Back to Journals » Clinical Ophthalmology » Volume 13
Real world use of loteprednol etabonate ophthalmic gel 0.5% in cases representative of comorbid pathologies responding to minimally invasive glaucoma surgery
Authors Sheppard JD, Singh IP
Received 23 February 2019
Accepted for publication 12 June 2019
Published 18 July 2019 Volume 2019:13 Pages 1279—1288
DOI https://doi.org/10.2147/OPTH.S206424
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
JD Sheppard,1 IP Singh2
1Department of Ophthalmology, Eastern Virginia Medical School, Norfolk, VA, USA; 2Department of Glaucoma, The Eye Centers of Racine & Kenosha, Racine, WI, USA
Purpose: With the increasing use of minimally invasive surgical techniques for intraocular pressure (IOP) lowering in glaucoma patients, there is a need to examine best practices regarding the postoperative management of these patients. Corticosteroids, though effective in controlling postoperative ocular pain and inflammation, present distinct challenges in glaucoma surgery patients, as their use can be associated with IOP elevation. Loteprednol etabonate (LE) is an ocular corticosteroid designed to have an improved safety profile relative to other corticosteroids.
Methods: We report here a representative selection of cases in which patients were successfully treated with LE ophthalmic gel 0.5% (LE gel) following a variety of minimally invasive glaucoma surgery (MIGS) procedures. Cases included patients undergoing various procedures including a Trabectome combined with cataract surgery; micro-stent surgery (iStent) combined with cataract surgery; supraciliary CyPass Micro-Stent placement combined with cataract surgery; Kahook Dual Blade goniotomy; and ab interno canaloplasty using the iTrack catheter.
Observations: In all cases, use of LE gel during the postoperative period appeared effective and safe in reducing inflammation and controlling pain. No adverse events or IOP elevations were noted, even in those patients continuing use of LE gel past the postoperative period for longer than six months with documented follow-up. In two cases, patients with elevated IOP using either prednisolone or difluprednate postoperatively were switched to LE gel, with a subsequent reduction in IOP.
Conclusions: This selection of cases involving patients undergoing MIGS suggests that LE gel may be an effective and safe option for treating postoperative inflammation and pain following such procedures with minimal to no effect on IOP or other negative sequalae.
Keywords: loteprednol etabonate, minimally invasive glaucoma surgery, intraocular pressure, postoperative pain and inflammation, safety
Introduction
As a leading cause of irreversible blindness worldwide, glaucoma encompasses a variety of progressive, chronic optic neuropathies associated with damage to the nerve fiber layer and reproducible visual field abnormalities.1 Currently, lowering intraocular pressure (IOP) is the only intervention proven to delay or prevent visual field loss, as demonstrated in a number of landmark studies.1–6 Initial IOP lowering is often achieved through pharmacological treatment or laser trabeculoplasty for patients in whom compliance with pharmacological dosing instructions may be an issue.1 When pharmacological and/or laser trabeculoplasty management are insufficient, incisional glaucoma surgery is indicated.1 Trabeculectomy (TE) is considered to be the standard surgical practice for lowering IOP in patients with uncontrolled glaucoma.4,7 However, due to the high frequency of complications and the need for prolonged follow-up management inherent to this procedure, interest in microinvasive, “bleb-free” techniques for lowering IOP has grown.1,7
Recent advancements in surgical technique have stimulated an expansion of such minimally invasive procedures in glaucoma. Microinvasive (or minimally invasive) glaucoma surgeries (MIGS) offer efficient IOP-lowering with the advantages of being faster than traditional glaucoma surgery, associated with fewer complications, and allowing for earlier intervention.8 Currently, there are four major categories of MIGS, each defined through the mechanism by which they aim to lower IOP (Table 1).
 |
Table 1 Minimally invasive glaucoma surgical proceduresa |
There is little published literature detailing postoperative management of patients undergoing MIGS procedures. However, control of ocular inflammation following traditional ocular surgeries (such as cataract removal and TE) can be a challenging aspect of postoperative patient care. When left untreated, the inflammatory response of the eye to traditional surgical approaches can result in pain, swelling, photophobia, itching, and/or potentially more serious postoperative complications, including cystoid macular edema and blurred vision.9–11 Postoperative treatment with topical corticosteroids has become a standard practice for patients undergoing a variety of ocular surgeries.1,12,13 Although their use has not been formally studied following MIGS, postoperative topical corticosteroid regimens tapered over four weeks have been used in association with various types of MIGS procedures, including Trabectome,14 ab interno canaloplasty,15,16 and iStent procedures.17,18
While effective in managing both pain and inflammation, use of topical corticosteroids poses certain potential risks, including IOP elevation, delayed wound healing, and cataract formation.19–21 Steroid-induced IOP elevation is a particular concern in glaucoma subjects, as recent studies suggest that eyes diagnosed with primary open-angle glaucoma (POAG) have decreased trabecular meshwork (TM) thickness and increased TM stiffness when compared to healthy eyes.22,23 They are therefore more susceptible to IOP spikes24 and the subsequent decrease in aqueous outflow through the TM related to corticosteroid use.19,20,25
Loteprednol etabonate (LE) is a topical corticosteroid approved by the FDA for postoperative inflammation and pain following ocular surgery and is marketed as a suspension, ointment, a gel formulation at a concentration of 0.5%, and most recently, a gel formulation at a concentration of 0.38%. The recommended dosing frequency for LE gel 0.5% is one to two drops into the conjunctival sac of the affected eye four times daily beginning the day after surgery and continuing throughout the first 2 weeks of the postoperative period. The LE molecule was specifically developed using retrometabolic drug design, with the goal of an improved safety profile relative to other corticosteroid compounds. The LE molecule differs from other ocular corticosteroids by having an ester rather than a ketone group at the carbon 20 position.26 By design, LE undergoes rapid conversion into inactive, nontoxic metabolites after binding to glucocorticoid receptors, thereby allowing for localized, controlled suppression of ocular inflammation with a limited potential for causing unwanted effects.27–29 Use of steroids such as LE in the glaucoma setting is particularly important vis a vis limiting the potential for steroid-induced IOP increase in this already vulnerable population. The safety and anti-inflammatory efficacy of LE gel 0.5% have been demonstrated in various ocular surgery settings including following cataract surgery,30–32 LASIK/PRK,33,34 and Descemet membrane endothelial keratoplasty.35
To date, three retrospective chart reviews have been published describing the use of LE suspension 0.5% in patients undergoing glaucoma surgery, all of which reported that postoperative treatment with LE 0.5% had a minimal or no effect on IOP following ab externo canaloplasty,36 selective laser trabeculoplasty (SLT),37 and trabecular micro-bypass stent18 procedures. However, currently, there are no published clinical data addressing the use of LE gel 0.5% specifically in patients undergoing glaucoma surgery. Glaucoma surgery patients are particularly vulnerable to risks associated with steroid-induced IOP elevation, and the low risk of IOP elevation with LE38 is particularly relevant for this population. The purpose of these case presentations is to share clinical experiences using LE gel 0.5% as part of routine postoperative care in patients with a range of comorbid pathologies undergoing a variety of MIGS procedures, specifically those which target trabecular outflow, with or without concomitant cataract surgery. The authors selected cases considered representative of the routine management of post-MIGS patients treated with LE gel 0.5% during the postoperative period. The collection of data reported in this paper, including the de-identified photographs, was submitted for IRB review (Advarra IRB, Columbia, MD). Using the Department of Health and Human Services regulations found at 45 CFR 46.104(d)(4), the IRB determined this research project to be exempt from IRB oversight. Data were kept confidential and did not include any personal health information identifiers.
Findings
Case 1—Trabectome®
A 40-year-old male presented with dense stromal scarring in the left eye (OS) due to herpes simplex virus (HSV) keratitis. His HSV keratitis was treated successfully 5 years earlier with penetrating keratoplasty (PKP), and the patient was maintained on acyclovir 800 mg BID and LE gel BID since the transplant. IOP was maintained below 20 mm Hg, the eye remained quiet, and the cornea stayed clear. Four years after this procedure, the patient began to experience gradual IOP elevations in the left eye to 25 mm Hg, felt to be a consequence of his underlying HSV status. Gonioscopy showed a Grade IV open angle with Grade III pigment deposition and no anterior synechiae. The stable examination suggested that the HSV was well controlled despite long-term damage to the cornea, and that IOP was gradually increasing due to reduced aqueous outflow via the trabecular meshwork. A diagnosis of open-angle glaucoma was made and treatment with levobunolol ophthalmic solution BID was initiated. On this new medication, IOP levels were maintained at 18 mm Hg without visual field loss, despite increasing punctate keratopathy presumably considered secondary to the topical beta-blocker.
This patient developed a posterior subcapsular cataract OS, a common postoperative complication of transplant surgery, with 20/100 visual acuity (VA) and 20/400 glare. A combined procedure of phacoemulsification with an Envista IOL and a Trabectome (Neomedix, Tustin, CA, USA) procedure to treat the glaucoma was performed without complication. The patient was successfully managed postoperatively with levobunolol BID and LE gel QID for 4 weeks, then tapered successfully off the levobunolol and returned to the preoperative LE gel BID maintenance dose. Six months postoperatively, best-corrected visual acuity (BCVA) was 20/25 and IOP was 15 mm Hg, with a quiet eye and no herpetic reactivations. The patient continued using LE gel BID on a regular basis while maintaining a stable IOP.
Case 2—Micro-stent surgery (iStent)
A 28-year-old female patient presented with bilateral (OU) sarcoid uveitis and grade 3 posterior subcapsular cataracts with 9 clock hours of posterior synechiae OU. The patient had been prescribed topical generic prednisolone acetate ophthalmic suspension 1% intermittently for many years with questionable compliance. Initially, the uveitis was well controlled with LE gel BID OU and intensive systemic therapy, including oral prednisone 50 mg QD and mycophenolate 1500 mg BID followed by oral prednisone 5 mg daily and mycophenolate 1000 mg twice daily for several years. VA during this time was 20/60 OD and 20/30 OS with IOPs of 12 mm Hg OU.
A mild flare and 1+ cell OU necessitated a sub-Tenon triamcinolone 40 mg injection OD in preparation for cataract surgery. The patient experienced postinjection IOP elevations to 40 mm Hg and required dorzolamide 20 mg/mL/timolol 5 mg/mL ophthalmic solution BID to maintain a stable IOP below 20 mm Hg. At this point, VA had deteriorated to 20/200 OD and 20/50 OS as the cataracts slowly progressed.
The patient was maintained on a treatment regimen of dorzolamide/timolol, LE gel, oral mycophenolate, and low dose oral steroid (all BID) for 3 months to ensure a sustained quiet eye, after which cataract surgery with posterior synechiolysis and a single iStent (Glaukos Corporation, Laguna Hills, CA, USA) placement was performed OD without complication.
During the postoperative period, the patient was managed with LE gel every three hours while awake and dorzolamide/timolol BID for 1 month, after which the LE gel was tapered to the preoperative BID dosage. The patient was maintained on an ongoing treatment regimen including LE gel BID, oral mycophenolate, and low dose oral prednisone. Throughout this treatment, the eye remained quiet, with a BCVA of 20/30, and a normal IOP once dorzolamide/timolol was discontinued. Three months after the initial surgery, a similar procedure was successfully performed OS, with an identical topical and systemic regimen. She remains a candidate for systemic adalimumab (Humira®, Abbvie) therapy, postponed by her needle phobia.
Case 3—Micro-stent surgery (iStent) plus cataract surgery
A 69-year-old female with a history of POAG OU was controlled on latanoprost ophthalmic solution 0.005% QD in the evening OU and brimonidine tartrate/timolol maleate ophthalmic solution 0.2%/0.5% BID OU for 3 years. When compliant with medication, her IOPs were on target, ranging in the upper teens, and appeared stable during office visits. However, the patient began to report subjective complaints of decreasing night vision, difficulty driving, and needing more light to read at home. The patient admitted to missing her medication doses.
Humphrey visual field (HVF) testing revealed a nasal step that had slightly progressed over the past few years. Slit lamp exam showed the conjunctiva and cornea to be quiet and normal in appearance. The anterior chamber (AC) was deep, with open angles to the ciliary body band (CBB) and 1–2+ trabecular meshwork pigmentation on gonioscopy. The lens revealed a progressing 3+ nuclear sclerotic cataract with 1+ cortical changes. Retina vessels were normal with a healthy macula. The optic nerve head (ONH) showed increased cupping OD (0.55 vertically) and OS (0.4 horizontally), with no obvious retinal nerve fiber layer (RNFL) bundle defect, rim loss, or disc hemorrhage, and an early nasal step on 24-2 HVF testing.
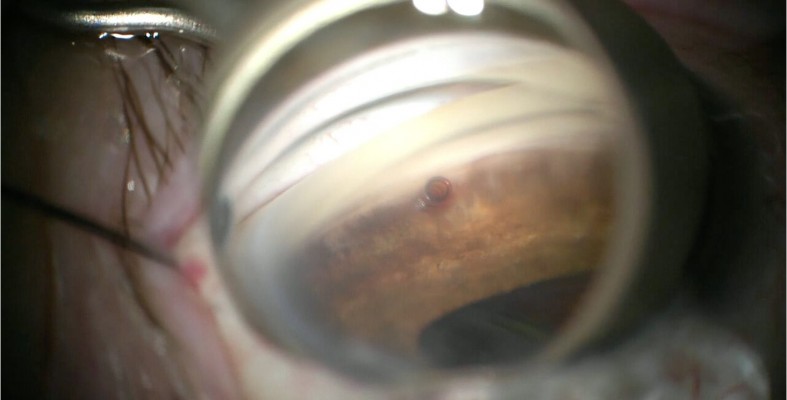
Based on the patient’s subjective complaints of daily functioning due to her vision, mild to moderate POAG and issues with medication compliance, along with evidence of a cataract, a combined cataract and iStent surgery OD (followed by OS) was planned. She started antibiotic drops (besifloxacin ophthalmic solution 0.6% BID) 3 days prior to the procedure (continued postoperatively for 1 week), as well as bromfenac ophthalmic solution 0.07% QD (continued for 6 weeks postoperatively). Her surgery was uneventful, with successful placement of an Akreos hydrophilic lens and a G1 iStent in the Schlemm’s canal (Figure 1).
On the first postoperative day, her VA was 20/30 with no complaints of pain or discomfort, and her IOP was 18 mm Hg. She had mild conjunctival injection and 1+ cell with no hemorrhage or hyphema. She was then started on LE gel QID for 1 week with a tapering dose over 4 weeks.
One week later, she returned to the office with an uncorrected VA of 20/20, and an IOP of 16 mm Hg while on LE gel TID and bromfenac QD. Her conjunctiva was quiet with no cells in the AC. At 1 month, when she had completed her LE gel regimen and was only on bromfenac, her IOP remained stable at 16 mm Hg, and her vision was 20/20. Two weeks after her right eye procedure, the same procedure was performed on her left eye, and she had a similar outcome on the same course of medications.
Case 4—Micro-stent surgery (supraciliary stent) and cataract surgery
This case was a 64-year-old African American male with a history of POAG OU on bimatoprost 0.01% solution QHS OU, brinzolamide/brimonidine tartrate ophthalmic suspension 1%/0.2% BID OU, and timolol 0.5% QD OU. In 2008, the patient underwent a successful express shunt surgery OD. However, the patient continued to require IOP-lowering medication to maintain an IOP in the lower teens OD and the middle to upper teens OS. The maximum IOP was 30–35 mm Hg for both eyes in the absence of IOP-lowering medications.
During routine office visits, the patient indicated he was having increased difficulty in remembering to administer his eye drops. He also stated he was not tolerating his medications well, and that his eyes were often red and irritated. He noticed a decrease in overall quality of vision, with more glare and halos around lights when driving. Upon exam, his BCVA was 20/25 OD and 20/40 OS with a constricted visual field OD, but a superior arcuate defect consistent with ONH and optical coherence tomography findings OD. Exam also revealed a 3+ NS cataract OS (pseudophakic OD).
Cataract surgery OS with CyPass (Alcon Inc., Fort Worth, TX, USA) supraciliary stent placement was planned, with the objective of reducing the necessity of IOP-lowering medications while avoiding a bleb. The patient was started on bromfenac ophthalmic solution 0.07% QD and besifloxacin ophthalmic solution 0.6% BID for 3 days prior to the procedure (to be continued for 1 week postoperatively). The surgery was uneventful, and the stent was in a good position nasally (Figure 2).
On the first postoperative day, uncorrected VA was 20/40, with 1–2+ cell, no hyphema, and an IOP of 8 mm Hg. LE gel was started QID for 1 week followed by a tapering dose over 3 weeks. At 1 week, IOP was 11 mm Hg with minimal inflammation (occasional cell). VA was 20/25, and the patient stated he was very happy with his visual recovery. One month later, the IOP was 12 mm Hg on bromfenac QD only, and the AC remained quiet. No IOP-lowering medications were needed, and the patient stated he would like another MIGS procedure OD to help reduce the need for IOP-lowering medications in that eye as well.
Case 5—Micro-stent (iStent) plus cataract surgery
A 67-year-old female with a history of mild POAG was treated with latanaprost ophthalmic solution 0.005% for over 7 years with stable IOP and developed a 2+ NS cataract in the right eye. The patient was scheduled for a phacoemulsification with placement of an iStent to manage both the cataract and the glaucoma diagnosis in a single procedure. Preoperative exam results included a VA of 20/30 (glare to 20/60), an IOP of 20 mm Hg, and a cup disk ratio of 0.5, with thinning of the inferior retinal never fiber layers on optical coherence tomography but otherwise healthy fields. The patient was started on besifloxacin 0.6% BID and bromfenac 0.07% QD three days before the surgical procedure was scheduled.
Following a successful combined cataract/iStent procedure, day 1 exam showed a deep chamber with an occasional cell, an uncorrected VA of 20/25, and an IOP of 16 mm Hg. The patient was continued on bromfenac QD and besifloxacin BID, in addition to difluprednate ophthalmic emulsion 0.05% QID for the first week with tapering of the dosing over the following 3 weeks. One week later, the patient returned with an elevated IOP of 34 mm Hg. The ONH remained healthy based on slit lamp biomicroscopy.
Assuming a likely steroid-induced IOP response had occurred, the difluprednate was replaced with LE gel BID until the next visit. The following week, IOP had decreased to 22 mm Hg and the LE gel dose was tapered. Three weeks later, it decreased further to 17 mm Hg without the addition of any IOP-lowering medications. She has maintained an IOP in the upper teens for two years of follow-up.
Case 6—Kahook dual blade goniotomy
A 72-year-old male patient with a history of successful cataract surgery and moderate POAG in both eyes was managed for over 5 years using 2 medications (bimatoprost ophthalmic solution 0.01% QD and timolol/brimonidine BID). The patient’s IOP ranged from 18–22 mm Hg while on medication, but he reported difficulty with medication compliance due to cost, forgetfulness, and medication side effects.
Although visual field and ONH head was stable (cup/disc of 0.5 OU and early nasal step), a Kahook Dual Blade (New World Medical, Rancho Cucamonga, CA, USA) goniotomy was performed successfully on the right eye to more adequately control the patient’s IOP while maintaining quality of life. On postoperative day 1, IOP was 12 mm Hg, uncorrected VA was 20/25, and the AC had 1+ cell with minimal red blood cells. Bromfenac 0.07% was initiated three days prior to surgery and continued QD postoperatively along with moxifloxacin 0.5% QID and prednisolone acetate 1% QID. No IOP-lowering medications were administered or prescribed.
One week later, the IOP OD had increased to 21 mm Hg, though the AC demonstrated only occasional cells and no heme. Bromfenac and prednisolone were continued TID for 1 week with tapering of the dosing over the following three weeks. When the patient returned after two weeks, the IOP OD had further increased to 28 mm Hg while on prednisolone 1% BID. Rare cells were noted in the AC, and the angle appeared open with a good-sized goniotomy visible (100 degrees).
Treatment with prednisolone was discontinued due to a suspected steroid-induced IOP response, and the patient began using LE gel BID for 1 week tapering to QD for the subsequent week. After two weeks of therapy with LE, IOP had lowered to 20 mm Hg OD and settled at 16 mm Hg upon completion of treatment. At the time of writing, the patient’s IOP was still well controlled between 15–17 mm Hg in the absence of any IOP-lowering medications.
Case 7—Ab interno canaloplasty using the iTrack catheter
A 71-year-old white male with a history of POAG OD was initially successfully managed on bimatoprost ophthalmic solution 0.01% and brimonidine tartrate ophthalmic solution 0.1% OD BID. With treatment, IOP OD was maintained between 20–22 mm Hg, with a maximum IOP of 29 mm Hg when IOP-lowering medications were discontinued. In 2014, the patient underwent SLT with 360 degrees of treatment OD. Minimal to no response was noted, and IOP-lowering medications were continued.
After 2 years of compliance with this regimen, the patient continued to experience difficulty driving at dusk and when raining. The patient’s BCVA OD was 20/30, but glare testing dropped the BCVA to 20/60. The conjunctiva and cornea were healthy (mild superficial punctate keratitis seen), and the lens revealed a 2+ NS with 2+ cortical changes. On examination of the ONH, the vertical cup/disc ratio was 0.6 with slight loss of inferior RNFL. The visual field also demonstrated a nasal/superior arcuate defect with a pattern standard deviation of 2.8 dB and mean deviation of −5.0 dB.
Cataract surgery with ab internal viscodilation of the Schlemm’s canal (using the iTrack [Ellex, Adelaide, Australia] catheter) was scheduled. The patient was started on bromfenac QD (continued for 6 weeks postoperatively) and besifloxacin BID (continued for 1 week postoperatively) OD 3 days prior to surgery. Surgery was successful and uneventful. On the first postoperative day, uncorrected VA OD was 20/50, with 1–2+ cell and a microhyphema. The patient reported no discomfort, and IOP was 12 mm Hg. LE gel was added for 1 week with QID dosing and a scheduled taper of 1 less drop per week over 3 more weeks.
At 1 week, uncorrected VA OD was 20/25, with an IOP of 14 mm Hg, and the patient stated he was able to drive better at night. The AC revealed rare cells, no heme cells were observed, and the conjunctiva and cornea were clear and quiet. At 1 month, when the LE gel regimen was completed, the IOP was 15 mm Hg, and the AC was quiet. VA remained stable at 20/25 uncorrected. The patient was seen again 3 months later, and the IOP OD was stable at 15 mm Hg while all IOP-lowering medications remained discontinued.
Discussion
As surgical techniques for glaucoma continue to evolve, best clinical practices regarding postoperative patient management need to be considered. The cases presented here illustrate both the safety and efficacy of LE gel 0.5% use for glaucoma patients following a variety of MIGS procedures (Trabectome, micro-stents, KDB goniotomy, and ABiC with iTrack), either with or without cataract removal. In patients for whom LE gel was included in the initial postoperative drug regimen, ocular inflammation and pain were successfully controlled. The treatment was well tolerated, there were no adverse events (AEs) attributed to treatment with LE gel, and there were no noted elevations in IOP. To date, neither author has experienced any MIGS case in which postoperative treatment with LE gel resulted in AEs or discontinuations in treatment.
In 2 of the 7 cases reported here, LE gel was instituted as replacement for initial postoperative treatment with a different corticosteroid (prednisolone acetate or difluprednate) which was associated with an elevation in postoperative IOP. Switching to LE gel led to a reduction of the steroid-induced IOP in these cases, without loss of subjective or objective improvements in ocular findings, such as postoperative pain and inflammation. In the uveitis patient, inflammation control also required periocular injection and systemic medications.
When LE gel was used during the immediate postoperative period for MIGS patients, it was most commonly prescribed on the first postoperative day as one drop QID with a tapering dose over 4 weeks. In steroid IOP-responsive patients, where LE gel replaced treatment with a different corticosteroid, LE gel was prescribed 2 to 4 times daily for the first week and then tapered as needed based on AC inflammation. However, there is no typical regimen, and treatment must be titrated for each patient based upon previous observation of the patient as well as their postoperative response.
These cases are consistent with previous reports on the use of LE gel 0.5% postoperatively. Two randomized, double-masked, parallel-group, vehicle controlled, multicenter trials evaluating the use of LE gel 0.5% for the treatment of postoperative inflammation and pain following cataract surgery found the drug to be both safe and effective without elevating IOP,30,31 while a retrospective chart review documented similar results for patients using LE gel 0.5% during the postoperative LASIK or PRK period.33 LE gel 0.5% was also studied in patients following Descemet membrane endothelial keratoplasty, where it both prevented immunologic graft rejection and caused significantly fewer IOP elevations when compared to treatment with prednisolone acetate ophthalmic solution 1%.35 A recent review of published data with LE noted low rates of clinically significant IOP elevation (≥10 mm Hg from baseline) ranging from 0.8% (14/1725 subjects) to 1.5% (21/1386 subjects) in short- and long-term use studies, respectively.38
Although LE gel has not been studied in MIGS procedures specifically, three retrospective chart reviews have evaluated the use of LE suspension 0.5% in patients undergoing non-MIGS procedures for glaucoma.18,36,37 In patients following combined phacoemulsification and trabecular micro-bypass stent implantation, treatment with LE suspension 0.5% had a minimal effect on IOP.18 There was also no difference in IOP in patients undergoing SLT who received LE suspension 0.5% at the time of surgery compared to those not receiving corticosteroid.37 In a retrospective evaluation of patients managed with LE suspension 0.5% post ab externo canaloplasty with or without cataract surgery, a small percentage of patients (5.3%) had an IOP ≥30 mm Hg one week post-surgery, but rarely thereafter and there were no treatment discontinuations secondary to IOP elevations.36
In the cases described herein, LE gel 0.5% was prescribed for MIGS patients based on the drug’s unique design and favorable safety profile. Accordingly, none of the MIGS patients experienced clinically significant elevations in IOP associated with the use of LE gel. This included patients who previously exhibited an IOP response to other ocular corticosteroids (prednisolone, difluprednate) a finding paralleled by prior reports on the use of LE suspension in known steroid responders.39,40
LE gel has other aspects that we find useful for controlling inflammation and pain post-MIGS. The gel formulation is non-settling, thus does not require vigorous shaking prior to instillation; this can be a benefit for older glaucoma patients who may have difficulty shaking medications because of arthritis or other physical weakness, as well as those who may have trouble remembering to shake a medication. The gel also has features designed to minimize ocular irritation, including a low concentration of the preservative benzalkonium chloride, a pH close to that of normal tears (6.5), and inclusion of known demulcents (glycerin and propylene glycol).41 None of the patients in these cases experienced any ocular surface disturbances or complained of dry eye or drop-induced ocular discomfort following surgery.
Conclusions
Topical corticosteroids, including LE gel 0.5%, are an important component of ocular postoperative care, despite some potential drawbacks as a class. Clinical outcomes of the cases described herein and the authors’ combined clinical experience suggest LE gel 0.5% safely and effectively treats inflammation and postoperative pain following a variety of MIGS procedures, with a high degree of tolerability and a low propensity for inducing IOP elevations, even in known corticosteroid responders. As a small selection of case reports, these vignettes are intended only to share our typical experiences using LE gel 0.5% postoperatively in patients undergoing MIGS procedures and should be interpreted carefully given their anecdotal nature. Additional formal studies with larger patient populations and possibly an active comparator are warranted to properly examine the benefit(s) of LE gel 0.5% use in the glaucoma surgery setting.
Acknowledgments
The authors acknowledge the writing assistance of Rachel Hathcock, RN of Churchill Communications, funded by Bausch + Lomb, Incorporated.
Disclosure
JD Sheppard serves as a consultant for Alcon, Bausch + Lomb, a division of Bausch Health US, LLC, and NeoMedix. He also reports grants from Bausch + Lomb, for the conduct of independent research studies. IP Singh receives honoraria for speaking and consulting for Bausch + Lomb. He is also a speaker and consultant for Allergan, Alcon, Glaukos, Ellex, and New World Medical. The authors report no other conflicts of interest in this work.
References
1. Prum BE, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern® guidelines. Ophthalmology. 2016;123(1):P41–P111. doi:10.1016/j.ophtha.2015.10.053
2. Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi:10.1001/archopht.120.10.1268
3. Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E; Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56. doi:10.1001/archopht.121.1.48
4. The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi:10.1016/S0002-9394(00)00538-9
5. Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126(4):487–497. doi:10.1016/S0002-9394(98)00223-2
6. Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385(9975):1295–1304. doi:10.1016/S0140-6736(14)62111-5
7. Rulli E, Biagioli E, Riva I, et al. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol. 2013;131(12):1573–1582. doi:10.1001/jamaophthalmol.2013.5059
8. Kaplowitz K, Schuman JS, Loewen NA. Techniques and outcomes of minimally invasive trabecular ablation and bypass surgery. Br J Ophthalmol. 2014;98(5):579–585. doi:10.1136/bjophthalmol-2013-304546
9. El-Harazi SM, Feldman RM. Control of intra-ocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2001;12:4–8. doi:10.1097/00055735-200102000-00002
10. Porela-Tiihonen S, Kaarniranta K, Kokki M, Purhonen S, Kokki H. A prospective study on postoperative pain after cataract surgery. Clin Ophthalmol. 2013;7:1429–1435.
11. Lundberg B, Jonsson M, Behndig A. Postoperative corneal swelling correlates strongly to corneal endothelial cell loss after phacoemulsification cataract surgery. Am J Ophthalmol. 2005;139(6):1035–1041. doi:10.1016/j.ajo.2004.12.080
12. Chuck RS, Jacobs DS, Lee JK, et al. American Academy of Ophthalmology Preferred Practice Pattern Refractive Management/Intervention Panel. Refractive errors & refractive surgery preferred practice pattern®. Ophthalmology. 2018;125(1):P1–P104. doi:10.1016/j.ophtha.2017.10.003
13. Olson RJ, Braga-Mele R, Chen SH, et al. Cataract in the adult eye preferred practice pattern®. Ophthalmology. 2017;124(2):P1–P119. doi:10.1016/j.ophtha.2016.09.027
14. Yildirim Y, Kar T, Duzgun E, Sagdic SK, Ayata A, Unal MH. Evaluation of the long-term results of trabectome surgery. Int Ophthalmol. 2016;36(5):719–726. doi:10.1007/s10792-016-0190-y
15. Khaimi MA. Canaloplasty: A minimally invasive and maximally effective glaucoma treatment. J Ophthalmol. 2015;2015:485056. doi:10.1155/2015/485065
16. Cagini C, Peruzzi C, Fiore T, Spadea L, Lippera M, Lippera S. Canaloplasty: current value in the management of glaucoma. J Ophthalmol. 2016;2016:7080475. doi:10.1155/2016/7080475
17. Arriola-Villalobos P, Martínez-de-la-Casa JM, Díaz-Valle D, et al. Mid-term evaluation of the new glaukos iStent with phacoemulsification in coexistent open-angle glaucoma or ocular hypertension and cataract. Br J Ophthalmol. 2013;97(10):1250–1255. doi:10.1136/bjophthalmol-2012-302394
18. Wang Q, Harasymowycz P. Short-term intraocular pressure elevations after combined phacoemulsification and implantation of two trabecular micro-bypass stents: prednisolone versus loteprednol. J Ophthalmol. 2015;2015:341450. doi:10.1155/2015/341450
19. McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25(1):33–55. doi:10.2165/00002018-200225010-00004
20. Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: A review of the literature. Eye. 2006;20(4):407–416. doi:10.1038/sj.eye.6701895
21. Dibas A, Yorio T. Glucocorticoid therapy and ocular hypertension. Eur J Pharmacol. 2016;787:57–71. doi:10.1016/j.ejphar.2016.06.018
22. Liu B, McNally S, Kilpatrick JI, Jarvis SP, O’Brien CJ. Aging and ocular tissue stiffness in glaucoma. Surv Ophthalmol. 2018;63(1):56–74. doi:10.1016/j.survophthal.2017.06.007
23. Wang K, Read AT, Sulchek T, Ethier CR. Trabecular meshwork stiffness in glaucoma. Exp Eye Res. 2017;158:3–12. doi:10.1016/j.exer.2016.07.011
24. Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol. 1963;70:492–499. doi:10.1001/archopht.1963.00960050494011
25. Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: are they all the same? Ophthalmol Ther. 2013;2(2):55–72. doi:10.1007/s40123-013-0020-5
26. Comstock TL, Sheppard JD. Loteprednol etabonate for inflammatory conditions of the anterior segment of the eye: twenty years of clinical experience with a retrometabolically designed corticosteroid. Expert Opin Pharmacother. 2018;19(4):337–353. doi:10.1080/14656566.2018.1439920
27. Wu WM, Huang F, Lee Y, Buchwald P, Bodor N. Pharmacokinetics of the sequential metabolites of loteprednol etabonate in rats. J Pharm Pharmacol. 2008;60(3):291–297. doi:10.1211/jpp/60.10.0006
28. Howes J, Novack GD. Failure to detect systemic levels, and effects of loteprednol etabonate and its metabolite, PJ-91, following chronic ocular administration. J Ocul Pharmacol Ther. 1998;14(2):153–158. doi:10.1089/jop.1998.14.229
29. Bodor N, Loftsson T, Wu WM. Metabolism, distribution, and transdermal permeation of a soft corticosteroid, loteprednol etabonate. Pharm Res. 1992;9(10):1275–1278. doi:10.1023/A:1015849132396
30. Rajpal RK, Roel L, Siou-Mermet R, Erb T. Efficacy and safety of loteprednol etabonate 0.5% gel in the treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2013;39(2):158–167. doi:10.1016/j.jcrs.2012.09.013
31. Fong R, Leitritz M, Siou-Mermet R, Erb T. Loteprednol etabonate gel 0.5% for postoperative pain and inflammation after cataract surgery: results of a multicenter trial. Clin Ophthalmol. 2012;6:1113–1124. doi:10.2147/OPTH.S32643
32. Abessi B, Brooksby L, Schultze RL. Comparison of efficacy of difluprednate 0.05% and loteprednol gel 0.5% after cataract surgery. Eye Contact Lens. 2018;44(Suppl 2):S37–S42. doi:10.1097/ICL.0000000000000407
33. Salinger CL, Gordon M, Jackson MA, Perl T, Donnenfeld E. A retrospective analysis of the postoperative use of loteprednol etabonate gel 0.5% following laser-assisted in situ keratomileusis or photorefractive keratectomy surgery. Clin Ophthalmol. 2015;9:2089–2097.
34. Mifflin MD, Betts BS, Frederick PA, et al. Efficacy and safety of a 3-month loteprednol etabonate 0.5% gel taper for routine prophylaxis after photorefractive keratectomy compared to a 3-month prednisolone acetate 1% and fluorometholone 0.1% taper. Clin Ophthalmol. 2017;11:1113–1118. doi:10.2147/OPTH.S138272
35. Price MO, Feng MT, Scanameo A, Price FW
36. Khaimi MA. A retrospective analysis of the use of loteprednol etabonate ophthalmic suspension 0.5% following canaloplasty. Clin Ophthalmol. 2018;12:319–329. doi:10.2147/OPTH.S153912
37. Rebenitsch RL, Brown EN, Binder NR, et al. Effect of topical loteprednol on intraocular pressure after selective laser trabeculoplasty for open-angle glaucoma. Ophthalmol Ther. 2013;2(2):113–120. doi:10.1007/s40123-013-0018-z
38. Sheppard JD, Comstock TL, Cavet ME. Impact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressure. Adv Ther. 2016;33(4):532–552. doi:10.1007/s12325-016-0315-8
39. Bartlett JD, Horwitz B, Laibovitz R, Howes JF. Intraocular pressure response to loteprednol etabonate in known steroid responders. J Ocul Pharmacol. 1993;9(2):157–165. doi:10.1089/jop.1993.9.157
40. Holland EJ, Djalilian AR, Sanderson JP. Attenuation of ocular hypertension with the use of topical loteprednol etabonate 0.5% in steroid responders after corneal transplantation. Cornea. 2009;28(10):1139–1143. doi:10.1097/ICO.0b013e3181a2ad81
41. Coffey MJ, DeCory HH, Lane SS. Development of a non-settling gel formulation of 0.5% loteprednol etabonate for anti-inflammatory use as an ophthalmic drop. Clin Ophthalmol. 2013;7:299–312.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


