Back to Journals » Journal of Pain Research » Volume 10
Real-world utilization of once-daily extended-release abuse deterrent formulation of hydrocodone: a comparison with the pre-approval randomized clinical trials
Authors Taber L , Bond TC, Wang X, Kadakia A, Mayne TJ
Received 3 May 2017
Accepted for publication 26 June 2017
Published 25 July 2017 Volume 2017:10 Pages 1741—1746
DOI https://doi.org/10.2147/JPR.S140990
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Louise Taber,1 T Christopher Bond,2 Xuezhe Wang,2 Aditi Kadakia,2 Tracy J Mayne2
1Arizona Research Center, Phoenix, AZ, USA; 2Purdue Pharma L.P., Stamford, CT, USA
Background and objective: Hydrocodone bitartrate extended release (Hysingla® ER, HYD) was previously studied in a 12-week randomized, double-blind, placebo-controlled trial and a 52-week open-label safety study. Both of these preapproval studies allowed dose titration to efficacy. The purpose of the present analysis was to compare dosing and utilization patterns in these previous clinical trials with real-world data (RWD) usage in a retrospective claim analysis performed 12–14 months post approval in the US.
Methods: In the claim analysis (Truven Health Analytics MarketScan® Research Database), patients prescribed HYD between January 1, 2015, and April 30, 2016, were followed for up to 6 months of continuous HYD use. Daily average consumption (DACON), initial dose, rescue opioid use and total milligram dose over time were also evaluated.
Results: HYD daily dose stabilized at ~60 mg dose once daily across all three studies. There was also a reduced need for rescue medication with HYD, resulting in a lower total opioid milligram dose over time. In the claim analysis, the mean monthly HYD dose increased from 49 to 55 mg in month 2 and then remained stable through month 6. The mean (standard deviation [SD]) time on drug was 79.5 days (61.42 days), and DACON was 1.04 pills/day, corresponding to the approved full prescribing information (FPI) and once-daily dosing.
Conclusion: In 12–14 months post approval, real-world dosing and utilization of HYD mirrored registration and open-label study findings, with stable once-daily dosing of ~60 mg and no increase in rescue medicine utilization.
Keywords: chronic low back pain, hydrocodone, bitartrate extended release, opioid, Hysingla ER, NCT01452529, NCT01400139
Background
Hydrocodone bitartrate extended release tablet (Hysingla® ER, HYD; Purdue Pharma L.P., Stamford, CT, USA) is a once-daily (every 24 hours) opioid formulation with properties intended to deter abuse. It is indicated for the management of pain severe enough to require daily, around-the-clock, long-term treatment and for which alternative treatment options are inadequate.1 HYD was approved for use in the US in November 2014 and commercialized in January 2015.
Previously, HYD demonstrated efficacy and safety in a Phase III randomized, double-blind, placebo-controlled, multicenter, 12-week clinical trial in both opioid-experienced and opioid-naïve patients with moderate-to-severe chronic low back pain who were unresponsive to prior analgesic therapy.2 Among the 905 patients treated with HYD in the initial open-label titration period, 588 (65%) successfully stopped their other chronic pain medications and achieved a stable HYD dose during the 45-day titration period. These patients were randomized to either continue on HYD (n=296) or be tapered off HYD and onto placebo (n=292). During the 12-week double-blind period, the average daily HYD dose was 57 mg (Figure 1A). Among HYD patients, 78% utilized optional rescue medication of 5 mg oxycodone immediate release (IR; ≤ maximum of 30 mg/day) versus 83% of placebo patients. The mean dose of rescue medication was 3.4 mg for HYD patients and 4.5 mg for placebo patients.
While randomized, placebo-controlled trials are necessary to understand safety and efficacy for the purpose of drug registration, the study sample and drug utilization in tightly controlled and monitored trials may not be representative of real-world use. Pain practice in a community setting was more closely approximated in a previous second study, an open-label trial of the long-term safety and effectiveness of HYD for patients with chronic moderate-to-severe nonmalignant/non-neuropathic pain.3 In this study, 922 patients enrolled, and after a dose-titration period of ≤45 days, 728 entered and 410 completed a 52-week maintenance period. The mean HYD daily dose at the end of the dose-titration period was 60.9 mg and averaged 62.6 mg throughout maintenance (Figure 1B). Most patients (66%) remained on the same HYD dose for the 52 weeks. During the titration period, the average daily dose of rescue opioid medications decreased from 19.9 to 4.0 mg. During the maintenance period, 48% of HYD-treated patients used rescue opioids, and the daily dose of these rescue medications remained stable at 3.1 mg.
Although the open-label trial more closely approximates real-world use than a double-blind randomized trial, it is still controlled and monitored. It is possible that this may not represent the real-world use seen post approval. Real-world data (RWD) are observations of effects based on what happens after a prescriptive treatment decision is made where the researcher cannot control who gets what treatment and cannot control the medical management of the patient beyond observing the outcomes. The goal of the present retrospective claim analysis was to examine actual use of HYD in an uncontrolled environment using observational insurance claim data. We examined these real-world treatment patterns to assess how closely they parallel more controlled clinical trials with regard to mean dose, daily average consumption (DACON), rescue opioid use and total opioid dose over time. As this was a claim analysis, no information about efficacy or safety relative to the label could be assessed.
Methods
Database and cohort definition
The methodology used on the clinical trial and open-label safety study has been reported elsewhere.2,3 A retrospective cohort study was performed using commercial claims from the Truven MarketScan Commercial Claims and Encounter data (Truvenhealth.com) for October 1, 2014, through July 31, 2016. This database, publicly available for a fee, provides medical claims linked to outpatient prescription drug claims and person-level enrollment data. It is a consolidation of longitudinal medical and prescription claims (and associated International Classification of Diseases, 9th Revision, Clinical Modification, ICD-9-CM codes) from 150 contributing employers, with 130 contributing unique carriers and 21 health care plans representing >98 million patients, and is representative of the US population in real-world settings.
Patients 18–64 years who had a recorded prescription filled for an opioid between January 1, 2015 and April 30, 2016 were eligible. Only first use for each patient was included, and no previous HYD use was possible because the observation period started at the introduction of the drug into the US market. There was no institutional review board (IRB) or ethics committee approval required for the retrospective data real-world evidence (RWE) analysis from this claims database, as all data are de-identified and Health Insurance Portability and Accountability Act (HIPAA) compliant.
Patients were required to be continuously enrolled during the 3-month pre-index baseline period and for at least 3 months (90 days) after the index date – defined as the first dispensed HYD prescription on or after January 1, 2015. Use of any ER or IR opioids was also tracked; however, baseline use was not an inclusion/exclusion criterion.
Patients were followed for up to 6 months of continuous HYD use. Follow-up months were included if at least 1 day was covered by a prescription for HYD. The total period of continuous use was calculated using a 15-day allowable gap between prescriptions. Mean HYD dose and standard deviation (SD) were calculated at each month using a generalized linear model (GLM) to adjust for intra-patient correlation. No other statistical analyses were performed.
Analysis of dose
Average daily dose calculations were based on prescribed dose, as actual compliance was not observed. For example, the following rules were used for HYD:
- Early refills at the same dose were carried forward to the scheduled refill date (eg, two 30-day prescriptions with the second filled 2 days early would be counted as a full 60 days at the same dose).
- For early refills at a different dose, the previous prescription was stopped the day before the second fill and the new prescription dose was applied to the following days (eg, if a 30-day prescription at 30 mg was followed by a 30-day prescription at 40 mg filled on day 29, days 1–28 would be assigned 30 mg and days 29–58 would be assigned 40 mg).
- For late (1–14 days) refills, the dose of the previous prescription was carried through the gap as the prescribed dose for that period.
Equivalent rules were used for rescue medicines after conversion to morphine equivalent (MEQ). These rules did not apply to DACON, as described later. For IR opioids, average daily dose calculations were based on the calculated use. It was assumed that available pills were taken as prescribed and no pills were taken during gaps between prescriptions.
Mean and SD of dose dispensed for all other ER opioids and all IR opioids – converted to MEQ – were reported for each of the 3 baseline months. All opioids were converted using Prescription Drug Monitoring Program Training and Technical Assistance Center Guide, eg, 1 mg HYD was considered to be 1 MEQ.4 For the 6 follow-up months, HYD dosing was reported, along with the doses of any rescue opioids and total opioid dose, including HYD.
Analysis of DACON
DACON was calculated for HYD group by dividing the total number of tablets dispensed during the first 90 prescription days by 90. For prescriptions extending beyond the 90th day, the average number of tablets was assessed for the entire prescription, but that average was applied only to the days through day 90. Days during a gap in prescription claims (≤15 days) were assigned a pill count of 0.5
Results
Demographics
This study included 2,163 patients dispensed HYD who met baseline data requirements. The most commonly diagnosed pain condition in the 3 months baseline for the HYD group was back/neck pain, recorded for 73.4% patients, and 15.6% reported osteoarthritis (Table 1). Patients could have more than one type of pain recorded, and the mean number of pain diagnoses per patient was 2.2. None of the prespecified pain conditions were recorded for 9.3% of HYD patients in the 3 months baseline (eg, a patient may have had a diagnosis for an injury/event but not pain related to that injury/event). The mean (SD) age was 48.6 years (9.91 years), and 57.7% of HYD patients were females.
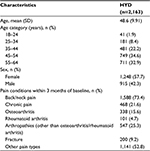  | Table 1 Demographics and clinical characteristics Abbreviations: ER, extended release; HYD, Hysingla® ER; SD, standard deviation. |
Opioid use during baseline period
The majority of HYD patients used an opioid during the 3-month baseline period (93.2%) and 26.5% used one or more ER opioids (Table 2). Among patients receiving opioid treatment in the baseline period, mean doses were 73.9 MEQ in the month before starting HYD and 77.3 MEQ in the month prior to that. The doses of IR opioids and ER opioids used during the baseline period were lower in the third month before the index date compared to the last 2 months prior to the index date.
  | Table 2 Opioids used during baseline period Abbreviations: ER, extended release; HYD, Hysingla® ER; IR, immediate release. |
Patients discontinuing therapy
Patients were dropped from the analysis due to discontinuation of HYD. This comprised ≤3% of patients in any given treatment month. The highest discontinuation rate was between month 1 and month 2 as 47% of patients discontinued HYD treatment after 1 month.
HYD dose and DACON during observation period
The mean (SD) starting dose for HYD was 49 mg (105 mg; Table 3), and the mean time on drug was 79.5 days (61.42 days). HYD dose increased to 55 mg (96 mg) in month 2 and was relatively stable through month 6 ending at 55 mg (87 mg; Figure 1C).
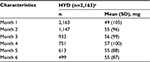  | Table 3 HYD dose during observation period Note: aMean (SD) time on drug = 79.5 days (61.42 days). Abbreviations: HYD, Hysingla® ER; SD, standard deviation; ER, extended release. |
HYD patients received a mean of 1.04 pills/day. Note that the full prescribing information (FPI) for HYD states to take once daily (every 24 hours).1,2
Concomitant opioid dose during observation period
Among the patients treated with HYD, the highest use of rescue opioids was in month 1 (85.3%). In months 2–6, the percentage of patients with a prescription fill for a rescue opioid ranged from 74.4% to 77.8%. Among patients prescribed rescue opioids in a given month, mean (SD) doses were 47 MEQ (83 MEQ) to 58 MEQ (122 MEQ). The dose for rescue opioids decreased slightly for HYD patients from 52 MEQ (100 MEQ) in month 2 to a low of 47 MEQ (83 MEQ) at the end of the study. Overall, including those not receiving a rescue opioid, the HYD group showed stable mean doses of rescue opioids of 36 MEQ (75 MEQ) in month 2 and 40 MEQ (90 MEQ) in month 6.
Total opioid dose during observation period
The mean total opioid dose, including HYD dose plus any rescue opioids, declined slightly for HYD patients from 98 MEQ in month 1 to 95 MEQ in month 6 (Figure 2).
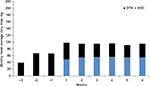  | Figure 2 Mean average HYD and OTH opioid dose by month. Notes: Mean OTH dose calculated for cohort as a whole (regardless of individual use). All opioids were converted using Prescription Drug Monitoring Program Training and Technical Assistance Center Guide, eg, 1 mg HYD was considered to be 1 MEQ.5 Abbreviations: HYD, Hysingla® ER; OTH, other opioids used for rescue; ER, extended release. |
Discussion
To fully understand how a medicine is utilized, one must study it using multiple designs and drawing on multiple data sources. While double-blind, placebo-controlled trials offer data that are scientifically suitable for regulatory approval and labeling, they are tightly controlled and not easily generalized to real-world use. Open-label trials approximate real-world use but are still vulnerable to the Hawthorne effect that observation affects the behavior being observed. Although subject to measured and unmeasured confounding, real-world observational data are unaffected by scientific observation. Examining medicine utilization in studies of all three designs provides the clearest picture and maximum insight into how the drug is and will be used in clinical practice.
In this analysis, HYD dosing in real-world data (DACON of 1.04 pills/day) corresponded to the approved FPI and once-daily (every 24 hours) dosing. In all three studies, patients achieved a stable, mean daily dose of ~60 mg: 57 mg in the 12-week registration trial, 62.6 mg in the 52-week open-label trial and 55 mg in the 6-month real-world study. The use of rescue opioids was similar in the registration trials (78%) and real-world study (78%), but lower in the open-label extension study (48%). Mean daily dose of rescue medication was 3.4 mg in the registration trial. In the open-label trial study, rescue opioid dose decreased from 19.9 to 4.0 mg during titration and remained stable at 3.1 mg during the 12-month maintenance phase. While dose of rescue opioid also decreased in the real-world study (52 to 47 MEQ), the dose was higher than in the more controlled studies.
Conclusion
In real-world use, HYD dosing corresponded very closely to the approved label of HYD administered orally once daily (every 24 hours) with a DACON of 1.04 pills/day. Patients on HYD maintained a stable dose of ~60 mg/day across all three studies. Rescue opioid use, while common, showed a consistent decreasing trend, which is reflected in a decrease in total opioid dose over time among patients treated with HYD. In totality, there is remarkable consistency across the controlled and the open-label registration trials as well as the real-world claim studies in HYD utilization.
Disclosure
Dr Taber was an investigator for the clinical studies. Dr Bond, Mr Wang, Ms Kadakia and Dr Mayne are employees of Purdue Pharma L.P., Stamford, CT, USA. The authors report no other conflicts of interest in this work.
References
HYSINGLA ER [webpage on the Internet] (hydrocodone bitartrate) extended release tablets for oral use, CII [full prescribing information]. Stamford, CT: Purdue Pharma L.P.; 2016. Available from: http://app.purduepharma.com/xmlpublishing/pi.aspx?id=h. Accessed 28 March 2017. | ||
Wen W, Sitar S, Lynch SY, He E, Ripa SR. A multicenter, randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of single-entity, once-daily hydrocodone tablets in patients with uncontrolled moderate to severe chronic low back pain. Expert Opin Pharmacother. 2015;16(11):1593–1606. | ||
Wen W, Taber L, Lynch SY, He E, Ripa S. 12-month safety and effectiveness of once-daily hydrocodone tablets formulated with abuse-deterrent properties in patients with moderate to severe chronic pain. J Opioid Manag. 2015;11(4):339–356. | ||
Prescription Drug Monitoring Program Training and Technical Assistance Center Guide No. 01-13. Calculating Daily Morphine Milligram Equivalents (MMEs). Brandeis University; 2013. Available from: http://www.pdmpassist.org/pdf/BJA_performance_measure_aid_MME_conversion.pdf. Accessed 26 April 2017. | ||
Rubino M, Summers KH, Puenpatom A, Fu C, Ohsfeldt RL, Ben-Joseph RH. A comparison of daily average consumption (DACON) of oxycodone and oxymorphone long-acting oral tablets. J Manag Care Pharm. 2011;17(5):367–376. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

