Back to Journals » International Journal of Nanomedicine » Volume 16
Recent Advances in the Use of Mesoporous Silica Nanoparticles for the Diagnosis of Bacterial Infections
Authors Şen Karaman D , Pamukçu A , Karakaplan MB , Kocaoglu O, Rosenholm JM
Received 30 April 2021
Accepted for publication 24 August 2021
Published 24 September 2021 Volume 2021:16 Pages 6575—6591
DOI https://doi.org/10.2147/IJN.S273062
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Didem Şen Karaman,1 Ayşenur Pamukçu,2 M Baran Karakaplan,3 Ozden Kocaoglu,1 Jessica M Rosenholm4
1Biomedical Engineering Department, Faculty of Engineering and Architecture, İzmir Katip Çelebi University, İzmir, 35620, Turkey; 2İzmir Kâtip Çelebi University, Graduate School of Natural and Applied Sciences, Department of Biomedical Technologies, İzmir, Turkey; 3İzmir Kâtip Çelebi University, Graduate School of Natural and Applied Sciences, Department of Biomedical Engineering, İzmir, Turkey; 4Pharmaceutical Sciences Laboratory, Faculty of Science and Engineering, Åbo Akademi University, Turku, 20520, Finland
Correspondence: Didem Şen Karaman Email [email protected]
Abstract: Public awareness of infectious diseases has increased in recent months, not only due to the current COVID-19 outbreak but also because of antimicrobial resistance (AMR) being declared a top-10 global health threat by the World Health Organization (WHO) in 2019. These global issues have spiked the realization that new and more efficient methods and approaches are urgently required to efficiently combat and overcome the failures in the diagnosis and therapy of infectious disease. This holds true not only for current diseases, but we should also have enough readiness to fight the unforeseen diseases so as to avoid future pandemics. A paradigm shift is needed, not only in infection treatment, but also diagnostic practices, to overcome the potential failures associated with early diagnosis stages, leading to unnecessary and inefficient treatments, while simultaneously promoting AMR. With the development of nanotechnology, nanomaterials fabricated as multifunctional nano-platforms for antibacterial therapeutics, diagnostics, or both (known as “theranostics”) have attracted increasing attention. In the research field of nanomedicine, mesoporous silica nanoparticles (MSN) with a tailored structure, large surface area, high loading capacity, abundant chemical versatility, and acceptable biocompatibility, have shown great potential to integrate the desired functions for diagnosis of bacterial infections. The focus of this review is to present the advances in mesoporous materials in the form of nanoparticles (NPs) or composites that can easily and flexibly accommodate dual or multifunctional capabilities of separation, identification and tracking performed during the diagnosis of infectious diseases together with the inspiring NP designs in diagnosis of bacterial infections.
Keywords: mesoporous silica nanoparticles, bacteria, antibacterial, bacterial separation, biosensors, biomedical imaging, MSN
Introduction
The incidence of antimicrobial resistance (AMR) and the persistent nature of biofilms in bacterial infections cause unfavorable outcomes during treatments.1,2 Without urgent action, we are heading towards what the World Health Organization (WHO) has coined the “post-antibiotic era”, in which infections that have been treatable for decades can kill again. The development of new principles and entirely new solutions are encouraged3 for diagnosis along with therapeutic, curative, or preventive measures against bacterial infectious diseases to overcome the failures in treatments.4,5 Adequate and cost-effective treatment of infection is only be possible by employing rapid, accurate, and precise diagnostics.
Diagnosis of bacterial infections and the quantification of bacteria in human clinical samples possess great importance to focus on appropriate antibacterial treatments. Usually, cross validation of the bacterial infection is required to affirm the bacterial infection and eliminate the inappropriate usage of antibiotics, which is regarded as the principal determinant of AMR.6 The Global AMR guide for the Global Antimicrobial Resistance Surveillance System (GLASS) has been published by WHO to support diagnostic stewardship and points out the important practices of robust microbiological diagnosis in patients.7 The guide also points out the need for a paradigm shift in the diagnosis of bacterial infections and in battling against AMR.
The Paradigm Shift in the Diagnosis of Bacterial Infection
The process of diagnosing bacterial infections starts with sampling and isolation of bacteria from the sampling matrix, which is crucial to obtain precision in diagnosis.8 Bacterial infection can occur in any part of the body; therefore, vast sampling matrices with heterogeneous distribution of pathogenic bacteria in infected tissue make the isolation of bacteria and diagnosis process challenging.9 Isolation of the pathogenic bacteria is critical to elucidate bacterial pathogenicity and assessing potential effects on the patient.10 The isolation of bacteria from complex sampling media and enrichment of the target organism is commonly performed by physical separation techniques, which decreases the recovery of target bacteria due to a series of steps containing washing and replications. Efficient approaches are required to improve the yield of isolated bacteria and focusing on the identification of bacteria prior to therapy.
Antibiotic susceptibility assays are standard methods for microbial identification.11 Numerous other methods are established for detection of bacteria, such as biochemical identification, immunoassays, and polymerase chain reaction (PCR). However, these generally require complex and time-consuming procedures (e.g., several hours to days for bacterial culture, bacterial metabolites extraction, and bacterial discrimination) to analyze the results.12 While being highly sensitive, the requirement for the use of costly equipment with specialized personnel hampers the practicality of these methods.13
The above-mentioned separation and identification of target pathogenic bacterial cells via conventional microbiological methods for diagnosing bacterial infections can lead to a multitude of issues. For instance: ex vivo cultivation of the anaerobic bacterium in question; contamination and practical errors during the processing; biased location of tissue sampling, and ignoring the temporal changes and treating as inherent properties due to use of a single time point sampling; especially in the circumstance of highly dynamic infection.14 Hence, a paradigm shift in bacterial infection diagnosis practices is direly needed to overcome the possible failures at the early diagnosis stages.
The recent advances in nanotechnology can speed up the development of a new generation of nanodiagnostics with improved precision and accuracy for diagnosis. Nanoparticles (NP) have become attractive for the diagnosis and treatment of infectious diseases, especially through the knowledge gained from the research in oncology-related nanomedicine.15 Among the vast array of NP, mesoporous silica nanoparticles (MSN) are seen as ideal due to their flexible design options and functionalities. First, its constituent, amorphous silicon dioxide (SiO2) is a GRAS (generally recognized as safe) classified material by the Food and Drug Administration (FDA).16 Second, while the characteristic properties of mesoporous material family are most recognized for their potential in drug delivery, the same characteristics can be exploited in the design of nanoscaled imaging probes. For instance, the encapsulation of fluorescent imaging probes or nuclear markers within their pores can be applied whilst good diffusion through their porous matrix could be retained, rendering MSN matrix as ideal candidates for sensing devices with outstanding features for further diagnosis.17,18 In our recent review,18 we outlined how the porous structure of MSN or MSN with voidal structure at the center possess cavities that can act as reservoirs to accommodate high amount of different small-molecular compounds, biomolecules, and organic or inorganic imaging agents to be employed in steps of diagnosis as also discussed in the below sections: separation, sensing, and imaging. The incorporation of imaging agents for imaging or affinity ligands for selectivity and separation into or onto the silica matrix can also be provided by the construction of layer-by-layer structures to obtain nanocomposites. Additionally, the extent of silanol groups on their surfaces are advantageous for the demanded biological interactions, and these can be readily functionalized to provide more specific or desired interactions at the nano-bio interface.
Furthermore, multimodal imaging modalities can be incorporated into MSN system, and the detection specificity can be introduced by employing the nanochemistry toolbox for MSN-based nanomedicine.19 Similar approaches have already been employed with different types of NP (e.g., magnetic NP, gold NP [AuNP], quantum dots [QDs]) for specific, selective, and fast bacterial detection by modifying them with antibiotics, antibodies, aptamers, peptides, and carbohydrates as the recognition entities.20
This review focuses on the introduction of nanodiagnostic approaches as the paradigm shift in the diagnosis of bacterial infections, with a special focus on strategies applied with numerous designs of MSN. The conferred design options of nanoscaled mesoporous silica matrices will be devoted to present their utilization in the separation of bacterial species of interest, biosensing of targeted bacterial species via integration into biosensor devices, in situ imaging, and theragnostic approaches for battling bacterial infections. Recent design options with MSN for improving the diagnosis selectivity and sensitivity will be evaluated. Finally, issues pertaining to the current applications and future outlook are presented.
Nanoparticles Aided Bacterial Separation from Sampling Matrix
Fast processing and accurate methodologies in separation of bacteria have a critical role to reduce diagnosis time and planning of therapy. An inevitable step before the separation of the target bacteria is enrichment step once the concentration of the bacteria is below the detection limit (sensitivity level). For methods like PCR and enzyme linked immunosorbent assay (ELISA) requiring a minimum of 103–104 and 104–105 colony forming unit per milliliter (CFU/mL) for detection, pre-enrichment steps is necessary prior to analysis.21 An ideal enrichment starts with high amount of bacteria capturing (separation) within minimum sample size. Immune-magnetic separation (IMS) is the preferred method, which is based on the capture of bacteria by magnetic materials coated with target-specific antibodies. Nevertheless, the utilization of IMS is restricted because of the high antibody production costs and low stability of antibodies in harsh environments.
The shortcomings of traditional methods have tried to be overcome with the aid of advanced NP designs.22 Identifying the key descriptors responsible for the substrate attachment efficiency to bacterial cells is crucial for the separation of bacteria from sampling. To substantiate the interaction of the NP with gram-positive and gram-negative bacteria Pajerski et al have tuned the shape and the surface chemistry of AuNPs.23 The electron microscopy observations of the adhered AuNPs to the bacterial cell walls revealed direct correlation between the number of the attached NP and the ζ-potential of the bacterial strains. There has been great effort to identify affinity ligands for removal of pathogens from blood since sepsis is a life-threatening disease requiring rapid detection and removal of pathogenic bacteria. To separate the pathogenic bacteria from the blood microbiome magnetic nanoparticles (MNP) modified with zinc(II)-bis(dipicolylamine) (ZnDPA) ligands and vancomycin targeting phosphatidylserines on gram-negative and positive bacteria surface, and peptidoglycans on gram-positive bacteria was prepared.24,25 Vancomycin conjugated MNP showed promise for the enrichment of S. aureus from the whole blood matrix within 2.5 hours.26,27 The selectivity of ZnDPA ligand for gram-negative bacteria has been also presented by employing bis-Zn-DPA-modified polyethylene glycol-coated (PEG) MNP for the separation of E. coli and endotoxins from blood samples and minimal interaction with red blood cells could be achieved.28
NP-aided colorimetric methods are attractive in the separation and enrichment of bacteria due to their convenience in operation and read-out quantifications. Mou et al achieved separation with the design of AuNPs presenting azide and alkyne groups on their surface and NP aggregates formed upon Cu+ production and resulted in a color change showing rapid detection of E. coli in blood samples.29 Moreover, the developed system was later combined with a magnetic separation process to assess selectivity and sensitivity. It was shown that E. coli bacteria with a concentration of 40 CFU/mL was able to be detected in complex sepsis blood samples comprising 2×103 CFU/mL S. aureus, B. subtilis, and P. aeruginosa within 1 hour.29
More recently, a core-shell structured NP-based a new capture platform (Figure 1) made of mesoporous titanium dioxide (TiO2) coated magnetic NPs functionalized with targeting aptamers (designated as AptFe3O4@mTiO2) was designed to identify the pathogen and confirm bacterial bloodstream infections.30 The designed platform could shorten the identification and capturing time down to 2h, whereas the conventional blood culture method could take 2–5 days.
 |
Figure 1 Schematic representation of the aptamer-based capture platform to identify the pathogen in human blood. Notes: Adapted from Shen H, Wang J, Liu H, et al. Rapid and Selective Detection of Pathogenic Bacteria in Bloodstream Infections with Aptamer-Based Recognition, ACS Appl. Mater. Interfaces. 2016; 8 (30): 19371–19378. Copyright © 2016, with permission from American Chemical Society.30 |
To sum up, augmenting separation and enrichment efficacy could be possible with novel nanocomposite designs possessing adhering features; MSN could aid separation by tuning their surface chemistry, composition and morphology as well by employing sol-gel chemistry and nano-chemistry toolbox. The recent advances in this subject are discussed in MSN designs for the separation of bacteria from sampling media.
Nanoparticles Based Biosensors for Identification of Bacterial Species
Long detection time and identification have a negative correlation in antibacterial therapy and the prevention of AMR bacterial infections.31 The acquisition of bacterial detection and identification are demanded by the health disciplines.32 It is known as each hour delay in the initiation of antibiotic therapy increases the risk of mortality by 7–12%.33 The success of the bacterial detection is assigned by the rapid, sensitive, reliable, and cost-effective processing.34
Biosensors offering an accurate response in short-term and real-time detection for clinical observations with high sensitivity (less than 103 CFU/mL), high selectivity, reproducibility, and reusability (good shelf-life in long periods and high temperature up to 45 °C) are required.35,36 To meet these requirements, a variety of sampling strategies and biosensor components with superior features need to be provided. On many occasions, sampling is performed after disruption of the bacterial cell integrity. In such a circumstance of E. coli detection, DNA probe integrated biosensing application could be provided.37 Although the amplification step of DNA was not performed, the detection takes 3–5 hours for quantification of 2–20 CFU/mL. Even though selectivity could be provided, the shortening of analysis time is still demanded. There is a need of eliminating steps prior to sampling which could be only achieved by providing biosensor designs for whole-cell detection. Farrow et al has performed the detection of protective antigen (PA) exotoxin from Bacillus anthracis via a peptide-based capturing agent against to PA. A very low limit of detection (LOD) for PA (170 pg/mL) could be achieved.38 A similar strategy has been also employed in Figure 2, where researchers designed an electrochemical biosensor that recognizes E.coli in 140 min with the LOD of 1 CFU/mL.39
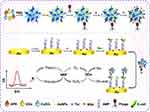 |
Figure 2 Design of organic-inorganic nanoflowers composites for integration in electrochemical detection of E. coli. Notes: Reprinted from Li Y, Xe G, Qu J, et al. A new biosensor based on the recognition of phages and the signal amplification of organic-inorganic hybrid nanoflowers for discriminating and quantitating live pathogenic bacteria in urine. Sensors and Actuators B: Chemical 2018; 258 (30): 803–812. Copyright © 2017, with permission from Elsevier.40 |
This specific design provided quantification of E.coli with organic-inorganic nanoflowers, AuNPs, T4 phages, Thionine biorecognition elements and amplification of the signals and the quantity of E. coli was provided by species selective antimicrobial peptides (AMP).40 Another outstanding approach was with a label-free, cost-effective and low LOD biosensor design in which the lowest 2.2 CFU/mL bacteria could be detected in 2 hours. Although the presented approaches are advantageous since no sample preparation is required, the authors emphasized the requirement of detection time shortening in order to meet the demands for the biosensor as point of care devices.41 Integration of synthetic biorecognition entities in biosensor designs is required instead of natural biorecognition elements due to the disadvantages of the stability of natural bio-recognition elements. Molecularly imprinted polymers (MIPs) have been employed to achieve analyte specificity. Superior chemical and thermal stability, a variety of immobilization/incorporation strategies, and long shelf-life could be provided by employing the MIP.42 However, the nonspecific interactions are ascribed as the hindering facts in this specific synthetic bio-recognition element.43 To overcome the nonspecific interactions with synthetic biorecognition elements, researchers have focused on the preparation of species selective synthetic antimicrobial peptides (sAMPs) as beneficial to provide accuracy with bio-recognition. To obtain quantitative data, researchers have designed sAMP coated gold surface and further employed electrochemical impedance spectroscopy transduction technique. The results showed that detection limits of 102 CFU/mL for E. coli, P. aeruginosa, S. aureus, and S. epidermidis in addition to the identification of dead and live cells was possible.44 Furthermore, MIP particles have been designed as a further step to improve the selectivity of biosensors. Ahari et al have designed a potentiometric biosensor encompassing selective patterns of MIP particles for S. aureus exotoxin detection.45 Additionally, MIP particles paved the way for improving the stability of the biosensor components that detect an exotoxin density up to 10–3M at 68 nm of synthesized MIP for 32 days.
To achieve high selectivity and sensitivity, accommodation of bio-recognition elements with free recognition sites is critically important. High selectivity in biosensors could be only possible by planning controlled interface chemistry interactions. There is a need of substrates with high surface area, applicable for flexible chemistry approaches for the immobilization process.46,47 Metal/metal oxide NP have great advantages to provide large surface areas, thus aiding in bio-recognition element immobilization with adequate ability for reaction and biocompatibility.48
Here, MSN could provide great advantages for sensitivity and selectivity by tuning the surface chemistry of MSN as well as shape and morphology by employing sol-gel chemistry and nano-chemistry toolbox.49 The design of MSN integrated biosensor systems to improve the selectivity will be discussed in the related section.50,51 MSN can be used alone and in combination with other classes of nanostructures to improve the signal amplification for improving the sensitivity, detection, and quantification of the analyte.
Nanoimaging Probes for in situ Diagnosis of Bacterial Infections
In situ imaging of bacterial infection is advantageous for diagnosis, monitoring patient outcomes and following the treatment in real-time. Numerous imaging techniques (optical imaging [OI], ultrasound [US], magnetic resonance imaging [MRI], computed tomography [CT], or positron emission tomography [PET]) are available for a fast and accurate diagnosis to enable antibiotic stewardship and to treat patients with a bacterial infection.52,53 However, these imaging modalities have limitations for distinguishing sites of bacterial infection and bacterial species from sterile inflammation.54 Recent advances in NP platform together with the improvement in biomedical imaging techniques offer novel systems for nano-diagnostic and even nanotheranostic applications.
An enormous amount of experience has been gained on NP-aided targeted multimodal imaging methods in oncology over the past ten years.54–56 Especially the modularity of NP typically offers advantages by tagging the nanomaterial with a specific label to track with the imaging modality in question. For tagging of the NP with any kind of targeting or labeling molecules, the surface chemistry of NP has to be tuned to prevent undesired bio-distribution after administration.18 The ideal nano-imaging probes are expected to possess inherent detectability by the desired imaging modality in use without post modifications, exemplified by the detection of luminescent QDs by OI and superparamagnetic iron oxide nanoparticles (SPION) by MRI. The novel nanoscopic imaging probes could offer the advantage of targeted and/or multimodality imaging for in situ bacterial infection, especially by employing post-modification strategies to accommodate species-selective affinity ligands such as antibodies, antibiotics, AMPs, metabolic compounds, bacteriophages, and DNA/RNA binding molecules.54
To date, researchers have designed different hybrid NPs either with inherent antibacterial properties or encapsulating the antibacterial drug to provide the therapeutic action, in addition to defined surface chemistry to avoid fast clearance and targeting of the infection site or in vitro species selectivity with high binding ability to the bacterial cells in question.57 To the best of our knowledge, hybrid MSN designs have a strong potential to combine diagnostic function, active targeting, and therapeutic delivery even though most of the targeting studies involve therapeutic drug delivery with NP formulations. In oncology-related studies, targeted multimodal theranostic MSN designs are exploited for targeted cancer cell tracking and treatment. Within this concept, the evolving structural composition of MSN for nanotheranostic designs is an attractive attempt to manage in situ imaging of bacterial infection and will be discussed in the following sections.
Nanoparticle-Aided Identification of Antibiotic Resistance
The persistent and progressive development of bacterial resistance has risen the problem of antimicrobial multi-resistance as a problematic hot topic and health concern worldwide.58 Antimicrobials those are used for multidrug resistance (MDR) infections are often limited and require a high dosage of antibiotics, which could lead to intolerable toxicity and side effects.59 These clinical obstacles necessitate the development of alternative and more effective antimicrobial strategies than the conventional ones. Rapid diagnosis and pathogen identification are essential for effective treatments. Along with innovative antimicrobial treatments, eliminating the possibility of bacteria developing AMR mechanisms is critically important. Exploration of new strategies capable of fast, precise, and accurate detection of multidrug-resistant bacterial infections together with the therapies are desired.58 Both directions, designing innovative antimicrobial strategies and designing reliable methods for monitoring their antimicrobial activity against MDR mechanisms are essential for making a sustainable plan for using antibiotics.58 Especially by knowing that an immense amount of time and resources are required to discover new safe and effective antibiotics, diagnostic approaches to identify the antibiotic resistance could help the medical community to preserve the utility of these precious drugs.
In clinical cases, to identify the AMR, the etiology and antibiotic susceptibility profiling are employed as conventional methods to optimize and narrow antimicrobial therapy.60 However, the receiving of the definite result by the employed common methods takes approximately 48h or even more. However, it is expected that rapid identification of AMR of the pathogen and antimicrobial susceptibility directly from clinical samples at the point-of-care would be available within approximately 30 min. Recently, a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) technique was ascribed as a rapid and direct technique for MDR bacterial detection.61–63 The application of MALDI-TOF MS technology to clinical diagnostic microbiology has provided a new, accurate, and robust tool allowing rapid and reliable microbial identification.64 The MALDI-TOF MS-aided assay has shorten the time for diagnosis of MDR bacteria down to 4h, whereas conventional antibiotic susceptibility takes 48h.65 The ongoing research activities focus on extending the detection limits and eliminating extra peaks in the obtained spectrum analysis of the MALDI-TOF MS-based assay. For this purpose, Zhu et al performed MALDI-TOF MS fingerprinting method after aqueous suspension of titanium dioxide (TiO2) was spotted on a stainless-steel target plate, and then TiO2 were sintered to the plate for having a steady layer on the surface.66 Then, E. coli suspension was placed on the plate surface and photoreactive TiO2 destroyed the bacterial cell wall. As a result, an efficient desorption/ionization occurred, which then allowed direct and fast detection of resistance-associated proteins from intact cells without requiring sample pre-treatment. The composite NP could offer a rapid analysis by eliminating the sample pre-treatments in MALDI-TOF-MS-based diagnostic approaches for AMR pathogens.
An important obstacle in pathogenic bacteria detection is macrophages, which usually compromise infection detection therefore selective determination of AMR pathogens over macrophage-like cells is required.67 In this regard, species selective pre-treatments with NPs would be helpful for pathogenic profiling of different antibiotic-resistant bacteria. Bhaisare et al employed MALDI-MS to pull out pathogenic bacteria from blood samples by designing ionic liquid-modified silica coated magnetite (Fe3O4@SiO2) NP and rapidly identifying them using mass spectrometric analysis.68 In their study, the core-shell Fe3O4@SiO2 NP were synthesized by the sol-gel method and grafted by 3-chloropropyltrimethoxysilane further reacted with N-methylimidazole. Bacteria cells were separated and the lowest detectable number of bacteria being 3.4x103, 3.2x103, and 4.2×103 CFU mL−1 for E. coli, P. aeruginosa, and S. aureus, respectively was analyzed through MALDI-MS.
From the practical point of view, the developments in MALDI-TOF-MS have a high potential for clinical practices and it is important to be aware of the requirements of sample preparation duration prior to analysis, both to extend the limit of detection and shortening the required time for analysis. It is foreseen that NP-aided assays would provide great advantages for both aspects of MALDI spectroscopy analysis methods.
Design of Mesoporous Silica Nanoparticles for Bacterial Infection Diagnosis
Preparation of Mesoporous Silica Nanoparticles
MSN are at the forefront in bacterial infection detection and treatment due to their large surface area enabling contact with the bacterial surface, and high physical and chemical stability important for surpassing different environmental conditions (e.g., pH, temperature). There has been a vast array of studies on the synthesis of MSN exploring the synthesis protocols for tuning size, shape, pore structure, and silica matrix chemistry.69
The first report on mesoporous silica matrices for application within the biomedical field (drug delivery) was published in 2001.70,71 To improve the bio-applicability of MSN, altering the silica matrix chemistry is commonly employed either via chemical conjugation or physical adsorption of functional moieties. Depending on the functional groups, the surface charge can be tuned to positive, negative, neutral, or zwitterionic. Facile sol-gel synthesis enables the tuning of the silica matrix chemistry. Modifying the surface groups of silica particles is a commonly employed to obtain functional silica NP. This can be provided via co-condensation or post-synthesis surface modification approaches.72 In the co-condensation approach, the functional groups are already introduced during the synthesis step in the form of organosilanes, whereas post-synthesis functionalization approaches are carried out after the synthesis of particles. Post-synthesis functionalization strategies encompass the use of organosilanes, but also polymers or inorganic species are utilized as surface modifiers.73,74 Possible advantages and disadvantages of different modification strategies have been investigated for the diagnostic process. For instance, while aiming for species selective applications, additional functionalities such as targeting moieties are recommended to be introduced to the outer most surface of particles.67,75 In the following sections, various MSN designs will be reviewed to accomplish cost and time-effective diagnosis for bacterial infections.
MSN Designs for the Separation of Bacteria from Sampling Media
Bacterial separation and detection systems based solely on silica NP have attracted interest and led to the development of different design strategies with the support of flexible design options provided by the silanol chemistry and sol-gel approaches. To accomplish successful separation of bacterial cells from a complex sampling matrix, or to eliminate the interference by the sampling matrix, a successful capturing of the bacteria in question is required. This could only be possible by fine-tuning of the interface between the bacterial cells and silica NP. The impact of tuning the surface chemistry of silica NP on the capturing of bacterial species capture has been demonstrated by Zheng et al. In their study, the surface grafting of silicon dioxide with different polymers was carried out to capture the bacteria E. coli, and S. epidermidis. SiO2-NH2 NP synthesized via the one-step Stöber method have been photo-conjugated with a pH- and temperature-responsive polymer, poly(N-isopropylacrylamide-co-glycidyl methacrylate (poly(NIPAm-co-GMA)) to which boronic acid was grafted. The bacteria-capturing nanocomposite, Si@poly(NIPAm-co-GMA)@PCAPBA interacted with bacteria surface through boronic ester bonds and captured gram-negative bacteria. It was also shown that bacterial capture using Si@poly(NIPAm-co-GMA)@PCAPBA did not affect bacterial cell viability, and can be employed as a successful design strategy for the enrichment of bacterial species prior to analysis or to investigate the pathogenicity of the bacteria in question.76 The same strategy could readily be employed also for MSN, since the required –NH2 group abundance to initiate the conjugation of pH and temperature-responsive polymers could be provided either by surface grafting or as performed by co-condensation method. By adopting MSN for the same, higher surface area for displaying boronic ester bonds could be provided,77 and the capturing efficiency could even have been improved.
Bacterial separation and detection over mammalian cells have gained interest in recent years. Selective separation of bacteria over macrophage-like cells using MSNs was carried out by Qi et al.67 Authors decorated MSN with vancomycin and assessed the selectivity of the MSNs-Van on E. coli and S. aureus cells. Results showed that MSNs-Van binds selectively to S. aureus cells due to the affinity of vancomycin to gram-positive bacteria. However, the designed MSN also demonstrated to reduce the viability of S. aureus, and therefore, the mentioned design could be employed both for separation and treating the bacterial cells.
Magnetic separation is another approach to have ultra-low concentration separation of bacteria from sampling matrix. To improve the magnetic separation, the colloidal dispersibility of magnetic NP could be improved by employing the siliceous matrix as a shell construct of core-shell NP or by integrating the magnetic NP into a silica matrix.28,77,78 Since bare magnetic NP have poor colloidal stability resulting in decreased bacterial capture efficiency, utilization of silica as a shell enhances colloidal stability, and thus prevents agglomeration of the core-shell NP in the samples. Li et al used polyethyleneimine (PEI) coated, tetramethylrhodamine-conjugated (TRITC) Fe3O4-SiO2 core-shell NP to capture E. coli at ultralow concentrations.79 With this highly cationic NP system, very low concentrations of E. coli, 10 and 100 CFU/mL, were captured within 1 hour without conducting a pre-enrichment process.79 In the light of the presented finding, concerns about the utilization of highly cationic charged NP was raised since unspecific binding between NP and bacteria lead to agglomeration, which is a possible reason behind the ineffective isolation and inaccurate bacteria cell counts. To overcome this problem, Kadam et al designed a biofunctionalized magnetic Janus NP with good stability and capture efficiency of over 80%. In the study, half of the magnetic Janus NPs were attached with PEG chains for the prevention of bacterial agglomeration, while the other half was coated with antibody molecules specific to E. coli to achieve species-selective separation.80 Functionalization with either PEG chains or anti-E. coli antibody was achieved via silanol functional groups upon coating of magnetic Janus NP with a SiO2 layer. The selectivity and capturing for E. coli and S. simulans with the thus designed NP was achieved. Exposure to NP did not further interfere with cell viability, which is a prerequisite for accurate quantification.80 Lee et al designed another approach for tuning the mesoporous silica matrices for magnetic separation.28 In their design, Ni+ doped heterogeneous magnetic mesoporous matrices (Ni-HMMS) was prepared with a significant incorporation of Fe particles within the silica mesopores by programed thermal hydrogen reaction and functionalized with Ni2+ ion on the surface by the wet impregnation process. Tuning the composition of silica matrices by incorporation of Fe particles and Ni2+ ion yielded with detection of pathogenic E. coli at ultralow concentration (1 Log10 CFU/mL) in the real samples.
The core-shell structured silica NP have advantageous features for enrichment prior to going for bacterial detection analyses of matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). To this end, ionic liquid-modified Fe3O4-SiO2 NP were used for separation and detection of three different bacteria, E. coli, S. aureus, and P. aeruginosa from mouse and sheep blood by MALDI-MS analysis. At this point, Fe3O4-SiO2 NP have been shown to detect and enrich mass signals of bacteria without further treatment.68 The recent literature investigations demonstrate the operability with MSN based nanocomposite designs in separation and enrichment methods of bacteria content in terms of reduced analysis time and complexity.
MSN Integrated Biosensors for Detection of Bacterial Infection
In the design of biosensors, high selectivity is demanded to identify the bacterial infection sources and to focus on appropriate therapy. To provide high selectivity, a sufficient amount of bio-recognition elements accommodation as biosensor components is critically important. This could only be provided by controlled interface chemistry between the substrate and recognition element in conjunction with a high surface area. From the perspective of improving the selectivity of biosensors, the unique properties of mesoporous silica matrices with flexible silanol chemistry and high surface-area-to-volume ratio provides good substrate properties. The presence of functional groups, structure, morphology, and matrix of MSN are the key parameters for improving selectivity of biosensors with MSN. Different types of ordered MSN such as Mobil Composition of Matter No.41 (MCM-41) and Santa Barbara Amorphous-15 (SBA-15) and mesocellular foams (MCF) are attractive for bio-immobilization.50 High surface area with the porous architecture of MSN is advantageous since the pore reservoirs could act for the analyte diffusion into the pores, which are decorated with bio-recognition elements via silanol functional groups.81 Since the increasing pore wall thickness directly affects the maximum binding analyte amount, it is an important parameter for porous structures.82 Silanol functional groups allow the design of different bio-recognition strategies and improve the accuracy of detection while providing time-efficient detection methods.69 For instance, Gu et al designed MSN deposited with hemin that is a chemiluminescence material, which could be capped with DNA.83 DNA nuclease enzyme as analyte for bacterial detection, binds the DNA on the MSN and cause release of hemin. Chemiluminescence activity is measured for bacterial detection.
Different biosensors are designed by employing surfaces modified with MSN to improve the detection limits and sensitivity as summarized in Table 1. In different designs, researchers have exploited the porous structure of MSN to guide the electrical field through analyte-filled pores and improve the sensitivity of biosensors.84 To exemplify this, Mathelié-Guinlet et al, designed an electrochemical biosensor, in which the presence of silica NP functionalized with specific polyclonal antibodies (Abs) was tested for targeting and binding of E.coli85,86 and changes in the responses of the transducer observed by Cyclic Voltammetry (CV). The obtained results showed the LOD could be lowered to the range between 103 to 106 CFU/mL, the time of detection can be lowered to only 5 min, the best LOD of 2×103 CFU/mL being achieved in a short term of 30 minutes incubation. Accuracy could be demonstrated with the unit of 1 µA change in CV assigned for 12–20 CFU/mL change during the incubation time with a linear increases from low to high concentrations. The threshold concentration of bacteria species is critically important such as 105 CFU/mL for urinary infection87 since the viable but nonculturable state of bacteria may cause untreatable symptoms88 caused by the destructive bacterial toxins for the host cells.89 To the best of our knowledge, the metabolites of nonculturable state of bacteria,90 can take a role as the analyte for biosensors. Therefore, LOD of a biosensor to detect a low amount of toxins could be employed to predict nonculturable bacteria quiddity. The accurate detection of bacterial toxins could be provided with the help of silicon-based NP by paving the way of eluding and filtering undesired bindings to the surface for biosensor applications.91
 |
Table 1 Designs of MSNs Employed in MSN Integrated Biosensors for Detection of Bacterial Infections |
Utilization of NP as transducers in biosensor designs to amplify the signal can distinctly increase the sensitivity of the biosensors. MSN can be used alone and in combination with other classes of transducer elements for improving the sensitivity, detection, and quantification; which are known as the other key descriptors for the performance of biosensors. Researchers have investigated the potential of silica NP in optical electrochemical and mass-based biosensors by exploiting the inherent properties of mesoporous silica matrix to take a role as transduction element.50
Several optical sensing mechanisms could be employed in optical biosensor designs for analyte detection such as absorption, fluorescence, or luminescence.92 In this sense, the optical properties of MSN are intensively exploited to design optical biosensors with the desired features for bacterial detection.93,94 Remarkably, MSN is inert against photons as well as optically transparent and presents fascinating platform to construct novel, stable optical sensors with sensitivity and rapid response.95,96 Sol-gel approaches are employed for the production of MSN and superior possibilities for the incorporation of conventional luminophores (organic dyes) and inorganic luminophores by employing in situ incorporation strategies, for instance by employing approaches in Figure 3A. Evenly distributed luminophores into silica matrices could be obtained, but part of some of the hybrid indicators may be inaccessible. Thus, future developments to obtain fully accessible optical indicators sacrificing less porosity, facilitating molecular diffusion, and adsorption kinetics are required. Another strategy for the accommodation of optical indicators into an MSN matrix could be via post-synthesis approaches that may be performed as presented in Figure 3B without changing the arhitecture of the silica framework. Various ionic, molecular, metal complex, and macromolecular photoactive units have been integrated with ordered mesoporous frameworks as advanced optical sensors.81
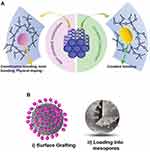 |
Figure 3 (A) Co-condensation of luminophores via covalent bonding and noncovalent interaction in mesoporous siliceous frameworks. (B) Post-synthesis approaches for the addition of optical units into MSN. Notes: (A) Reprinted from Gao M, Zeng J, Liang K, Zhao D, Kong B. Interfacial Assembly of Mesoporous Silica‐Based Optical Heterostructures for Sensing Applications. Adv. Funct. Mater. 2020, 1906950. Copyright 2020 with permission from WILEY‐VCH.81 |
However, the interaction surfaces may be hindered due to the presence of luminophores accumulation on MSN. MSN with controlled synthesis protocols need to be developed for the next generation of hybrid optical indicators. From the standpoint of silica based transducer development, Jenie et al have developed Rhodamine B (RB), a well-known as a pink fluorescent dye, incorporated silica NPs (SNP-RB) from natural amorphous silica and investigated its performance as E. coli biosensor.94 The sensing principle was designed by the fluorescence-quenching mechanism of SNP-RB and a wide linear E. coli concentration range of 10–105 CFU/mL could be obtained with a LOD of 8 CFU/mL.94 In practice, the preparation of SNP-RB was carried out by employing co-condensation of RB into silica matrix and no structural changes of SNP was presented to compare with/without RB incorporation, and the obtained results were only concluded as to be sufficient enough to meet the demanded sensitivity and selectivity for E. coli. However, it could be beneficial to investigate the dispersibility of SNP from the point of quenching aspects since the aggregation‐caused quenching (ACQ) and leakage of dyes at high loading of fluorophores are important challenges of such designs.97
The electrochemical sensing approaches represent the needs of advantageous properties of an open framework like the MSN matrix.98 MSN can enable effective and fast access to active functionalities immobilized at the electrode/solution interface99 as most electron transfer processes are diffusion controlled. Especially by employing sol-gel approaches, different compositions of organic functional groups modified silica matrices could be provided. This opens up the possibility of electrochemical sensors and biosensors.100 By making use of the advantages of silica matrices for the incorporation of electrochemical responsive dopants, Mathelié-Guinlet et al designed an electrochemical biosensor, based on an amplification method of differently sized silica NP for E. coli detection.85 However, the study encompasses the operability of silica NP by employing two steps spin coating protocol enabled immobilization of silica NP to be used in combination with polyelectrolytes as a transducer; not a detailed description of how the bacterial detection mechanism got affected from the different sizes of silica NP.
The multimodal action of MSN in biosensor designs, such as selective separation of species and as a transducer was exemplified by aptamer functionalized magnetic silica NP employed for isolation of S. aureus from blood serum.101 In this study, 2 minutes to release of MNase enzyme (analyte), which is specific for S. aureus, was carried out to bind oligonucleotides on the MSN that carry fluorophores. The analyte allowed the release of fluorophores and the designed system had a LOD of 682 CFU/mL.
Ordered mesoporous composites possessing dual or multifunctional capabilities of detection, adsorption, separation, and easy regeneration are likely to trigger new advances. From the aspect of practical implementation, spherical or monolithic adsorbents may be more applicable because they are easier to operate, and, more importantly, they exhibit better adsorption kinetics than powders. Furthermore, as an expedient strategy signal, indicators can be embedded into the silica matrix to obtain inherent signal transducers. This could be used to recognize the significant signal changes after the attachment of the analyte through the bio-recognition entities accommodated into the pores of silica NP.94
MSN Designs for in situ Imaging of Bacterial Infection
MSN are commonly recognized as potential carriers for active pharmaceutical ingredients (API), providing benefits for improving bioavailability, solubility, targeted delivery, and sustained/controlled release.102 The same benefits gained by the incorporated API can be provided in the design of nano-imaging probes by encapsulating molecular imaging agents instead of drugs. MSN-based imaging probes could be designed to be employed as imaging probes in biomedical or contrast agents in medical imaging; improving the imaging clarity and highlighting the specific physiological states/regions.103 In the literature, a broad range of MSN-based imaging/contrast agents have been designed, and their advantages over conventional molecular analogs have been claimed.18 Post-synthesis and in situ synthesis protocols have been reported for preparing MSN-based imaging probes. The in situ synthesis protocols rely heavily on the co-condensation of organosilanes that have first been conjugated to the molecular imaging agent in question. The agent is typically a fluorescent dye, which is very straightforward to conjugate to, e.g., amino-silanes such as aminopropyltriethoxysilane (APTES) since most fluorophores are available in an amine-reactive form to be easily conjugated to biomolecules. The molecular agent can also be an organic chelating agent that is later on used for complexation with an inorganic ion, such as Gd3+ for achieving paramagnetic activity or Eu3+ or Tb3+ for achieving optical activity. The same chelates can also be used to complex radionuclides for nuclear imaging (PET and single photon emission computed tomography [SPECT]). In general, this approach works quite well but water has to be absent in the pre-conjugation reaction so as not to induce self-conjugation of silanes; and this approach is not suitable for incorporating large amounts of agents into the silica framework. Depending on the foreseen application, it has been shown that molecular agents embedded inside the pores may not even be accessible and thus “silent” upon especially MR-imaging.104 To avoid this, organosilanes may also be used for post-synthesis labeling of molecular imaging agents. In this case, the imaging agent may either be pre-conjugated to the silane or the MSN may first be silanized with the organosilane, where after the imaging agent is coupled to the functional groups after silanization. Using post-synthesis approaches, any type of chemical grafting strategies can be employed utilizing, e.g., polymers for higher grafting density.77,105 Introduction of inorganic species can also be attempted by in situ approaches such as doping of the silica framework, or the doping can take place post-synthesis (Figure 4). The major limitation with grafted molecular species (usually fluorescent dyes) is the imminent probability of detachment of the dye linkage, either due to cleavage of the actual bond but perhaps more likely due to the rapid dissolution (via hydrolysis) of MSN in an aqueous environment.106,107 If such MSN are to be used as imaging probes, it should be certain that the signal detected upon imaging is in fact stemming from the MSN itself and not detached dye molecules. Another drawback in occupying the pore space with imaging agents may be if the MSN are to be used to carry additional substances in their pores, such as drugs. Not only do these occupy space in the pores, thus hindering high drug loading; but also will the presence of drug molecules affect the fluorescent signal of the incorporated dyes. Fluorophores are extremely sensitive to the most immediate surroundings and thus, everything from pore size to (grafting) density of dyes and even surface functionalization will affect the fluorescence signal and thus, needs to be carefully taken into account in the design of MSN-based imaging probes.107–109
A completely different approach that is used to fully separate the imaging signal from the possible active substance carrying modality is the construction of core-shell designs. Virtually any inorganic NP can serve as the core, which can subsequently be coated with a mesoporous shell for further loading/grafting of molecular agents.110 Consequently, multimodal imaging probes can readily be designed via this approach; combining one imaging modality in the core with another molecular entity incorporated in the pores of the shell. The core can also be utilized for other functionalities besides imaging; whereas the molecular imaging agent in the pores will serve for the imaging signal. In such designs, the core activity can be exploited, e.g., for its magnetic activity in cell/bacterial separation, antibacterial or antioxidant properties.28,111
 |
Figure 4 Strategies for incorporation of inorganic species into MSN. |
On the utilization of MSN-based imaging probes for bacterial detection, Qi et al designed vancomycin-modified fluorescent MSNs (Van-MSNs) for detection of gram-positive bacteria.67 For enabling visualization, they covalently coupled fluorescein isothiocyanate (FITC) molecules in the pores of MSN. To test this strategy, they used two mixtures, the first with S. aureus, E. coli, and mouse leukemic monocyte-macrophage cells; the second with only the S. aureus and E. coli. These cell mixtures were incubated with fluorescent Van-MSN, where after the cells were imaged using confocal laser scanning microscopy. A higher fluorescence signal was observed in S. aureus cells due to the hydrogen bonding between Van and D-alanyl-D-alanine. In another study, a fluorescent silica core-shell structured nanocomposite was employed to study morphology and temporal evolution of pH microenvironments in E. coli (PHL628) and mixed-culture by implementing with 3D (tomographic) fluorescence imaging. The study provided the understanding of non-uniform pH profiles in biofilm infections ascribed to be positively correlated with the development of antibiotic resistance from a clinical standpoint.112 From a biomedical imaging practicality point, for the precision of the imaging and elimination of contrast changes at the site of interest, the contrasting agent should not lead to any toxicity. This practicality could be achieved by providing methylene blue doped mesoporous silica NP (MB-MSN) as imaging agents for bacteria.113 In that study, MB-MSN as bioimaging agents were investigated and the obtained results revealed the interaction with E. coli and B. subtilis with bright fluorescence in confocal imaging, and no apparent toxicity towards bacterial cells was observed.
Due to its high spatial resolution and excellent soft-tissue contrast, MRI is used to image inflammatory processes upon bacterial infections. Here, researchers have put efforts into the detection of distinct bacterial cell populations by employing contrast agents with enhanced relaxivity for MRI. Recently, Xue et al have prepared positively charged ultra-small gadolinium oxide (Gd2O3) NP to be embedded in MSN and the resulting composite (Gd2O3@MSN) exhibited enhanced r1 value and T1-weighted MRI performance. Furthermore, the maltodextrin modification on the composite structure was provided for targeted diagnosis with impressive biocompatibility.114 The conceivable application was ascribed as a bacteria-targeted, promising MRI contrast agent for effective discrimination of bacterial infections from a tumor.
Conclusion and Future Outlook
MSN aided diagnosis have great potential to provide sensitive, selective and fast diagnosis of bacterial infections. Although most of the studies have focused on the treatment of bacterial infection with MSN based drug delivery systems, diagnosis aspects with MSN have recently begun to surface. So far, there is not a single diagnosis modality that could meet the demands of the whole diagnosis process. However, the MSN matrix with flexible design options offer new and improved strategies to achieve selective, sensitive and fast diagnosis since it can be implemented in separation, identification, and during the in situ imaging processes. The well-known advantages of MSN based drug delivery systems can also be combined with the diagnostic approaches to provide MSN based nanotheranostics to fight against bacterial infection.
It is essential to develop multimodal MSN designs while paying attention to improve their species-selective and signal amplifying features for meeting the requirements of early detection of bacterial infection. Additionally, pre-clinical investigations has to be performed under clinically relevant conditions. This has enormous importance in medicine, and commonly most of the performed investigations lack the mentioned concerns. MSN present an excellent substrate and matrix for the development of nanotheranostics. This approach could yield stimuli responsive core-shell structures with a porous silica shell loaded with selected antibacterial drugs, subsequently closing the pores with appropriate species-selective synthetic affinity ligands. There is still need for further research on multimodal MSN preparation for both treatment and diagnosis of bacterial infections, which will be highly important for future applications of these materials in biology and medicine.
On a greater scale, also the nanotechnology field, along with virtually all other fields and industries, is currently expanding into making greater use of AI (artificial intelligence) and machine learning approaches, not only to design but also to more rapidly and accurately predict the performance of the nanosystems in specific applications. Recently, advances in AI and machine learning have immensely helped to decode and empower cell-nanomaterial interaction modeling in predicting both biosafety and efficacy of nanodrugs115–117 with in silico methods potentially being able to decipher quantitative nanostructure activity-relationships (Nano-QSAR).116,118 While the pharmaceutical industry is expecting this paradigm-shifting technology to solve the current problems mainly in the areas of novel drug discovery, drug repurposing, and clinical trials; the nanomedicine field is taking to the AI toolbox for more detailed understanding of the interactions at the nano–bio interface, which in turn may aid in the proper and more rapid design of sophisticated nanosystems. With the integration of synthetic and systems biology approaches powered by AI,122 highly precise and accurate nanosystems are likely to be the way of the future.
Acknowledgments
Funding support from the Sigrid Jusélius Foundation (Finland), Magnus Ehrnrooth Foundation (Finland), and Medicinska Understödsföreningen Liv & Hälsa (Finland) is greatly acknowledged. This work was suppoterd by The Scientific and Technological Research Council of Turkey, TUBITAK, # 319S024 (D.S.K) TUBITAK, Directorate of Science Fellowships and Grant Programmes-2218, # 118C488 (O.K). This work is also part of the activities within the strategic research profiling area Solutions for Health at Åbo Akademi University (Academy of Finland, # 336355).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nigam A, Gupta D, Sharma A. Treatment of infectious disease: beyond antibiotics. Microbiol Res. 2014;169(9–10):643–651. doi:10.1016/j.micres.2014.02.009
2. Hand WL. Current challenges in antibiotic resistance. Adolesc Med. 2000;11(2):427–438.
3. Horizon 2020-Work Programme 2018–2020. Health, demographic change and wellbeing; 2017 [cited 2018 Feb 9]. Available from: http://ec.europa.eu/research/participants/data/ref/h2020/wp/2018-2020/main/h2020-wp1820-health_en.pdf.
4. Pandey AT, Pandey I, Hachenberger Y, et al. Emerging paradigm against global antimicrobial resistance via bioprospecting of mushroom into novel nanotherapeutics development. Trends Food Sci Technol. 2020;106:333–344. doi:10.1016/j.tifs.2020.10.025
5. Tiwari Pandey A, Pandey I, Zamboni P, et al. Traditional herbal remedies with a multifunctional therapeutic approach as an implication in COVID-19 associated co-infections. Coatings. 2020;10(8):761. doi:10.3390/coatings10080761
6. Banoo S, Bell D, Bossuyt P, et al. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol. 2006;4(S9):S21–S31. doi:10.1038/nrmicro1523
7. World Health Organization. Diagnostic Stewardship: a guide to implementation in antimicrobial resistance surveillance sites. World Health Organization; 2016. Available from: https://apps.who.int/iris/handle/10665/251553.
8. Tsalik EL, Bonomo RA, Fowler VG. New molecular diagnostic approaches to bacterial infections and antibacterial resistance. Annu Rev Med. 2018;69(1):379–394. doi:10.1146/annurev-med-052716-030320
9. Jakobsen TH, Xu Y, Bay L, et al. Sampling challenges in diagnosis of chronic bacterial infections. J Med Microbiol. 2021;70(3). doi:10.1099/jmm.0.001302
10. OECD. Safety assessment of transgenic organisms in the environment, Volume 5: OECD consensus documents. OECD; 2016. doi:10.1787/9789264253018-en.
11. Khan ZA, Siddiqui MF, Park S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics. 2019;9(2):49. doi:10.3390/diagnostics9020049
12. Tang J, Chu B, Wang J, et al. Multifunctional nanoagents for ultrasensitive imaging and photoactive killing of gram-negative and gram-positive bacteria. Nat Commun. 2019;10(1):4057. doi:10.1038/s41467-019-12088-7
13. Wang Z, Cai R, Gao Z, Yuan Y, Yue T. Immunomagnetic separation: an effective pretreatment technology for isolation and enrichment in food microorganisms detection. Compr Rev Food Sci Food Saf. 2020;19(6):3802–3824. doi:10.1111/1541-4337.12656
14. Northrup JD, Mach RH, Sellmyer MA. Radiochemical approaches to imaging bacterial infections: intracellular versus extracellular targets. Int J Mol Sci. 2019;20(22):5808. doi:10.3390/ijms20225808
15. Zazo H, Colino CI, Lanao JM. Current applications of nanoparticles in infectious diseases. J Control Release. 2016;224:86–102. doi:10.1016/j.jconrel.2016.01.008
16. Younes M, Aggett P, Aguilar F, et al.; EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Re‐evaluation of silicon dioxide (E 551) as a food additive. EFSA J. 2018;16(1):e05088. doi:10.2903/j.efsa.2018.5088
17. Garcia-Bennett AE. Synthesis, toxicology and potential of ordered mesoporous materials in nanomedicine. Nanomedicine. 2011;6(5):867–877. doi:10.2217/nnm.11.82
18. Karaman DŞ, Sarparanta MP, Rosenholm JM, Airaksinen AJ. Multimodality imaging of silica and silicon materials in vivo. Adv Mater. 2018;30(24):1703651. doi:10.1002/adma.201703651
19. Karaman DS. Nano-chemistry: the toolbox for nanoparticle based diagnosis and theraphy. Ann Chem Sci Res. 2018;1(1). doi:10.31031/ACSR.2018.01.000502
20. Pardhi DM, Şen Karaman D, Timonen J, et al. Anti-bacterial activity of inorganic nanomaterials and their antimicrobial peptide conjugates against resistant and non-resistant pathogens. Int J Pharm. 2020;586:119531. doi:10.1016/j.ijpharm.2020.119531
21. Wang Y, Salazar JK. Culture‐independent rapid detection methods for bacterial pathogens and toxins in food matrices. Compr Rev Food Sci Food Saf. 2016;15:183–205. doi:10.1111/1541-4337.12175
22. Chen CT, Yu JW, Ho YP. Identification of bacteria in juice/lettuce using magnetic nanoparticles and selected reaction monitoring mass spectrometry. J Food Drug Anal. 2019;27(2):575–584. doi:10.1016/j.jfda.2018.09.006
23. Pajerski W, Ochonska D, Brzychczy-Wloch M, et al. Attachment efficiency of gold nanoparticles by Gram-positive and Gram-negative bacterial strains governed by surface charges. J Nanopart Res. 2019;21(8):186. doi:10.1007/s11051-019-4617-z
24. Zhu M, Liu W, Liu H, et al. Construction of Fe 3 O 4 /vancomycin/PEG magnetic nanocarrier for highly efficient pathogen enrichment and gene sensing. ACS Appl Mater Interfaces. 2015;7(23):12873–12881. doi:10.1021/acsami.5b02374
25. Lu HD, Yang SS, Wilson BK, et al. Nanoparticle targeting of Gram-positive and Gram-negative bacteria for magnetic-based separations of bacterial pathogens. Appl Nanosci. 2017;7(3–4):83–93. doi:10.1007/s13204-017-0548-0
26. Hassan MM, Ranzoni A, Phetsang W, Blaskovich MAT, Cooper MA. Surface ligand density of antibiotic-nanoparticle conjugates enhances target avidity and membrane permeabilization of vancomycin-resistant bacteria. Bioconjugate Chem. 2017;28(2):353–361. doi:10.1021/acs.bioconjchem.6b00494
27. Hassan MM, Ranzoni A, Cooper MA. A nanoparticle-based method for culture-free bacterial DNA enrichment from whole blood. Biosens Bioelectron. 2018;99:150–155. doi:10.1016/j.bios.2017.07.057
28. Lee SY, Lee J, Lee HS, Chang JH. Rapid pathogen detection with bacterial-assembled magnetic mesoporous silica. Biosens Bioelectron. 2014;53:123–128. doi:10.1016/j.bios.2013.09.052
29. Mou XZ, Chen XY, Wang J, et al. Bacteria-instructed click chemistry between functionalized gold nanoparticles for point-of-care microbial detection. ACS Appl Mater Interfaces. 2019;11(26):23093–23101. doi:10.1021/acsami.9b09279
30. Shen H, Wang J, Liu H, et al. Rapid and selective detection of pathogenic bacteria in bloodstream infections with aptamer-based recognition. ACS Appl Mater Interfaces. 2016;8(30):19371–19378. doi:10.1021/acsami.6b06671
31. van de Beek D, de Gans J, Tunkel AR, Wijdicks EFM. Community-acquired bacterial meningitis in adults. N Eng J Med. 2006;354(1):44–53. doi:10.1056/NEJMra052116
32. Xu S. Electromechanical biosensors for pathogen detection. Microchim Acta. 2012;178(3–4):245–260. doi:10.1007/s00604-012-0831-4
33. Pant A, Mackraj I, Govender T. Advances in sepsis diagnosis and management: a paradigm shift towards nanotechnology. J Biomed Sci. 2021;28(1):6. doi:10.1186/s12929-020-00702-6
34. Shukla SK, Govender PP, Tiwari A. Polymeric micellar structures for biosensor technology. In: Advances in Biomembranes and Lipid Self-Assembly. Vol. 24. Elsevier; 2016:143–161. doi:10.1016/bs.abl.2016.04.005
35. Ahmed A, Rushworth JV, Hirst NA, Millner PA. Biosensors for whole-cell bacterial detection. Clin Microbiol Rev. 2014;27(3):631–646. doi:10.1128/CMR.00120-13
36. Singh R, Mukherjee MD, Sumana G, Gupta RK, Sood S, Malhotra BD. Biosensors for pathogen detection: a smart approach towards clinical diagnosis. Sens Actuators B Chem. 2014;197:385–404. doi:10.1016/j.snb.2014.03.005
37. Paniel N, Baudart J. Colorimetric and electrochemical genosensors for the detection of Escherichia coli DNA without amplification in seawater. Talanta. 2013;115:133–142. doi:10.1016/j.talanta.2013.04.050
38. Farrow B, Hong SA, Romero EC, et al. A chemically synthesized capture agent enables the selective, sensitive, and robust electrochemical detection of anthrax protective antigen. ACS Nano. 2013;7(10):9452–9460. doi:10.1021/nn404296k
39. Templier V, Roux A, Roupioz Y, Livache T. Ligands for label-free detection of whole bacteria on biosensors: a review. Trends Analyt Chem. 2016;79:71–79. doi:10.1016/j.trac.2015.10.015
40. Li Y, Xie G, Qiu J, et al. A new biosensor based on the recognition of phages and the signal amplification of organic-inorganic hybrid nanoflowers for discriminating and quantitating live pathogenic bacteria in urine. Sens Actuators B Chem. 2018;258:803–812. doi:10.1016/j.snb.2017.11.155
41. Zaraee N, Kanik FE, Bhuiya AM, et al. Highly sensitive and label-free digital detection of whole cell E. coli with interferometric reflectance imaging. Biosens Bioelectron. 2020;162:112258. doi:10.1016/j.bios.2020.112258
42. Crapnell RD, Dempsey-Hibbert NC, Peeters M, Tridente A, Banks CE. Molecularly imprinted polymer based electrochemical biosensors: overcoming the challenges of detecting vital biomarkers and speeding up diagnosis. Talanta Open. 2020;2:100018. doi:10.1016/j.talo.2020.100018
43. Verheyen E, Schillemans JP, van Wijk M, Demeniex M-A, Hennink WE, van Nostrum CF. Challenges for the effective molecular imprinting of proteins. Biomaterials. 2011;32(11):3008–3020. doi:10.1016/j.biomaterials.2011.01.007
44. Liu X, Marrakchi M, Xu D, Dong H, Andreescu S. Biosensors based on modularly designed synthetic peptides for recognition, detection and live/dead differentiation of pathogenic bacteria. Biosens Bioelectron. 2016;80:9–16. doi:10.1016/j.bios.2016.01.041
45. Ahari H, Hedayati M, Akbari-adergani B, Kakoolaki S, Hosseini H, Anvar A. Staphylococcus aureus exotoxin detection using potentiometric nanobiosensor for microbial electrode approach with the effects of pH and temperature. Int J Food Prop. 2017;20:1578–1587. doi:10.1080/10942912.2017.1347944
46. Azharuddin M, Zhu GH, Das D, et al. A repertoire of biomedical applications of noble metal nanoparticles. Chem Comm. 2019;55(49):6964–6996. doi:10.1039/C9CC01741K
47. Du H, Li Z, Wang Y, Yang Q, Wu W. Nanomaterial-based optical biosensors for the detection of foodborne bacteria. Food Rev Int. 2020:1–30. doi:10.1080/87559129.2020.1740733
48. Malekzad H, Sahandi Zangabad P, Mirshekari H, Karimi M, Hamblin MR. Noble metal nanoparticles in biosensors: recent studies and applications. Nanotechnol Rev. 2017;6(3):301–329. doi:10.1515/ntrev-2016-0014
49. Chen Z, Tan Y, Xu K, et al. Stimulus-response mesoporous silica nanoparticle-based chemiluminescence biosensor for cocaine determination. Biosens Bioelectron. 2016;75:8–14. doi:10.1016/j.bios.2015.08.006
50. Hasanzadeh M, Shadjou N, de la Guardia M, Eskandani M, Sheikhzadeh P. Mesoporous silica-based materials for use in biosensors. Trends Analyt Chem. 2012;33:117–129. doi:10.1016/j.trac.2011.10.011
51. Yang X, Qiu P, Yang J, et al. Mesoporous materials–based electrochemical biosensors from enzymatic to nonenzymatic. Small. 2021;17(9):1904022. doi:10.1002/smll.201904022
52. Ohlsen K, Hertlein T. Towards clinical application of non-invasive imaging to detect bacterial infections. Virulence. 2018;9(1):943–945. doi:10.1080/21505594.2018.1425072
53. Mills B, Bradley M, Dhaliwal K. Optical imaging of bacterial infections. Clin Transl Imaging. 2016;4(3):163–174. doi:10.1007/s40336-016-0180-0
54. van Oosten M, Hahn M, Crane LMA, et al. Targeted imaging of bacterial infections: advances, hurdles and hopes. FEMS Microbiol Rev. 2015;39(6):892–916. doi:10.1093/femsre/fuv029
55. Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol. 2010;65(7):500–516. doi:10.1016/j.crad.2010.03.011
56. Joshi BP, Wang TD. Targeted optical imaging agents in cancer: focus on clinical applications. Contrast Media Mol Imaging. 2018;2018:1–19. doi:10.1155/2018/2015237
57. Jiang L, Lee HW, Loo SCJ. Therapeutic lipid-coated hybrid nanoparticles against bacterial infections. RSC Adv. 2020;10(14):8497–8517. doi:10.1039/C9RA10921H
58. Vukomanovic M, Torrents E. High time resolution and high signal-to-noise monitoring of the bacterial growth kinetics in the presence of plasmonic nanoparticles. J Nanobiotechnol. 2019;17(1):21. doi:10.1186/s12951-019-0459-1
59. Lee NY, Ko WC, Hsueh PR. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front Pharmacol. 2019;10:1153. doi:10.3389/fphar.2019.01153
60. Burnham CAD, Leeds J, Nordmann P, O’Grady J, Patel J. Diagnosing antimicrobial resistance. Nat Rev Microbiol. 2017;15(11):697–703. doi:10.1038/nrmicro.2017.103
61. Valéria Dos Santos K, Diniz CG, de Castro Veloso L, et al. Proteomic analysis of Escherichia coli with experimentally induced resistance to piperacillin/tazobactam. Res Microbiol. 2010;161(4):268–275. doi:10.1016/j.resmic.2010.03.006
62. Hrabák J, Chudáčková E, Walková R. Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry for detection of antibiotic resistance mechanisms: from research to routine diagnosis. Clin Microbiol Rev. 2013;26(1):103–114. doi:10.1128/CMR.00058-12
63. Pinto L, Poeta P, Vieira S, et al. Genomic and proteomic evaluation of antibiotic resistance in Salmonella strains. J Proteomics. 2010;73(8):1535–1541. doi:10.1016/j.jprot.2010.03.009
64. Florio W, Tavanti A, Barnini S, Ghelardi E, Lupetti A. Recent advances and ongoing challenges in the diagnosis of microbial infections by MALDI-TOF mass spectrometry. Front Microbiol. 2018;9:1097. doi:10.3389/fmicb.2018.01097
65. Sauget M, Bertrand X, Hocquet D. Rapid antibiotic susceptibility testing on blood cultures using. PLoS One. 2018;13(10):e0205603. doi:10.1371/journal.pone.0205603
66. Zhu Y, Gasilova N, Jović M, et al. Detection of antimicrobial resistance-associated proteins by titanium dioxide-facilitated intact bacteria mass spectrometry. Chem Sci. 2018;9(8):2212–2221. doi:10.1039/C7SC04089J
67. Qi G, Li L, Yu F, Wang H. Vancomycin-modified mesoporous silica nanoparticles for selective recognition and killing of pathogenic gram-positive bacteria over macrophage-like cells. ACS Appl Mater Interfaces. 2013;5(21):10874–10881. doi:10.1021/am403940d
68. Bhaisare ML, Abdelhamid HN, Wu BS, Wu HF. Rapid and direct MALDI-MS identification of pathogenic bacteria from blood using ionic liquid-modified magnetic nanoparticles (Fe3O4 @SiO2). J Mater Chem B. 2014;2(29):4671–4683. doi:10.1039/C4TB00528G
69. Slowing II, Trewyn BG, Giri S, Lin VSY. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater. 2007;17(8):1225–1236. doi:10.1002/adfm.200601191
70. Vallet-Regi M, Rámila A, Del Real RP, Pérez-Pariente J. A new property of MCM-41: drug delivery system. Chem Mater. 2001;13(2):308–311. doi:10.1021/cm0011559
71. Lin YS, Hurley KR, Haynes CL. Critical considerations in the biomedical use of mesoporous silica nanoparticles. J Phys Chem Lett. 2012;3(3):364–374. doi:10.1021/jz2013837
72. Stein A, Melde BJ, Schroden RC. Hybrid inorganic-organic mesoporous silicates—nanoscopic reactors coming of age. Adv Mater. 2000;12(19):1403–1419. doi:10.1002/1521-4095(200010)12:19<1403::AID-ADMA1403>3.0.CO;2-X
73. Rahikkala A, Rosenholm JM, Santos HA. Biofunctionalized mesoporous silica nanomaterials for targeted drug delivery. In: Biomedical Applications of Functionalized Nanomaterials. Elsevier; 2018:489–520. doi:10.1016/B978-0-323-50878-0.00016-1
74. Zhang J, Rosenholm JM. Molecular and nanoscale engineering of porous silica particles for drug delivery. In: Nanoengineered Biomaterials for Advanced Drug Delivery. Elsevier; 2020:395–419. doi:10.1016/B978-0-08-102985-5.00017-6
75. Colilla M, Vallet-Regí M. Targeted stimuli-responsive mesoporous silica nanoparticles for bacterial infection treatment. Int J Mol Sci. 2020;21(22):8605. doi:10.3390/ijms21228605
76. Zheng H, Gong H, Cao L, Lin H, Ye L. Photoconjugation of temperature- and pH-responsive polymer with silica nanoparticles for separation and enrichment of bacteria. Colloids Surf B Biointerfaces. 2021;197:111433. doi:10.1016/j.colsurfb.2020.111433
77. Gulin-Sarfraz T, Zhang J, Desai D, et al. Combination of magnetic field and surface functionalization for reaching synergistic effects in cellular labeling by magnetic core–shell nanospheres. Biomater Sci. 2014;2(12):1750–1760. doi:10.1039/C4BM00221K
78. Wen CY, Jiang YZ, Li XY, et al. Efficient enrichment and analyses of bacteria at ultralow concentration with quick-response magnetic nanospheres. ACS Appl Mater Interfaces. 2017;9(11):9416–9425. doi:10.1021/acsami.6b16831
79. Li Z, Ma J, Ruan J, Zhuang X. Using positively charged magnetic nanoparticles to capture bacteria at ultralow concentration. Nanoscale Res Lett. 2019;14(1):195. doi:10.1186/s11671-019-3005-z
80. Kadam R, Maas M, Rezwan K. Selective, agglomerate-free separation of bacteria using biofunctionalized, magnetic janus nanoparticles. ACS Appl Bio Mater. 2019;2(8):3520–3531. doi:10.1021/acsabm.9b00415
81. Gao M, Zeng J, Liang K, Zhao D, Kong B. Interfacial assembly of mesoporous silica‐based optical heterostructures for sensing applications. Adv Funct Mater. 2020;30(9):1906950. doi:10.1002/adfm.201906950
82. RoyChaudhuri C. A review on porous silicon based electrochemical biosensors: beyond surface area enhancement factor. Sens Actuators B Chem. 2015;210:310–323. doi:10.1016/j.snb.2014.12.089
83. Gu Z, Fu A, Ye L, Kuerban K, Wang Y, Cao Z. Ultrasensitive chemiluminescence biosensor for nuclease and bacterial determination based on hemin-encapsulated mesoporous silica nanoparticles. ACS Sensors. 2019;4(11):2922–2929. doi:10.1021/acssensors.9b01303
84. Ciampi S, Böcking T, Kilian KA, Harper JB, Gooding JJ. Click chemistry in mesoporous materials: functionalization of porous silicon rugate filters. Langmuir. 2008;24(11):5888–5892. doi:10.1021/la800435d
85. Mathelié-Guinlet M, Gammoudi I, Beven L, et al. Silica nanoparticles assisted electrochemical biosensor for the detection and degradation of Escherichia coli bacteria. Procedia Eng. 2016;168:1048–1051. doi:10.1016/j.proeng.2016.11.337
86. Mathelié-Guinlet M, Cohen-Bouhacina T, Gammoudi I, et al. Silica nanoparticles-assisted electrochemical biosensor for the rapid, sensitive and specific detection of Escherichia coli. Sens Actuators B Chem. 2019;292:314–320. doi:10.1016/j.snb.2019.03.144
87. Coulthard MG. Defining urinary tract infection by bacterial colony counts: a case for 100,000 colonies/mL as the best threshold. Pediatr Nephrol. 2019;34(10):1639–1649. doi:10.1007/s00467-019-04283-x
88. Zhao X, Zhong J, Wei C, Lin CW, Ding T. Current perspectives on viable but non-culturable state in foodborne pathogens. Front Microbiol. 2017;8:580. doi:10.3389/fmicb.2017.00580
89. Schmitt CK, Meysick KC, O’Brien AD. Bacterial toxins: friends or foes? Emerg Infect Dis. 1999;5(2):224–234. doi:10.3201/eid0502.990206
90. Li L, Mendis N, Trigui H, Oliver JD, Faucher SP. The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol. 2014;5:258. doi:10.3389/fmicb.2014.00258
91. Reta N, Saint CP, Michelmore A, Prieto-Simon B, Voelcker NH. Nanostructured electrochemical biosensors for label-free detection of water- and food-borne pathogens. ACS Appl Mater Interfaces. 2018;10(7):6055–6072. doi:10.1021/acsami.7b13943
92. Yin M, Gu B, An QF, Yang C, Guan YL, Yong KT. Recent development of fiber-optic chemical sensors and biosensors: mechanisms, materials, micro/nano-fabrications and applications. Coord Chem Rev. 2018;376:348–392. doi:10.1016/j.ccr.2018.08.001
93. Huang F, Guo R, Xue L, et al. An acid-responsive microfluidic salmonella biosensor using curcumin as signal reporter and ZnO-capped mesoporous silica nanoparticles for signal amplification. Sens Actuators B Chem. 2020;312:127958. doi:10.1016/j.snb.2020.127958
94. Jenie SNA, Kusumastuti Y, Krismastuti FSH, et al. Rapid fluorescence quenching detection of Escherichia coli using natural silica-based nanoparticles. Sensors. 2021;21(3):881. doi:10.3390/s21030881
95. Montalti M, Prodi L, Rampazzo E, Zaccheroni N. Dye-doped silica nanoparticles as luminescent organized systems for nanomedicine. Chem Soc Rev. 2014;43(12):4243–4268. doi:10.1039/C3CS60433K
96. Park JH, Baek SD, Cho JI, et al. Characteristics of transparent encapsulation materials for OLEDs prepared from mesoporous silica nanoparticle-polyurethane acrylate resin composites. Compos B Eng. 2019;175:107188. doi:10.1016/j.compositesb.2019.107188
97. Andreiuk B, Reisch A, Bernhardt E, Klymchenko AS. Fighting aggregation‐caused quenching and leakage of dyes in fluorescent polymer nanoparticles: universal role of counterion. Chem Asian J. 2019;14(6):836–846. doi:10.1002/asia.201801592
98. Walcarius A, Mandler D, Cox JA, Collinson M, Lev O. Exciting new directions in the intersection of functionalized sol–gel materials with electrochemistry. J Mater Chem. 2005;15(35–36):3663. doi:10.1039/b504839g
99. Walcarius A. Silica-based electrochemical sensors and biosensors: recent trends. Curr Opin Electrochem. 2018;10:88–97. doi:10.1016/j.coelec.2018.03.017
100. Wang J. Sol–gel materials for electrochemical biosensors. Anal Chim Acta. 1999;399(1–2):21–27. doi:10.1016/S0003-2670(99)00572-3
101. Borsa BA, Tuna BG, Hernandez FJ, et al. Staphylococcus aureus detection in blood samples by silica nanoparticle-oligonucleotides conjugates. Biosens Bioelectron. 2016;86:27–32. doi:10.1016/j.bios.2016.06.023
102. Jadhav KS, Dumbare PS, Pande VV. Mesoporous silica nanoparticles (MSN): a nanonetwork and hierarchical structure in drug delivery. JNMR. 2015;2(5):1–8. doi:10.15406/jnmr.2015.02.00043
103. Yuan D, Ellis CM, Davis JJ. Mesoporous silica nanoparticles in bioimaging. Materials. 2020;13(17):3795. doi:10.3390/ma13173795
104. Carniato F, Tei L, Arrais A, Marchese L, Botta M. Selective anchoring of Gd III chelates on the external surface of organo-modified mesoporous silica nanoparticles: a new chemical strategy to enhance relaxivity. Chem Eur J. 2013;19(4):1421–1428. doi:10.1002/chem.201202670
105. von Baeckmann C, Guillet-Nicolas R, Renfer D, Kählig H, Kleitz F. A toolbox for the synthesis of multifunctionalized mesoporous silica nanoparticles for biomedical applications. ACS Omega. 2018;3(12):17496–17510. doi:10.1021/acsomega.8b02784
106. Kettiger H, Sen Karaman D, Schiesser L, Rosenholm JM, Huwyler J. Comparative safety evaluation of silica-based particles. Toxicol in Vitro. 2015;30(1):355–363. doi:10.1016/j.tiv.2015.09.030
107. Desai D, Karaman DS, Prabhakar N, et al. Design considerations for mesoporous silica nanoparticulate systems in facilitating biomedical applications. Mesoporous Biomater. 2014;1(1):16–43. doi:10.2478/mesbi-2014-0001
108. Zhang J, Rosenholm JM, Gu H. Molecular confinement in fluorescent magnetic mesoporous silica nanoparticles: effect of pore size on multifunctionality. ChemPhysChem. 2012;13(8):2016–2019. doi:10.1002/cphc.201100943
109. Rosenholm JM, Gulin-Sarfraz T, Mamaeva V, et al. Prolonged dye release from mesoporous silica-based imaging probes facilitates long-term optical tracking of cell populations in vivo. Small. 2016;12(12):1578–1592. doi:10.1002/smll.201503392
110. Girija AR, Balasubramanian S. Theragnostic potentials of core/shell mesoporous silica nanostructures. Nanotheranostics. 2019;3(1):1–40. doi:10.7150/ntno.27877
111. Parra-Robert M, Zeng M, Shu Y, et al. Mesoporous silica coated CeO2 nanozymes with combined lipid-lowering and antioxidant activity induce long-term improvement of the metabolic profile in obese Zucker rats. Nanoscale. 2021;13(18):8452–8466. doi:10.1039/D1NR00790D
112. Hidalgo G, Burns A, Herz E, et al. Functional tomographic fluorescence Imaging of pH microenvironments in microbial biofilms by use of silica nanoparticle sensors. Appl Environ Microbiol. 2009;75(23):7426–7435. doi:10.1128/AEM.01220-09
113. Kirla H, Hughes L, Henry DJ. Carbohydrate coated fluorescent mesoporous silica particles for bacterial imaging. Colloids Surf B Biointerfaces. 2020;188:110751. doi:10.1016/j.colsurfb.2019.110751
114. Xu C, Li Z, Akakuru OU, et al. Maltodextrin-conjugated Gd-Based MRI contrast agents for specific diagnosis of bacterial infections. ACS Appl Bio Mater. 2020;4(5):3762–3772. doi:10.1021/acsabm.0c01246
115. Singh AV, Ansari MHD, Rosenkranz D, et al. Artificial intelligence and machine learning in computational nanotoxicology: unlocking and empowering nanomedicine. Adv Healthcare Mater. 2020;9(17):1901862. doi:10.1002/adhm.201901862
116. Singh AV, Maharjan RS, Kanase A, et al. Machine-learning-based approach to decode the influence of nanomaterial properties on their interaction with cells. ACS Appl Mater Interfaces. 2021;13(1):1943–1955. doi:10.1021/acsami.0c18470
117. Singh AV, Rosenkranz D, Ansari MHD, et al. Artificial intelligence and machine learning empower advanced biomedical material design to toxicity prediction. Adv Intell Syst. 2020;2(12):2000084. doi:10.1002/aisy.202000084
118. Singh AV, Jahnke T, Wang S, et al. Anisotropic gold nanostructures: optimization via in silico modeling for hyperthermia. ACS Appl Nano Mater. 2018;1(11):6205–6216. doi:10.1021/acsanm.8b01406
119. Singh AV, Ansari MHD, Laux P, Luch A. Micro-nanorobots: important considerations when developing novel drug delivery platforms. Expert Opin Drug Deliv. 2019;16(11):1259–1275. doi:10.1080/17425247.2019.1676228
120. Patel GM, Patel GC, Patel RB, Patel JK, Patel M. Nanorobot: a versatile tool in nanomedicine. J Drug Target. 2006;14(2):63–67. doi:10.1080/10611860600612862
121. Tezel G, Timur SS, Kuralay F, et al. Current status of micro/nanomotors in drug delivery. J Drug Target. 2021;29(1):29–45. doi:10.1080/1061186X.2020.1797052
122. Singh AV, Laux P, Luch A, Balkrishnan S, Prasad Dakua S. Bottom-UP assembly of nanorobots: extending synthetic biology to complex material design. Front Nanosci Nanotech. 2019;5:1–2. doi:10.15761/FNN.1000S2005
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
