Back to Journals » International Journal of General Medicine » Volume 15
Rebamipide as a Potential Alternative Gastroprotective Agent to Proton Pump Inhibitor in Elderly Chronic Nonsteroidal Anti-Inflammatory Drug Users without Risk Factors
Authors Lee MY, Lee S, Heo KN, Kim WY, Jung SH, Ah YM , Lee JY
Received 16 December 2021
Accepted for publication 23 February 2022
Published 10 March 2022 Volume 2022:15 Pages 2835—2845
DOI https://doi.org/10.2147/IJGM.S353098
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mee Yeon Lee,1 Suhyun Lee,1 Kyu-Nam Heo,1 Woo-Youn Kim,1 Sun Hoi Jung,2 Young-Mi Ah,3 Ju-Yeun Lee1
1College of Pharmacy and Research Institute of Pharmaceutical Sciences, Seoul National University, Seoul, Republic of Korea; 2Department of Pharmacy, Seoul National University Boramae Medical Center, Seoul, Republic of Korea; 3College of Pharmacy, Yeungnam University, Gyeongsan-si, Republic of Korea
Correspondence: Ju-Yeun Lee, College of Pharmacy and Research Institute of Pharmaceutical Sciences, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul, 08826, Republic of Korea, Tel +82-2-3668-7472, Email [email protected]
Purpose: The use of proton pump inhibitors (PPI) is recommended to prevent nonsteroidal anti-inflammatory drug (NSAID)-induced gastrointestinal (GI) complications. The incidence of several adverse effects during the long-term use of PPI prompts the search for other alternatives. Limited studies have evaluated the efficacy of rebamipide, a widely used mucoprotective drug, as a gastroprotective agent (GPA) compared to PPI, focusing on the elderly chronic NSAID users, nor with GI risk stratification. We aimed to determine the population who would get benefit from the use of rebamipide as an alternative to PPI to prevent traditional nonsteroidal anti-inflammatory drug (tNSAID)-associated GI complications.
Patients and Methods: We identified 41,889 and 35,708 elderly chronic tNSAID users with PPI and rebamipide co-therapy, respectively, from the national claims database. Outcome was defined as hospitalization or emergency department visits due to serious GI complications. Propensity score-matched cohorts were constructed and compared within risk strata.
Results: In high and moderate risk groups with two risk factors, rebamipide showed a higher risk of serious GI complication compared to PPI (aHR 2.63, 95% CI 1.24– 5.59 and aHR 2.42, 95% CI 1.21– 4.83, respectively). However, in elderly patients without risk factors, there was no significant difference in the risk of serious GI complications between PPI and rebamipide (aHR 0.69, 95% CI 0.27– 1.76).
Conclusion: This study suggested that rebamipide can be considered as an alternative to PPI in elderly chronic tNSAID users without risk factors. However, elderly patients with other risk factors should use PPI rather than rebamipide. Therefore, the presence of GI risk factors needs to be evaluated in elderly chronic tNSAID users to prescribe the most suitable GPA in clinical practice.
Keywords: aged, gastroprotective agent, nonsteroidal anti-inflammatory drug, rebamipide, risk group
Introduction
The incidence of serious gastrointestinal (GI) complications in nonsteroidal anti-inflammatory drugs (NSAIDs) users during treatment has been reported to be 1–2% annually.1 However, about a quarter of patients aged ≥ 65 years use NSAIDs, and one-third of elderly patients who had taken NSAIDs were chronic users.2 Previous studies have shown that chronic NSAID use in the elderly increased the risk of adverse effects including peptic ulcer disease, which was prominent within the first month of treatment and remained high over time.3
Clinical guidelines categorize risk grade for NSAID-induced GI complications based on the number and profile of risk factors and recommend the concomitant use of gastroprotective agents (GPAs) with NSAIDs to reduce the risk of GI complications.4–8 The American Gastroenterological Association (AGA) recommends proton-pump inhibitor (PPI) as the treatment of choice for the prevention of GI complications in patients with traditional NSAIDs (tNSAIDs) or high risk patients with selective cyclooxygenase-2 (COX-2) inhibitors.4 A recently updated evidence-based clinical practice guideline by the Japanese Society of Gastroenterology recommends PPI with or without COX-2 inhibitors for patients with a previous history of GI complications and also strongly recommends the use of PPI as a GPA in elderly patients.9 However, long-term treatment with PPI was associated with an increased risk of Clostridium difficile infection, pneumonia, gastric cancer, bone fractures, and cognitive impairment.10,11
Rebamipide, a mucoprotective agent enhancing mucosal defense against gastric acid, is another class of GPA, and little has been reported on its long-term adverse effects. It has been approved for the treatment of gastric ulcers and gastritis. However, it is also widely used for the prevention of GI complications in clinical practice.
Pooled analysis showed that the use of rebamipide was effective in preventing NSAID-induced gastric injury compared to non-users. A recent single-center study in patients aged ≥ 20 years concluded that rebamipide was not inferior to PPI in protecting against NSAID-induced GI complication, which was in line with result from previous systematic review and meta-analysis, but concurrent users of anticoagulants, antiplatelets, and steroids were excluded.12–15
To the best of our knowledge, limited studies have been conducted to evaluate the gastroprotective effects of rebamipide among elderly chronic NSAID users compared to PPI nor with GI risk stratification. It is necessary to compare GI protective effects in the risk-stratified population to identify which patients can benefit from using rebamipide other than PPI in clinical practice.
Therefore, we aimed to evaluate the effectiveness of rebamipide in preventing serious GI complications associated with chronic NSAID use in elderly patients compared to PPI with stratification based on NSAID-induced GI complication risk grade and the number of risk factors.
Patients and Methods
Data Source
This study was performed using National claims database provided by the Korean Health Insurance Review and Assessment Service (HIRA), and was approved by the institutional review board of Seoul National University (SNU IRB No. E2002/001-008). Informed consent was waived as de-identified information was used.
Study Design and Population
In this retrospective cohort study, we screened patients aged ≥ 65 years who were diagnosed with arthritis and had at least one prescription for tNSAIDs from 2016 to 2018.
First, we identified incident chronic tNSAID users during the enrollment period (July 1, 2016 to June 30, 2017). Incident chronic tNSAID users were defined as patients who were prescribed tNSAIDs for at least 30 consecutive days during the enrollment period without prior history of tNSAID use for more than 15 consecutive days or 25 cumulative days during the 6 months prior to cohort entry.
The PPI and rebamipide cohorts included patients who started each GPA as monotherapy at the time of tNSAID initiation and maintained at least 24 consecutive days, which is 80% of the minimum chronic tNSAID use. The treatment episode started with the first date of the tNSAID and GPA co-therapy, and ended when tNSAID treatment or initial GPA were terminated. We allowed a gap of 30 days between the supplies of tNSAIDs and GPAs for continuous use. For treatment ending, we allowed a grace period considering 80% adherence after the end of the medication supply. The analyses were performed based on the treatment episodes. The treatment episode that occurred 180 days after the end of the previous episode was considered as a new episode.
Patients were excluded from the analysis who received combination therapy with other GPAs including histamine-2 receptor antagonists (H2RA), misoprostol, potassium-competitive acid blocker (i.e., prazan), and Artemisia herb soft extract at the start of the treatment episode; who had a history of esophageal varices or gastrointestinal cancer; and who were without a minimum follow-up period of 30 days after the end of the treatment episode.
The study population was stratified into the high risk group with more than two risk factors or history of serious GI complication, moderate risk group with two risk factors, the elderly users aged ≥ 65 years without additional risk factors other than age, and low risk group without risk factors. Risk factors were defined as high dose NSAID therapy, previous history of non-serious GI complications, concurrent use of aspirin, corticosteroids, or anticoagulants, and age ≥ 65 years according to the clinical guidelines and expert consensus on NSAID use.4,6 Given the limited consensus on cut-off values for high dose NSAIDs associated with upper GI complications, high dose NSAID therapy was defined as NSAIDs with > 1 defined daily dose (DDD), a unit of measurement indicating assumed average maintenance dose per day established by the World Health Organization.16 As our dataset only included patients aged ≥ 65 years, no patient was classified as the low risk group in this study.
Baseline comorbidities and concomitant medications known to have a significant effect on NSAID-induced GI complications during the 6 months before cohort entry were identified. Anticoagulants, antiplatelets, and selective serotonin reuptake inhibitors (SSRIs) administered over 80% of the treatment episode, or steroids administered for more than 7 consecutive days during the treatment episode were included as co-medications.
For each risk group, the PPI cohort was matched to the rebamipide cohort using a propensity score (Supplementary Table S1). The matching was performed using a greedy algorithm with a width of caliper equal to 0.2 standard deviations of the logit of the propensity score without replacement.
Outcome Definition
The study outcome was defined as hospitalization or emergency department visits due to serious GI complications until 30 days after the treatment episode. Serious GI complications include upper and lower GI bleeding, perforation, or obstruction identified with the main diagnosis code (Supplementary Table S2). The patients were followed up for up to 18 months. Patients were censored immediately when one of the following occurred: start combination with or switch to other GPAs, new diagnosis of gastrointestinal cancer or esophageal varices, or end of claims data.
Statistical Analysis
The incidence rate of NSAID-induced GI complications was calculated as the number of events divided by the total amount of time at risk, and the incidence proportion was obtained by dividing the number of events by the total number of persons at risk.
Cumulative incidence estimations were performed using Kaplan-Meier method, and comparisons were analyzed using Gray’s test. A multivariate Cox proportional hazard model was used to estimate the adjusted hazard ratios, and the PPI cohort served as the reference.
Data management and statistical analyses were performed using SAS version 6.1 (SAS Institute, Cary, NC).
Results
Demographic and Clinical Characteristics
The number of patients aged over 65 years, diagnosed with arthritis, and using tNSAIDs for more than 30 days from 2016 to 2018 was 1,236,235. After applying exclusion criteria, a PPI cohort of 41,889 patients and a rebamipide cohort of 35,708 patients were constructed. After stratifying patients based on the risk grade of NSAID-induced GI complications, the final cohort of each stratum after matching is shown in Figure 1.
Patients aged 65–107 years were included in the analysis, and the mean ages of the matched cohorts were 74.4 years for both PPI and rebamipide cohorts. Hypertension, gastroesophageal reflux disease, and diabetes mellitus were the most common comorbidities. In the high risk group, most patients had three risk factors (82.7% for PPI; 85.4% for rebamipide), followed by four risk factors (12.9% for PPI; 9.9% for rebamipide), and those who had a history of serious GI bleeding or perforation were 4.9% for PPI, and 5.1% for rebamipide group. The mean Charlson Comorbidity Index (CCI) score was higher in the high risk group than the moderate risk group and was similar in both cohorts (Table 1).
 |
Table 1 Baseline Characteristics of Chronic tNSAID Users According to Risk Grade and GPA |
PPI Vs Rebamipide in High Risk Group
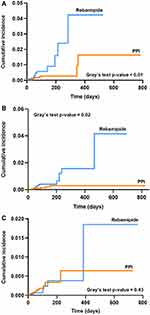
The PPI cohort showed significantly lower incidence proportions (0.16% for PPI, 0.35% for rebamipide, P = 0.02) and incidence rates of NSAID-induced serious GI complications compared to the rebamipide cohort (Table 2). With a median follow-up period of 43–49 days, PPI showed a cumulative incidence of 1.63%, while rebamipide showed 4.23% (P < 0.01) (Figure 2A). In the multivariate Cox proportional hazard model adjusted for well-known risk factors presented in the methods, using rebamipide as GPA showed a significantly higher risk of serious GI complications (aHR 2.63, 95% CI 1.24–5.59) compared with PPI (Figure 3). In addition, concurrent use of anticoagulants, antiplatelets, SSRI, and age ≥ 85 years were other significant risk factors for NSAID-induced serious GI complications (Supplementary Table S3).
 |
Table 2 Incidence of tNSAID-Induced Serious GI Complications in Chronic tNSAID Users by Risk Grade and Number of Risk Factors |
PPI Vs Rebamipide in Moderate Risk Group with Two Risk Factors
Incidence rates were significantly higher in the rebamipide cohort (0.58/100 PY for PPI, 1.17/100 PY for rebamipide, P = 0.03), while no significant difference was observed in the incidence proportions (0.11% for PPI, 0.19% for rebamipide, P = 0.09). During a median follow-up of 42–46 days, the cumulative incidence of PPI was 0.30% and 4.16% for rebamipide (P = 0.02) (Figure 2B). In multivariate analysis, rebamipide showed significantly less effective GI protection from tNSAID than PPI (aHR 2.42, 95% CI 1.21–4.83). Furthermore, co-administration of SSRI, age ≥ 85 years, male sex, and CCI score of 2–4 significantly increased the risk of serious GI complications (Supplementary Table S4).
PPI Vs Rebamipide in Elderly Patients without Risk Factors
There was no significant difference in the incidence proportion (0.13% for PPI, 0.09% for rebamipide, P = 0.30) and incidence rate (0.73/100 PY for PPI, 0.51/100 PY for rebamipide, P = 0.40) between the PPI and rebamipide cohorts. In addition, both cohorts showed no difference in cumulative incidence with 42–44 median days of follow-up (0.64% for PPI, 1.85% for rebamipide, P = 0.43) (Figure 2C). In multivariate analysis, rebamipide was not inferior to PPI in preventing NSAID-induced serious GI complications (aHR 0.69, 95% CI 0.27–1.76). Instead, concurrent use of antiplatelets and age of 75–84 years was shown to be risk factor for serious GI complications (Supplementary Table S5).
Discussion
In this study, we compared the GI protective effects of PPI and rebamipide in chronic tNSAID users with stratification based on each patient’s risk grade of NSAID-induced GI complications. In patients in the high risk group and moderate risk group with two risk factors, PPI provided significantly more effective GI protection than rebamipide. Specifically, rebamipide showed a 2.6- and 2.4-fold risk of serious GI complications in the high risk group and moderate risk group with two risk factors, respectively. This result was in line with the current AGA consensus, which recommends PPI as the treatment of choice for GPA in patients with high GI complication risk.7
However, in elderly patients without additional risk factors other than age, there was no significant difference in incidence proportion, incidence rate, cumulative incidence, and risk of serious GI complications between PPI and rebamipide after adjustment of confounders. These results suggest that rebamipide can provide a similar extent of GI protection as PPI in the chronic tNSAID-using elderly without risk factors.
Although PPI is recommended in elderly tNSAID users, PPI has also been widely reported to increase a risk of serious adverse effects, including pneumonia, gastric cancer, and cognitive impairment, with its long-term use.9–11
Considering that rebamipide is a tolerable agent without significant adverse drug events, the results of our study suggest the possibility of rebamipide being an alternative to PPI in tNSAID-using elderly patients with no additional risk factors for GI complications.17
A recent single-center cohort study was done in patients who were prescribed with NSAIDs for at least 1 month from the outpatient department and compared the effect of GPAs on preventing NSAID-induced occult GI bleeding.15 There was no significant difference in GI bleeding risk between rebamipide and PPI. However, the results were obtained from the adult population of all ages. Furthermore, they excluded patients with concurrent use of anticoagulants, antiplatelets, or steroids, and those with a history of peptic ulcer disease, each of which is an independent risk factor for NSAID-induced GI complications. Patients with diseases such as chronic kidney disease, chronic liver disease, or malignancy, which are common comorbidities in the elderly, were also excluded. Thus, the results may fit more to patients with relatively low GI risk, which results in a comparable hazard ratio of rebamipide to PPI with the result from elderly patients without risk factors in our study. Therefore, we cannot recommend the use of rebamipide instead of PPI in the elderly population who have a higher risk of GI complications, patients with a history of serious GI complications, and patients with several GI risk factors.
Another meta-analysis also concluded that there is no difference between rebamipide and PPI in the GI protective effect against NSAID. However, studies included have not been conducted in the elderly population, and GI risk factors were not considered.14 Thus, the results from the meta-analysis cannot be extrapolated to the elderly, each with different risk grades.
In this study, we evaluated elderly patients including all conditions related to GI risk factors and compared rebamipide with PPI for each risk group after adjustment for all conditions that are independent risk factors for serious GI outcomes. Since this study was conducted with elderly patients aged ≥ 65 years, all subjects had at least one risk factor of NSAID-induced GI complications. Therefore, the results from our study can simply be applied in practice as follows: As recommended by the current guideline, PPI is preferred as GPA monotherapy for NSAID rather than rebamipide among elderly patients aged ≥ 65 years who have previous history of serious GI complications or have at least one risk factor other than age. However, rebamipide can also be used as a GPA monotherapy in elderly patients aged ≥ 65 years, who have no additional risk factors other than age.
To the best of our knowledge, this study is the first nationwide study to compare rebamipide and PPI in elderly patients with risk stratification of GI complications. We determined the population in whom rebamipide showed GI protective effect similar to that of PPI by comparing both agents in chronic tNSAID users with stratification based on each patient’s risk grade of NSAID-induced GI complications. The results from our study might enable clinical practitioners to determine proper GPA in chronic tNSAID users after assessment of GI risk factors, and provide new and reliable evidence, which is essential while updating guidelines on the drug-related peptic ulcers in Korea.6 Moreover, it is meaningful to understand the substitutability of rebamipide for PPI therapy, as rebamipide provides higher cost-effectiveness and possibly minimizes the adverse effects caused by the long-term use of PPI.10,11,14 Thus, we suggest that the potential of rebamipide as an alternative GPA monotherapy be investigated with large-scale prospective randomized controlled studies in elderly chronic tNSAID users having low GI complication risk.
Our study has several limitations. First, medications available without prescription were excluded, as our study was performed based on the claims data. Nonetheless, chronic NSAID users are highly likely to receive prescriptions rather than purchase over-the-counter medication; therefore, the clinical impact underestimated in this study might not be significant. Second, we were not able to determine whether the patient actually took the medication with claims data. However, adherence measured with claims data was shown to be in concordance with actual drug exposure in many other studies.18 Third, due to the nature of claims data, no endoscopic data were available and diagnostic codes may be inaccurate for mild GI complications. Therefore, only serious GI complications related to hospitalization or emergency department visits were regarded as outcomes. Fourth, Helicobacter pylori infection, which is an independent risk factor for serious GI complications, was not considered in our study due to the limited information, so we might have underestimated the proportion of patients at risk. Fifth, we did not compare the effect of rebamipide and tNSAID co-prescription with that of COX-2 inhibitor, which is another GI protective strategy. Finally, although our study analyzed chronic tNSAID users who were prescribed with tNSAIDs for more than 30 days, the actual median follow-up duration was 42–49 days due to the relatively short duration of using GPA as monotherapy. Thus, the observation was not long enough to follow up during the chronic use of tNSAIDs.
Conclusion
This study suggests that elderly patients with a history of GI bleeding or perforation or with at least one risk factor other than age showed a significantly higher risk of serious GI complications with the use of rebamipide as GPA monotherapy compared to PPI, supporting the current guideline that suggests PPI as GPA monotherapy for chronic tNSAID use. However, in elderly patients without additional risk factors other than age nor previous history of GI bleeding or perforation, rebamipide could be an alternative to PPI as a GPA with tNSAID use.
Abbreviations
AGA, American Gastroenterological Association; aHR, adjusted hazard ratio; CCI, Charlson Comorbidity Index; CI, confidence interval; COX-2, cyclooxygenase-2; DDD, defined daily dose; GI, gastrointestinal; GPA, gastroprotective agent; H2RA, histamine-2 receptor antagonist; HIRA, Health insurance review and assessment service; IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton-pump inhibitor; PY, person-year; SD, standard deviation; SSRI, selective serotonin reuptake inhibitor; tNSAID, traditional nonsteroidal anti-inflammatory drug.
Data Sharing Statement
The data that support the findings of this study are available from HIRA, but restrictions apply to the availability of these data, which were used under license for the current study, and therefore are not publicly available. However, core data generated and analyzed during this study are included in this published article and its Supplementary Information Files. The HIRA, the data provider, requires all involved Researchers to pledge not to share, release, or review the data with other entities due to possibility of compromising ethical standards.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A2C110097111).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sostres C, Gargallo CJ, Lanas A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res Ther. 2013;15(Suppl 3):S3. doi:10.1186/ar4175
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs aging. 2003;20(9):701–710. doi:10.2165/00002512-200320090-00006
3. Marcum ZA, Hanlon JT. Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Ann Longterm Care. 2010;18(9):24–27.
4. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Am J Gastroenterol. 2010;105:2533–2549. doi:10.1038/ajg.2010.445
5. Yuan JQ, Tsoi KK, Yang M, et al. Systematic review with network meta-analysis: comparative effectiveness and safety of strategies for preventing NSAID-associated gastrointestinal toxicity. Aliment Pharmacol Ther. 2016;43(12):1262–1275. doi:10.1111/apt.13642
6. Joo MK, Park CH, Kim JS, et al. Clinical Guidelines for Drug-Related Peptic Ulcer, 2020 Revised Edition. Gut Liver. 2020;14(6):707–726. doi:10.5009/gnl20246
7. Wilcox CM, Allison J, Benzuly K, et al. Consensus development conference on the use of nonsteroidal anti-inflammatory agents, including cyclooxygenase-2 enzyme inhibitors and aspirin. Clin Gastroenterol Hepatol. 2006;4(9):1082–1089. doi:10.1016/j.cgh.2006.04.010
8. Lazzaroni M, Porro GB. Management of NSAID-induced gastrointestinal toxicity: focus on proton pump inhibitors. Drugs. 2009;69(1):51–69. doi:10.2165/00003495-200969010-00004
9. Kamada T, Satoh K, Itoh T, et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J Gastroenterol. 2021;56(4):303–322. doi:10.1007/s00535-021-01769-0
10. Jaynes M, Kumar AB. The risks of long-term use of proton pump inhibitors: a critical review. Ther Adv Drug Saf. 2019;10:2042098618809927. doi:10.1177/2042098618809927
11. Kinoshita Y, Ishimura N, Ishihara S. Advantages and Disadvantages of Long-term Proton Pump Inhibitor Use. J Neurogastroenterol Motil. 2018;24(2):182–196. doi:10.5056/jnm18001
12. Guo CG, Leung WK. Potential Strategies in the Prevention of Nonsteroidal Anti-inflammatory Drugs-Associated Adverse Effects in the Lower Gastrointestinal Tract. Gut Liver. 2020;14(2):179–189. doi:10.5009/gnl19201
13. Kim JH, Park SH, Cho CS, et al. Preventive efficacy and safety of rebamipide in nonsteroidal anti-inflammatory drug-induced mucosal toxicity. Gut Liver. 2014;8(4):371–379. doi:10.5009/gnl.2014.8.4.371
14. Zhang S, Qing Q, Bai Y, et al. Rebamipide helps defend against nonsteroidal anti-inflammatory drugs induced gastroenteropathy: a systematic review and meta-analysis. Dig Dis Sci. 2013;58(7):1991–2000. doi:10.1007/s10620-013-2606-0
15. Kim TJ, Kim ER, Hong SN, et al. Effectiveness of acid suppressants and other mucoprotective agents in reducing the risk of occult gastrointestinal bleeding in nonsteroidal anti-inflammatory drug users. Sci Rep. 2019;9(1):11696. doi:10.1038/s41598-019-48173-6
16. Castellsague J, Riera-Guardia N, Calingaert B, et al. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf. 2012;35(12):1127–1146. doi:10.1007/BF03261999
17. Park SH, Cho CS, Lee OY, et al. Comparison of Prevention of NSAID-Induced Gastrointestinal Complications by Rebamipide and Misoprostol: a Randomized, Multicenter, Controlled Trial-STORM STUDY. J Clin Biochem Nutr. 2007;40(2):148–155. doi:10.3164/jcbn.40.148
18. Fairman KA, Motheral B. Evaluating Medication Adherence: which Measure Is Right for Your Program? J Manag Care Pharm. 2000;6(6):499–506. doi:10.18553/jmcp.2000.6.6.499
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.