-linked immunosorbent assay and has high sensitivity. G-FET biosensor is used in the early detection of tumors because of its high electron mobility, specific surface graphene area, good sensitivity, and specificity. However, the zero band gap characteristics of graphene limit its ability to detect biomolecules. Therefore, further improvement is necessary.43
Feng et al44 employed the N-doped graphene method of adjusting the band gap called polypyrrole conversion nitrogen-doped minority graphene (PPy-NDFLG) by using polypyrrole as an N source and a chemical vapor deposition production through micro and nanofabrication. A PPy-NDFLG-FET was prepared through a process. The expression of VEGF plays an important role in tumor growth and metastasis. The authors used VEGF RNA aptamers as capture probes to modify graphene to further enhance the capture probe affinity. The use of the surface, PPy-NDFLG, and VEGF aptamers greatly improves the detection performance of the sensor in actual samples.
Conclusions and Perspectives
The advantages of nanomaterials in the treatment of oncology are that magnetic, optical, or special construction specially exist in nanoparticles. When these materials are combined with the ligands of specifically targeted tumors, including small molecular, peptides, and mAbs, these nanomaterials can target the biomarkers and vasculatures of tumors with high sensitivity and specificity. Over recent years, great progress has been made in the production of nanoprobes for the imaging of molecules, targeted nanomaterials for the treatment of cancers, and nanodevices for the early diagnosis of cancers. This review shows that nanomaterials have made great contributions to cancer diagnosis and treatment.
Moreover, microrobot devices (nanorobots) can be designed using molecular tools, which can locate and recognize cancer cells in vivo.45 Nanorobots carry a biosensor that can recognize cancer cells and release anti-cancer drugs when they encounter cancer cells, thereby inducing cell apoptosis. During the treatment with nanorobots, external devices can be used to monitor their activities in vivo. Considering that a broad spectrum of anticancer drug has not been discovered, computer programs can be used to match cancer types with the most appropriate reagents. In addition, nanorobots can be used in healthy people for early cancer diagnosis and prevention. However, certain challenges in the application of nanomaterials remain unsolved.
The nano-field effect transistor is a biosensor based on charge changes and detects biological markers associated with the early stage of tumors with high sensitivity. Nano-field effect transistor biosensor has been widely used in detecting nucleic acids, proteins, and other medical testing because of its high sensitivity and selectivity, high analysis speed, markup absence, miniaturization, and easy operation. For example, G-FET achieves the real-time detection of tumor marker CEA by quantitatively detecting the change in current.46 These data show that nano-field effect transistors can be used in the early diagnosis of various tumors.
Nanomagnetic materials, magnetic nanorobots, and nano-field effect transistors have underwent several attempts and exerted potent effects on various cancer treatments in animal models and humans clinical trials. However, the therapeutic effects of these agents need improvement. In addition, the safety of these therapeutic and diagnostic agents should be considered.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Spriggs DR, Zivanovic O. Ovarian cancer treatment- are we getting warmer? N Engl J Med. 2018;378(30):293–294. doi:10.1056/NEJMe1714556
3. Wanqing C, Kexin S, Rongshou Z, et al. Cancer incidence and mortality in China 2014. Chin J Cancer Res. 2018;30(1):1–12. doi:10.21147/j.issn.1000-9604.2018.01.01
4. Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol. 2008;26(11):1774–1777. doi:10.1200/JCO.2007.15.7438
5. Senter PD. Potent antibody drug conjugates for cancer therapy. Curr Opin Chem Biol. 2009;13(3):235–244. doi:10.1016/j.cbpa.2009.03.023
6. Betker JL, Gomez J, Anchordoquy TJ. The effects of lipoplex formulation variables on the protein corona and comparisons with in vitro transfection efficiency. J Control Release. 2013;171(3):261–268. doi:10.1016/j.jconrel.2013.07.024
7. Remaut K, Lucas B, Raemdonck K, Braeckmans K, Demeester J, De Smedt SC. Can we better understand the intracellular behavior of DNA nanoparticles by fluorescence correlation spectroscopy? J Control Release. 2007;121(1–2):49–63. doi:10.1016/j.jconrel.2007.04.008
8. Subramanian G, Hjelm RP, Deming TJ, Smith GS, Li Y, Safinya CR. Structure of complexes of cationic lipids and poly (glutamic acid) polypeptides: a pinched lamellar phase. J Am Chem Soc. 2000;122(2):26–34. doi:10.1021/ja991905j
9. Smisterova J, Wagenaar A, Stuart MC, et al. Molecular shape of the cationic lipid controls the structure of cationic lipid/dioleylphosphatidylethanolamine-DNA complexes and the efficiency of gene delivery. J Biol Chem. 2001;276(50):47615–47622. doi:10.1074/jbc.M106199200
10. Li JH, Zu XY, Liang GF, et al. Octopod PtCu nanoframe for dual-modal imaging-guided synergistic photothermal radiotherapy. Theranostics. 2018;8(4):1042–1058. doi:10.7150/thno.22557
11. Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12(2):89–103. doi:10.1038/nrc3205
12. Baker JH, Lindquist KE, Huxham LA, Kyle AH, Sy JT, Minchinton AI. Direct visualization of heterogeneous extravascular distribution of trastuzumab in human epidermal growth factor receptor type 2 overexpressing xenografts. Clin Cancer Res. 2008;14(7):2171–2179. doi:10.1158/1078-0432.CCR-07-4465
13. Ji F, Zhang K, Li J, Gu Y, Zhao J, Zhang J. A dual pH/magnetic responsive nanocarrier based on PEGylated Fe3O4 nanoparticles for doxorubicin delivery. J Nanosci Nanotechnol. 2018;18(7):4464–4470. doi:10.1166/jnn.2018.15275
14. Siontorou CG. Nanobodies as novel agents for disease diagnosis and therapy. Int J Nanomedicine. 2013;8:4215–4227. doi:10.2147/IJN.S39428
15. Lee TW. Fighting fire with fire: the revival of thermotherapy for gliomas. Anticancer Res. 2014;34(2):565–574.
16. Kamitakahara M, Ohtoshi N, Kawashita M, et al. Spherical porous hydroxyapatite granules containing composites of magnetic and hydroxyapatite nanoparticles for the hyperthermia treatment of bone tumor. J Mater Sci Mater Med. 2016;27(5):93. doi:10.1007/s10856-016-5704-7
17. Xu C, Zheng Y, Gao W, et al. Magnetic hyperthermia ablation of tumors using injectable Fe3O4/calcium phosphate cement. ACS Appl Mater Interfaces. 2015;7(25):1386–13875.
18. Guan J, Stavridi E, Leeper DB, et al. Effects of hyperthermia on p53 protein expression and activity. J Cell Physiol. 2002;190(3):365–374. doi:10.1002/jcp.10069
19. Sottile ML, Losinno AD, Fanelli MA, et al. Hyperthermia effects on Hsp27 and Hsp72 associations with mismatch repair (MMR) proteins and cisplatin toxicity in MMR-deficient/proficient colon cancer cell lines. Int J Hyperthermia. 2015;31(5):464–475. doi:10.3109/02656736.2015.1026848
20. Zhou J, Shum KT, Burnett JC, Rossi JJ. Nanoparticle-based delivery of RNAi thera- peutics: progress and challenges. Pharmaceuticals (Basel). 2013;6(1):85–107. doi:10.3390/ph6010085
21. Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3(4):406–417. doi:10.1158/2159-8290.CD-12-0429
22. Pardon E, Laeremans T, Triest S, et al. A general protocol for the generation of nanobodies for structural biology. Nat Protoc. 2014;9(3):674–693. doi:10.1038/nprot.2014.039
23. Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431(7006):371–378. doi:10.1038/nature02870
24. Cortez-Retamozo V, Backmann N, Senter PD, et al. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res. 2004;64(8):2853–2857. doi:10.1158/0008-5472.CAN-03-3935
25. Yang Z, Song JB, Dai YL, et al. Self-assembly of semiconducting-plasmonic gold nanoparticles with enhanced optical property for photoacoustic imaging and photothermal therapy. Theranostics. 2017;7(8):2177–2185. doi:10.7150/thno.20545
26. Mahmoudi K, Bouras A, Bozec D, Ivkov R, Hadjipanayis C. Magnetic hyperthermia therapy for the treatment of glioblastoma: a review of the therapy’s history, efficacy and application in humans. Int J Hyperthermia. 2018;34(8):1316–1328. doi:10.1080/02656736.2018.1430867
27. Rajkumar S, Prabaharan M. Multi-functional core-shell Fe3O4 @Au nanoparticles for cancer diagnosis and therapy. Colloids Surf B Biointerfaces. 2019;174:252–259. doi:10.1016/j.colsurfb.2018.11.004
28. Wood DW, Camarero JA. Intein applications: from protein purification and labeling to metabolic control methods. J Biol Chem. 2014;289(21):14512–14519. doi:10.1074/jbc.R114.552653
29. Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–1170. doi:10.1038/nbt1340
30. Behdani M, Zeinali S, Karimipour M, et al. Development of VEGFR2-specific nanobody pseudomonas exotoxin A conjugated to provide efficient inhibition of tumor cell growth. N Biotechnol. 2013;30(2):205–209. doi:10.1016/j.nbt.2012.09.002
31. D’ Huyvetter M, Vincke C, Xavier C, et al. Targeted radionuclide therapy with a 177Lu-labeled anti-HER2 nanobody. Theranostics. 2014;4(7):708–720. doi:10.7150/thno.8156
32. Muyldermans S, Baral TN, Retamozzo VC, et al. Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol. 2009;128(1–3):178–183. doi:10.1016/j.vetimm.2008.10.299
33. Doughton JA, Hofman MS, Eu P, Hicks RJ, Williams S. A first-in-human study of 68Ga-nanocolloid PET/CT sentinel lymph node imaging in prostate cancer demonstrates aberrant lymphatic drainage pathways. J Nucl Med. 2018;59(12):1837–1842. doi:10.2967/jnumed.118.209171
34. Semkina AS, Abakumov MA, Skorikov AS, et al. Multimodal doxorubicin loaded magnetic nanoparticles for VEGF targeted theranostics of breast cancer. Nanomedicine. 2018;14(5):1733–1742. doi:10.1016/j.nano.2018.04.019
35. Xiong F, Huang S, Gu N. Magnetic nanoparticles: recent developments in drug delivery system. Drug Dev Ind Pharm. 2018;44(5):697–706. doi:10.1080/03639045.2017.1421961
36. Arriortua OK, Insausti M, Lezama L, et al. RGD-functionalized Fe3O4 nanoparticles for magnetic hyperthermia. Colloids Surf B Biointerfaces. 2018;165:315–324. doi:10.1016/j.colsurfb.2018.02.031
37. Badrigilan S, Shaabani B, Gharehaghaji N, Mesbahi A. Iron oxide/bismuth oxide nanocomposites coated by graphene quantum dots: “three-in-one” theranostic agents for simultaneous CT/MR imaging-guided in vitro photothermal therapy. Photodiagnosis Photodyn Ther. 2018;25:504–514. doi:10.1016/j.pdpdt.2018.10.021
38. El-Boubbou K. Magnetic iron oxide nanoparticles as drug carriers: preparation, conjugation and delivery. Nanomedicine. 2018;13(8):929–952. doi:10.2217/nnm-2017-0320
39. Cardoso VF, Francesko A, Ribeiro C, Banobre-López M, Martins P, Lanceros-Mendez S. Advances in magnetic nanoparticles for biomedical applications. Adv Healthc Mater. 2018;7:5.
40. Tseng YJ, Chou SW, Shyue JJ, et al. A versatile theranostic delivery platform integrating magnetic resonance imaging/computed tomography, pH/ cis -diol controlled release, and targeted therapy. ACS Nano. 2016;10(6):5809–5822. doi:10.1021/acsnano.5b08130
41. Mosayebi J, Kiyasatfar M, Laurent S. Synthesis functionalization and design of magnetic nanoparticles for theranostic applications. Adv Healthc Mater. 2017;6(23):1700306.
42. Zeng Y, Wang L, Zhou Z, et al. Gadolinium hybrid iron oxide nanocomposites for dual T1-and T2-weighted MR imaging of cell labeling. Biomater Sci. 2016;5(1):50–56. doi:10.1039/C6BM00706F
43. Yin W, Yan L, Yu J, et al. High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano. 2014;8(7):6922–6933. doi:10.1021/nn501647j
44. Feng L, Yang D, He F, et al. A core-shell-satellite structured Fe3O4@g-C3N4-UCNPs-PEG for T1/T2-weighted dual-modal MRI-guided photodynamic therapy. Adv Healthc Mater. 2017;6(18). doi:10.1002/adhm.201700502
45. Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82:775–797. doi:10.1146/annurev-biochem-063011-092449
46. Hofman EG, Ruonala MO, Bader AN, et al. EGF induces coalescence of different lipid rafts. J Cell Sci. 2008;121(Pt 15):2519–2528. doi:10.1242/jcs.028753
47. Charbonneau DM, Tajmir-Riahi HA. Study on the interaction of cationic lipids with bovine serum albumin. J Phys Chem B. 2010;114(2):1148–1155. doi:10.1021/jp910077h
48. Yoo D,Lee JH,Shin TH,Cheon J. Theranostic magnetic nanoparticles [J]. Acc. Chem. Res. 2011;4(10):863–874.
49. Stine R, Mulvaney SP, Robinson JT, Tamanaha CR, Sheehan PE. Fabrication, optimization, and use of graphene field effect sensors[J]. Anal Chem. 2013;85(2):509–521.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
