Back to Journals » Clinical Ophthalmology » Volume 16
Real-World Use of Loteprednol Etabonate 0.5%/Tobramycin 0.3% Ophthalmic Suspension for the Treatment of Ocular Surface Inflammatory Conditions
Authors Deom JE, Kannarr S, Vollmer P
Received 27 September 2022
Accepted for publication 7 November 2022
Published 17 November 2022 Volume 2022:16 Pages 3803—3809
DOI https://doi.org/10.2147/OPTH.S389688
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
James E Deom,1,2 Shane Kannarr,3 Patrick Vollmer4,5
1The Dry Eye Center of Northeastern Pennsylvania, Hazleton, PA, USA; 2Hazleton and Stroudsburg Eye Specialists, Hazle Township, PA, USA; 3Kannarr Eye Care, LLC, Pittsburg, KS, USA; 4Vita Eye Clinic, Shelby, NC, USA; 5Synvenio Group, Shelby, NC, USA
Correspondence: James E Deom, Hazleton Eye Specialists, 281 Airport Road, Hazle Township, PA, 1820, USA, Tel +1 570 453 2020, Fax +1 570 453 1020, Email [email protected]
Purpose: Use of a combination corticosteroid/antibiotic product is common in ocular surface inflammatory conditions for which corticosteroid therapy is indicated and there exists a risk of superficial bacterial infection. Combination loteprednol etabonate 0.5% and tobramycin 0.3% (LE/T) has been evaluated for blepharokeratoconjunctivitis in two trials, but there has been limited reporting on its real-world use.
Patients and Methods: This was a retrospective chart review conducted at three optometry practices in the USA. Data were collected from cases in which LE/T was used and data were recorded for the period commencing with therapy with a minimum of one follow-up visit (within 2 months). Data abstracted included patient demographics, diagnosis, LE/T dosing regimen, pre- and post-treatment ocular signs and symptoms, intraocular pressure (IOP) measurements, adverse event (AE) reports, visual acuity (VA), and any notations as to resolution of baseline condition. Primary outcomes of interest included IOP changes and AEs.
Results: Ninety-six patient charts were extracted, and data from 87 charts (115 LE/T-treated eyes) were included. Mean (SD) years of age was 45.6 (19.7), most patients were white (83.9%), and just over half were female (58.6%). Common baseline conditions were conjunctival injury/corneal abrasion (25.3%), keratitis (18.4%), viral conjunctivitis (16.1%), and blepharitis/eyelid inflammation/MGD (11.5%). The most common LE/T dosing regimen was one drop QID. Mean (SD) IOP was 15.2 (4.4) mm Hg at baseline and 15.7 (4.4) mm Hg at the first follow-up visit (p = 0.2467). No AEs were recorded, and there were no significant changes in mean VA. Where recorded, most patients (83%) were noted as having their condition resolved/resolving at the first or second follow-up visit.
Conclusion: LE/T appears to have a high level of safety when used for the management of various ocular surface inflammatory conditions encountered in optometric practice.
Keywords: corticosteroid, ocular, retrospective, intraocular pressure, blepharokeratoconjunctivitis, real-world
Introduction
Use of a combination corticosteroid and antibiotic product is common in ocular surface inflammatory conditions for which corticosteroid therapy is indicated and there exists a risk of superficial bacterial infection. Topical corticosteroids have well-established efficacy in the management of ocular inflammation, but also carry potential risks including intraocular pressure (IOP) elevation.1,2 The combination product loteprednol etabonate 0.5% plus tobramycin 0.3% (“LE/T”; Zylet®, Bausch & Lomb Incorporated, Rochester, New York) combines a topical corticosteroid (loteprednol etabonate; LE) with an aminoglycoside antibacterial (tobramycin; T) in the form of an ophthalmic suspension.3 LE is a C-20 ester corticosteroid that is metabolized rapidly to inactive metabolites following corticosteroid receptor activation, a characteristic designed with the goal of enhanced safety.4 LE has been shown to be efficacious in a range of inflammatory ocular conditions along with a safety profile including a low risk of IOP elevation, even among known steroid responders.5,6 Tobramycin is an aminoglycoside with broad-spectrum antibacterial activity, including efficacy against penicillinase-resistant staphylococcal organisms.3,7 In a recent in vitro study involving 487 unique bacterial isolates (14 genera; 67 species) commonly associated with blepharitis, tobramycin demonstrated low minimum inhibitory concentrations (MICs) against most isolates, including staphylococci (overall, as well as most methicillin-resistant strains).8
The FDA-approved indication for LE/T is for use in steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection exists.3 While LE/T has been evaluated for the treatment of blepharokeratoconjunctivitis in two prospective clinical trials,9,10 and for blepharitis specifically in a pooled subanalysis,11 there has been limited reporting on real-world use of this combination product in a broader range of ocular inflammatory conditions. Thus, we designed a retrospective chart review study to assess the real-world use of LE/T in patients with ocular surface inflammatory conditions with a focus on dosing regimens used, IOP and other safety outcomes, and clinical disease resolution.
Materials and Methods
Study Design and Patients
This was a noninterventional retrospective chart review study of patients from three optometry practices in the US (Pennsylvania, Kansas, North Carolina). The study was conducted in compliance with the Declaration of Helsinki and all amendments and was granted an exemption from informed consent requirements by Advarra Institutional Review Board. All patient information was anonymized and kept confidential.
Cases were selected from the medical records of patients who had been treated by the study investigators. Eligibility requirements included age ≥18 years, diagnosis of an ocular inflammatory condition treated with LE/T in at least one eye, chart documentation of a baseline diagnostic visit, and a minimum of one follow-up visit within two months following commencement of LE/T. Exclusion criteria included: the use of systemic or topical ophthalmic non-steroidal anti-inflammatory agents (daily aspirin use was permitted as it was unlikely to impact outcomes),12,13 analgesics, and antihistamines; ocular surgery (including laser surgery) within the previous month; participation in an ophthalmic clinical trial within the previous month, and use of LE/T for a condition inconsistent with its labelled indication.
Outcomes of Interest
The primary outcomes of interest were treatment-emergent adverse event (AE) reports and changes in IOP from baseline to follow-up. Secondary outcomes included dosing patterns of LE/T, changes in visual acuity (VA), and clinical resolution of the inflammatory condition being treated.
Statistical Analysis
Basic descriptive statistics were used to characterize the endpoints of interest with comparisons at baseline and follow-up through the most recent patient visit. A paired t-test was used to compare changes from baseline in IOP and VA. Calculations were based on the number of eyes for which non-missing values were provided for the endpoint being summarized. Statistical analyses were conducted using Statistix version 10 (Analytical Software).
Results
A total of 96 patient charts were extracted, and data from 87 patient charts (a total of 115 LE/T treated eyes) met inclusion criteria and were included in the analysis. Baseline patient characteristics are presented in Table 1. Common diagnoses included conjunctival injury/corneal abrasion (25.3% of patients), keratitis (18.4%), viral conjunctivitis (16.1%), and blepharitis/eyelid inflammation/meibomian gland dysfunction (MGD; 11.5%). Most patients (n = 59; 67.8%) had one affected eye treated with LE/T, while 28 (32.2%) patients had two affected eyes treated with LE/T. Among the patients with both eyes treated with LE/T, common diagnoses were bacterial or viral conjunctivitis (n = 10), blepharitis (n = 8), and keratitis (n = 7). Ocular examination findings relevant to ocular inflammation and reported symptoms at baseline are presented in Figure 1. Most ocular findings were noted to be mild or moderate in severity. Conjunctival hyperemia was the most commonly noted sign, recorded for 63 (54.8%) eyes and was rated as moderate or severe in about two-thirds of the eyes (41/63; 65.1%). Mild or moderate corneal staining was noted in 44 (38.3%) eyes. Other ocular examination findings noted in ≥20 eyes included lid hyperemia (20 eyes; 17.4%), lid scaling and crusting (23 eyes; 20.0%), and corneal edema (24 eyes; 20.9%). With regard to symptoms, painful or sore eyes were reported for 84 (73.0%) eyes, and close to half of eyes were noted to have blurred vision with light sensitivity (55 eyes; 47.8%) and/or tearing (46 eyes; 40.0%).
 |
Table 1 Summary of Baseline Patient Demographics and Ocular Diagnoses |
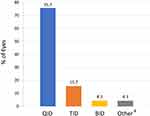
The most common LE/T dosing regimen was four times daily (n = 87 eyes; 75.7%) (Figure 2). Other dosing regimens included three times daily (n = 18 eyes; 15.7%), twice daily (n = 5; 4.3%), and other (n = 5; 4.3%; see footnote to Figure 2 for details). All eyes were dosed at one drop per dose, except for one eye which was dosed as two drops twice daily.
Per protocol, all 87 patients had one follow-up visit recorded; of these, 16 patients had two follow-up visits recorded. The mean time from the initial visit (when LE/T was prescribed) to first follow-up was 12 days (range, 1–72 days); the mean time from the initial visit to second follow-up was 20 days (range, 2–92 days). There were no AEs recorded. Among eyes for which IOP was recorded at both baseline and follow-up (n = 64), mean IOP (SD) at baseline was 15.2 (4.4) mm Hg. No significant changes from baseline were noted in the mean IOP at first follow-up (n = 64 eyes; mean change, −0.55 mm Hg; 95% CI: −1.481, 0.388; p = 0.25) or second follow-up (n = 8 eyes; mean change, −2.88 mm Hg; 95% CI: −7.101, 1.351; p = 0.15).
There were no significant changes from baseline to follow-up in mean VA among eyes for which VA was recorded at both baseline and follow-up. All VA assessments were performed using a Snellen chart (higher scores indicate worse VA). At the first follow-up visit, the mean changes from baseline in VA in the right eye (OD) (n = 63) and left eye (OS) (n = 46) were 3.73 (95% CI, −3.285, 10.745; p = 0.29) and 5.22 (95% CI, −4.220, 14.655; p = 0.27), respectively. At second follow-up, the mean changes from baseline in VA in OD (n = 14) and OS (n = 8) were −11.43 (95% CI, −32.776, 9.919; p = 0.27) and −8.13 (95% CI, −41.034, 24.784; p = 0.58), respectively.
At the first follow-up visit, the medical charts of 40 patients (46.0%) included notations to continue LE/T treatment. For 47 patients (54.0%), medical chart notations did not indicate the need to continue LE/T beyond the first follow-up visit; for 42 (89.4%) of these 47 patients, the treated condition was noted in the chart as “resolved” or “resolving.” One patient treated for iritis and whose condition was noted as “resolving” at initial follow-up was switched to prednisolone acetate. For the remaining 5 patients who did not continue on LE/T after the first follow-up, their condition was noted as “unchanged.” Of these five patients, two (one with corneal abrasion; one with keratitis) were switched to tobramycin/dexamethasone; one (diagnosis of conjunctival laceration) was switched to prednisolone acetate and besifloxacin; one (original diagnosis of eyelid inflammation) was referred to an ophthalmologist for pyogenic granuloma; and one with a diagnosis of allergic conjunctivitis was switched to therapy with LE ophthalmic suspension and a nasal steroid spray. No additional follow-up documentation was available for these five patients.
Among 16 patients with chart documentation of a second follow-up visit, ten were noted as having their condition “resolved” or “resolving” and no additional LE/T was prescribed. Of the six remaining patients/charts, four (two cases with corneal abrasion; one case with keratitis; one case with viral conjunctivitis) had notations to continue treatment and/or taper off LE/T for a set period of time beyond the visit and then discontinue. One patient with keratitis was instructed to continue LE/T at a reduced dosing frequency of BID rather than QID along with artificial tears as needed. One patient with a baseline diagnosis of neurotrophic keratoconjunctivitis was switched to cenegermin-bkbj (OxervateTM).
Discussion
This review of 87 patients treated for ocular surface inflammatory conditions with, or at risk for, bacterial infection provides insight into real-world use of LE/T for a variety of indications. The official dosing recommendations for LE/T suggest one or two drops every four to six hours; dosing every one to two hours may be used during the first 24 to 48 hours of treatment.3 In this real-world study, the vast majority of patients (75.7%) used the product four times a day, one drop per dose; only three patients were dosed every one to two hours for the first day (two with corneal abrasions, one with keratitis). Only one patient used two drops per dose.
There were no AEs noted in the medical charts reviewed in this study and no apparent changes in VA, supporting a high level of safety and tolerability of LE/T in this real-world cohort. In prospective studies of LE/T for blepharoconjunctivitis in which participants were proactively queried and examined for AEs, reports of AEs were generally of low incidence, non-serious, and non-severe.9–11 In the current retrospective study, the lack of noted AEs in the medical charts does not rule out the occurrence of AEs; it does, however, suggest an absence of AEs that rose to the importance of being mentioned to the healthcare practitioner and/or recorded in the patient’s chart. Similarly, it could be assumed that any relevant adverse findings noted by the healthcare practitioner during a follow-up visit examination would have been noted in the chart.
Although sequential IOP data were not available for all patients in this study, there were no significant changes in IOP noted among those who did have IOP data; this supports prior study findings of minimal IOP risk with LE/T, particularly as compared to other ocular corticosteroids.6 LE was retrometabolically designed to minimize the risk of adverse event risks while maintaining a high degree of anti-inflammatory activity.5 The chemical structure of LE was predicated on the prednisolone molecule, but modified to have an ester moiety at the C-20 position instead of a ketone group. The intended consequence of this modification is that the LE molecule is de-esterified to an inactive metabolite quickly after exerting its desired pharmacological activity, thus lessening exposure of local tissue to ongoing and potentially adverse corticosteroid effects.5,6 The absence of notable safety findings in this chart review provides further clinical evidence to support the notion that LE does, in fact, appear to have a high degree of clinical safety in real-world practice.
While efficacy was not able to be formally assessed in this retrospective review, among charts with explicit notes related to efficacy, most patient charts reflected the resolution of the baseline condition with the use of LE/T at their first follow-up visit. The size of the study population overall and variety of diagnoses treated precluded in-depth evaluations of efficacy in any one diagnosis; yet the multiple indications treated could be viewed as an observational strength. Additional limitations of this chart review include those typical for any retrospective study, namely that collection of data was limited to what was recorded during the course of routine medical practice, and thus not every patient had data for all of the study endpoints. Further, timing of recorded assessments was not uniform across patients. In addition, patient adherence with treatment was not able to be assessed.
Conclusion
Despite noted limitations, this retrospective chart review provides a clinically useful glimpse into real-world use of LE/T in a wide range of ocular surface inflammatory conditions with a potentially infective component. LE/T appears to have a high level of safety when used for the management of various ocular surface inflammatory conditions encountered in optometric practice.
Acknowledgments
This study was conducted by Churchill Outcomes Research. Medical writing support was provided by Sandra Westra, PharmD, through Churchill Communications, and funded by Bausch & Lomb Incorporated. Preliminary findings from this study were presented, in part, at the annual meeting of the Association of Research in Vision and Ophthalmology, held in Denver, CO, May 1–4, 2022, and virtually [Invest Ophthalmol Vis Sci 2022;62(7):3967-A0248].
Funding
This study was funded by Bausch & Lomb Incorporated.
Disclosure
Each of the authors received honoraria from Bausch and Lomb for work performed as part of this retrospective chart review study. The authors report the following additional disclosures, outside of this submitted work: Dr James E Deom is a paid consultant for Bausch Health. Dr Shane Kannarr reports personal fees from Alcon, Allergan, Bausch and Lomb, Cooper, Essilor, Johnson and Johnson, Kala, Novartis, Ocuphire, RVL, Sight Sciences, Tarsus, Vision Source, and Visus. Dr Patrick Vollmer has no other disclosures to report.
References
1. McGhee CNJ, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids. Benefits and risks. Drug Saf. 2002;25(1):33–55. doi:10.2165/00002018-200225010-00004
2. Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: are they all the same? Ophthalmol Ther. 2013;2:55–72. doi:10.1007/s40123-013-0020-5
3. Zylet (loteprednol etabonate and tobramycin ophthalmic suspension 0.5%/0.3%) [package insert]. Rochester, NY: Bausch & Lomb Incorporated; 2021.
4. Druzgala P, Hochhaus G, Bodor N. Soft drugs–10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: loteprednol etabonate. J Steroid Biochem Molec Biol. 1991;38:149–154. doi:10.1016/0960-0760(91)90120-T
5. Comstock TL, Sheppard JD. Loteprednol etabonate for inflammatory conditions of the anterior segment of the eye: twenty years of clinical experience with a retrometabolically designed corticosteroid. Expert Opin Pharmacother. 2018;19:337–353. doi:10.1080/14656566.2018.1439920
6. Sheppard JD, Comstock TL, Cavet ME. Impact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressure. Adv Ther. 2016;33:532–552. doi:10.1007/s12325-016-0315-8
7. Mah FS, Karpecki PM. Review of loteprednol etabonate 0.5%/tobramycin 0.3% in the treatment of blepharokeratoconjunctivitis. Ophthalmol Ther. 2021;10:859–875. doi:10.1007/s40123-021-00401-x
8. Deom JE, Cavet ME, Sanfilippo CM, DeCory HH. In vitro potency of tobramycin against common bacterial pathogens implicated in blepharitis. Invest Ophthalmol Vis Sci. 2021;62:414.
9. White EM, Macy JI, Bateman KM, Comstock TL. Comparison of the safety and efficacy of loteprednol 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of blepharokeratoconjunctivitis. Curr Med Res Opin. 2008;24:287–296. doi:10.1185/030079908X253898
10. Comstock TL, DeCory HH. Loteprednol etabonate 0.5%/tobramycin 0.3% compared with dexamethasone 0.1%/tobramycin 0.3% for the treatment of blepharitis. Ocul Immunol Inflamm. 2017;25:267–274. doi:10.3109/09273948.2015.1115879
11. Chen M, Gong L, Sun X. A multicenter, randomized, parallel-group, clinical trial comparing the safety and efficacy of loteprednol etabonate 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of Chinese patients with blepharokeratoconjunctivitis. Curr Med Res Opin. 2012;38:1–10.
12. Khawaja AP, Chan MPY, Broadway DC, et al. Systemic medication and intraocular pressure in a British population. Ophthalmology. 2014;121(8):1501–1507. doi:10.1016/j.ophtha.2014.02.009
13. Linden C, Alm A. Acetylsalicylic acid does not reduce the intraocular pressure variation in ocular hypertension or glaucoma. Exp Eye Res. 2000;70(3):281–283. doi:10.1006/exer.1999.0786
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.