Back to Journals » Drug Design, Development and Therapy » Volume 15
Real-World Use of Dalbavancin in the Era of Empowerment of Outpatient Antimicrobial Treatment: A Careful Appraisal Beyond Approved Indications Focusing on Unmet Clinical Needs
Authors Gatti M, Andreoni M, Pea F , Viale P
Received 23 June 2021
Accepted for publication 21 July 2021
Published 3 August 2021 Volume 2021:15 Pages 3349—3378
DOI https://doi.org/10.2147/DDDT.S313756
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Milo Gatti,1,2 Massimo Andreoni,3,4 Federico Pea,1,2 Pierluigi Viale1,5
1Department of Medical and Surgical Sciences, Alma Mater Studiorum University of Bologna, Bologna, Italy; 2SSD Clinical Pharmacology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; 3Department of Systems Medicine, University of Rome “Tor Vergata”, Rome, Italy; 4Infectious Diseases Clinic, University Hospital “Tor Vergata”, Rome, Italy; 5Infectious Disease Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
Correspondence: Milo Gatti
Department of Medical and Surgical Sciences, Alma Mater Studiorum University of Bologna, Via Massarenti 9, Bologna, 40138, Italy
Tel +39 051 214 3627
Email [email protected]
Abstract: Dalbavancin is a novel, long-acting lipoglycopeptide characterized by a long elimination half-life coupled with excellent in vitro activity against multidrug-resistant Gram-positives. Although it is currently approved only for the treatment of acute bacterial skin and skin structure infections, an ever-growing amount of evidence supports the efficacy of dalbavancin as a long-term therapy in osteomyelitis, prosthetic joint infections, endocarditis, and bloodstream infections. This article provides a critical reappraisal of real-world use of dalbavancin for off-label indications. A search strategy using specific keywords (dalbavancin, osteomyelitis, endocarditis, long-term suppressive therapy, bloodstream infection, pharmacokinetic/pharmacodynamic profile) until April 2021 was performed on the PubMed-MEDLINE database. As for other novel antibiotics, a conundrum between approved indications and potential innovative therapeutic uses has emerged for dalbavancin as well. The promising efficacy in challenging scenarios (i.e., osteomyelitis, endocarditis, prosthetic joint infections), coupled with the unique pharmacokinetic/pharmacodynamic properties, makes dalbavancin a valuable alternative to daily in-hospital intravenous or outpatient antimicrobial regimens in the treatment of long-term Gram-positive infections. This makes dalbavancin valuable in the current COVID-19 scenario, in which hospitalization and territorial medicine empowerment are unavoidable.
Keywords: dalbavancin, osteomyelitis, endocarditis, long-term suppressive therapy, PK/PD properties, COVID-19
Introduction
Dalbavancin is a novel, long-acting lipoglycopeptide active against Gram-positive pathogens, including multi-drug resistant isolates.1 Long elimination half-life and good tissue penetration represent the main pharmacokinetic features of dalbavancin,2 allowing for long-term efficacy despite the simplified weekly administration regimens. It was approved by the US Food and Drug Administration and the European Medicines Agency in 2014 and 2015 for the management of acute bacterial skin and skin structure infections (ABSSSIs) on an intravenous dosing regimen of 1500 mg as a single infusion or 1000 mg followed by 500 mg one week apart.3,4 Currently, ABSSSIs remain the only approved indication for dalbavancin, although this could represent a non-innovative and inefficient exploitation of its pharmacokinetic/pharmacodynamic (PK/PD) properties. Indeed, as reported for other novel antibiotics (e.g., ceftazidime-avibactam),5 a conundrum between approved indications and potential innovative therapeutic uses has emerged for dalbavancin.6 An ever-growing amount of evidence supports the efficacy of dalbavancin as a long-term therapy for off-label indications, namely osteomyelitis, prosthetic joint infections, endocarditis, and bloodstream infections,6–8 in which a treatment for at least 6 weeks is usually required.9,10 In the current COVID-19 era, patients requiring prolonged antibiotic therapy after hospital discharge due to severe bacterial infections (e.g., endocarditis or osteomyelitis) are at increased risk for contracting and/or transmitting COVID-19 due to extensive contact with the healthcare system.11 By virtue of its PK/PD properties, dalbavancin could be a valuable alternative to daily in-hospital intravenous or outpatient antimicrobial regimens in the treatment of long-term Gram-positive infections, providing an added value in the current COVID-19 scenario, in which ineluctable hospitalization and empowerment of territorial medicine are strongly required. We performed a critical reappraisal of real-world use of dalbavancin for off-label indications, suggesting therapeutic algorithms according to different clinical scenarios.
Materials and Methods
A literature search was conducted on PubMed-MEDLINE (from inception until April 2021) in order to retrieve randomized controlled trials (RCTs), prospective or retrospective observational studies, case series, and case reports investigating the use of dalbavancin for off-label indications. Six different main topics focusing on innovative use of dalbavancin were identified by three experts in the field (PV, MA, and FP), namely PK/PD properties, osteomyelitis, endocarditis and bloodstream infections, long-term suppressive therapy, safety, and quality of life. The following terms were searched on PubMed in combination:
dalbavancin, endocarditis, bloodstream infection, osteomyelitis, bone, prosthetic joint, long-term, off-label indication, pharmacokinetic, PK/PD, safety, quality of life.
Only studies in which clinical outcome associated with dalbavancin use was reported for each off-label indication were included. Articles were excluded if: only cumulative efficacy of dalbavancin for different off-label indications was provided; different long-acting lipoglycopeptides were investigated and clinical outcome for each single agent was not provided. There were no language restrictions. For each included study, the following information was extracted: (a) study author and year of publication; (b) study characteristics including study design and sample size; (c) features of the patients including site of infection, proportion of prior antibiotic therapy and duration, dalbavancin dosing schedule, isolated pathogens, and duration of follow-up; (d) types of outcome measurements, including rate of clinical success or improvement, clinical and/or microbiological failure rate, mortality rate, relapse rate, resistance development, and overall proportion of adverse events. Cumulative incidence of the different outcomes was calculated according to each specific dalbavancin off-label indication.
PK/PD Properties
Dalbavancin exhibits peculiar PK/PD properties, consisting in a long terminal half-life (approximately 14.4 days), high binding protein (93%), a predominant non-renal clearance, good tissue penetration, and high susceptibility rate (respectively 99.6% and 100.0%) against methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible Staphylococcus aureus (MSSA) isolates according to EUCAST clinical breakpoint (namely 0.125 mg/l).12–15 The clearance of dalbavancin was not affected by the presence of cytochrome P450 substrates, cytochrome P450 inhibitors, cytochrome P450 inducers, or selected concomitant medications. Furthermore, age, gender and race had no impact on the pharmacokinetic profile of dalbavancin.16 The ratio between the mean free-area under the curve and minimum inhibitory concentration (fAUC/MIC) represents the PK/PD parameter best correlating with in vivo efficacy of dalbavancin.17 The fAUC/MIC values for net stasis, 1-log kill, and 2-log kill against Staphylococcus aureus were respectively 27.1, 53.3, and 111.1.18 No dalbavancin dose adjustment is required in mild-moderate renal impairment, any degree of hepatic impairment, and different modalities of renal replacement therapy (i.e., intermittent haemodialysis, peritoneal dialysis, or continuous renal replacement therapy).19–21 Dose reduction should be implemented only in patients affected by severe renal impairment.19 Dalbavancin exhibits in vitro a potent activity against established biofilms due to Staphylococcus aureus, Staphylococcus epidermidis, and vancomycin-susceptible Enterococci,22–24 thus possibly playing a crucial role in the management of relevant infections characterized by bacterial biofilm production (e.g., endocarditis, osteomyelitis, device-related infections). Notably, dalbavancin is characterized by good tissue penetration in different sites of infection. Nicolau et al.25 reported a mean dalbavancin penetration into skin blister fluid of 59.6% after a single 1000 mg infusion, resulting in tissue concentrations well above the MIC90 of common gram-positive pathogens implicated in ABSSSIs (including MRSA) for up to 7 days. In a phase I study including 35 healthy subjects receiving a single infusion of 1500 mg dalbavancin, Rappo et al. found a penetration into the epithelial lining fluid of 36%, resulting in dalbavancin lung concentrations exceeding the MIC90 of Streptococcus pneumoniae and Staphylococcus aureus for at least 7 days.26 Notably, Dunne et al.2 found a mean bone/plasma AUC of 13.1%, suggesting that two-doses dalbavancin 1500 mg infusion administered one week apart may provide tissue exposure over the MIC for Staphylococcus aureus for 8 weeks. These findings were recently confirmed in a population PK study including 15 patients affected by osteoarticular infections, in which a two 1500 mg dosing regimen of dalbavancin one week apart may ensure efficacy against both MSSA and MRSA for up to 5 weeks.27 Consequently, these unique PK/PD properties make dalbavancin a valuable alternative to daily in-hospital intravenous or outpatient antimicrobial regimens in the treatment of long-term Gram-positive infections, posing the basis for its use beyond approved indications.
Real-World Use of Dalbavancin for off-Label Indications
Comparative Studies
Three studies (two RCTs and one retrospective case-control study)28–30 investigated efficacy and safety of dalbavancin compared with standard-of-care (SOC) treatment in different off-label settings (Table 1). Rappo et al.28 assessed 80 patients affected by osteomyelitis, 70 of whom were randomized to dalbavancin 1500 mg at day 1 and 8 and the other 10 received SOC (including vancomycin followed by linezolid, levofloxacin, or a third-generation cephalosporin). Staphylococcus aureus was the most frequent isolated pathogen. No difference in clinical cure rate between dalbavancin and SOC was found at the end of treatment (at 42 days; 97% vs 88%). The six-months and one-year follow-up assessment confirmed these findings (95% vs 88% and 94% vs 88%, respectively). Only one patient in the dalbavancin group required additional antibiotic therapy for relapse. There was no difference between groups in overall rate of drug-related AEs (1.4% vs 0.0%). Raad et al.29 assessed in a phase II RCT 67 patients affected by catheter-related bloodstream infections (CR-BSIs), 33 of whom were treated with dalbavancin (1000 mg at day 1 followed by 500 mg at day 8) and the other 34 with vancomycin (1 g q12h for 14 days). At 14-day intervals, dalbavancin showed a significantly higher clinical success rate compared with vancomycin (87.0% vs 50.0%; 95% CI 73.2–100.0% vs 31.5–68.5%). No infection relapse was found in dalbavancin group. Furthermore, no difference in safety was reported between the two groups, and dalbavancin reported no serious AE. Veve et al.30 retrospectively evaluated 215 patients affected by osteoarticular infections, infective endocarditis, and other BSIs (70 treated with dalbavancin and 145 receiving vancomycin or daptomycin). All patients treated with dalbavancin received prior antibiotic treatment. MRSA was isolated in 82.4% of cases. No difference in 90-day infection-related readmission was found between patients treated with dalbavancin or vancomycin/daptomycin (17% vs 28%; p = 0.12). Notably, dalbavancin use was independently associated with lower infection-related readmission at multivariate analysis (odds ratio [OR] 0.10; 95% CI 0.04–0.31). A significant lower AE rate was reported with dalbavancin (3% vs 14%; p = 0.013).
 |
Table 1 Summary of the Evidence Comparing the off-Label Use of Dalbavancin for the Treatment of Gram-Positive Infections with Standard of Care |
Infective Endocarditis
Eleven observational studies31–41 and five case reports8,42–45 assessed the efficacy and safety of dalbavancin for the treatment of infective endocarditis (IE; Table 2). Overall, 148 patients affected by infective endocarditis were treated with dalbavancin, resulting in a clinical success rate of 81.1%. Different dalbavancin regimens in terms of dose and duration were administered. Relapse was reported in up to 14.3% of cases. Only three cases of dalbavancin resistance have been reported.32,44,45 In the DALBACEN cohort study,31 34 patients affected by IE (15 prosthetic valve IE, 11 native valve IE, and eight cardiac device-related endocarditis) receiving at least one dose of dalbavancin were assessed. Prior antibiotic therapy implementation was reported in 100.0% of cases, with daptomycin (68.6%) being the most frequent agent used. Dalbavancin was administered as a single-dose or loading dose (LD) of 1000–1500 mg followed by 500–1000 mg weekly for up to 10 weeks. Coagulase-negative Staphylococci (CoNS) were the most frequent isolated pathogens. At 1-year, clinical success was documented in 85.3%. No relapse was reported. AEs occurred in 4.8% of patients, although none of these were serious. Interestingly, in all the three cases of IE caused by Enterococcus faecalis a positive clinical outcome was reported. Tobudic et al.32 retrospectively assessed 27 patients affected by IE (16 native valve IE, 6 prosthetic valve IE, and 5 cardiac device-related endocarditis) treated with dalbavancin. Prior antibiotic therapy use was reported in 88.9% of cases. Dalbavancin was administered as a LD of 1000 mg followed by 500 mg once-weekly, or 1000 mg biweekly after 1500 mg LD. Median duration was 6 weeks. Staphylococcus aureus (33.3%) was the most frequently isolated pathogen. At 6 months, clinical success and microbiological eradication was respectively found in 92.6% and 92.6% of patients. A case of resistance development occurred in a patient MSSA cardiac device-related endocarditis who was treated with dalbavancin on a once-weekly basis for 30 weeks. AEs occurred in 7.4% of patients. The other two cases reported the emergence of dalbavancin resistance in an IE setting.44,45 Steele et al.44 described a case of a 27-year pregnant woman affected by a native tricuspid-valve IE caused by MRSA receiving dalbavancin for 4 weeks after clinical failure with the use of vancomycin and daptomycin. Eleven days after the last dose of dalbavancin, a vancomycin-intermediate Staphylococcus aureus showing a dalbavancin MIC of 0.5 mg/L grew from blood cultures, and antibiotic therapy was switched to daptomycin in association with ceftaroline. Kussmann et al.45 described a case of a 36-year-old man affected by cardiac device-related endocarditis caused by MSSA/MRSA receiving long-term suppressive therapy for 6 months with dalbavancin. Two different MRSA strains exhibiting dalbavancin MIC of 0.5 mg/L and 1 mg/L were isolated from blood cultures. Antibiotic treatment was switched to fusidic acid in association with rifampicin, resulting in negative blood cultures and improved clinical conditions.
 |
 |
 |
 |
Table 2 Summary of the Evidence Investigating the off-Label Use of Dalbavancin for the Treatment of Endocarditis |
Bloodstream and Vascular Infections
Ten observational studies, one case series, and six case reports31,33–41,46–52 assessed the efficacy and safety of dalbavancin for the treatment of bloodstream infections (BSIs) and vascular infections (Table 3). Overall, 144 patients affected by BSIs or vascular infections were treated with dalbavancin, resulting in a clinical success of 81.3%. Different dalbavancin regimens in terms of dose and duration were administered. In a case of prosthetic graft infection due to Enterococcus faecium, dalbavancin was successfully administered as long-term suppressive therapy for a total of 62 weeks.40 Overall, relapse was reported in 3.5% of cases. The development of resistance to dalbavancin has emerged in only one case.49 In the DALBACEN cohort study,31 49 patients affected by BSIs (of which 69.4% were complicated) receiving at least one dose of dalbavancin were assessed. 93.9% of patients received dalbavancin for a median duration of 8 days, daptomycin and vancomycin being the most frequent agents used. Dalbavancin was administered as a single-dose of 1000–1500 mg, or 1000 mg LD followed by 500 mg at day 8. Staphylococcus aureus was the most frequent pathogen isolated (49.0%). Clinical success was documented in 100.0% of patients at 90 days (including two cases of BSI caused by Enterococcus faecium), with no case of relapse or resistance development. AEs occurred in 4.8% of patients, although none of these was serious. Different retrospective cohort studies and case series34,37,41,46 reported the efficacy of dalbavancin for the treatment of endovascular infections, with a clinical success ranging from 50–80% of cases. Notably, Werth et al.49 reported a case of occurrence of dalbavancin resistance in a 34-year man affected by a CR-BSI due to MRSA. The patient received a single dalbavancin dose of 1000 mg after eight days of ineffective therapy with vancomycin because of inability to achieve optimal serum concentrations. After 12 days, a VISA was isolated from urine culture exhibiting a 4-fold increase in MIC for dalbavancin and vancomycin compared with previous isolate retrieved in blood cultures.
 |
 |
 |
 |
Table 3 Summary of the Evidence Investigating the off-Label Use of Dalbavancin for the Treatment of Bloodstream Infections |
Bone and Joint Infections
Bone and joint infections represent the most frequent dalbavancin off-label indication. Specifically, one RCT,28 12 observational studies,33–35,37,38,40,41,53–57 one case series,36 and 11 case reports,8,58–67 assessed the efficacy and safety of dalbavancin for the treatment of bone and joint infections (Table 4). Overall, 483 patients affected by bone and joint infections were treated with dalbavancin, resulting in a clinical success rate of 84.5%. Different dalbavancin regimens in terms of dose and duration were administered. Relapse occurred in up to 12.5% of cases. Notably, no case of resistance development to dalbavancin was reported. As previously mentioned, Rappo et al.28 found no difference in clinical cure rate at the end of treatment in 70 patients affected by osteomyelitis randomized to dalbavancin compared with 10 subjects receiving SOC (97% vs 88%; p = NS). Morata et al.54 retrospectively collected 64 patients with bone and joint infections (namely 45 and 19 respectively affected by implant-associated infections and osteomyelitis). Staphylococcus epidermidis was isolated in almost half of cases. A clinical success or improvement was respectively reported in 97.7% and 89.5% of subjects with implant-associated infections and osteomyelitis. Relapse was described in only two patients. Wunsch et al.33 retrospectively assessed 62 patients (32 affected by prosthetic joint infections and 30 with osteomyelitis) receiving dalbavancin as second-line therapy for bone and joint infections. Dalbavancin was administered as a 1500 mg single dose, 1000 mg LD followed by 500 mg at day 8, or two 1500 mg doses one week apart. Clinical success was reported in 93.6% of patients, and no case of relapse was documented. Overall, AEs were reported in only 3% of cases. Interestingly, Bai et al.38 stratified clinical outcome according to different type of bone and joint infection in 50 patients receiving dalbavancin (1500 mg single dose or 1000 mg LD followed by 500 mg at day 8). At 6 months, clinical success was respectively reported in 89.7% of osteomyelitis/spondylodiscitis, 76.5% of prosthetic joint infections, and 75% of septic arthritis. Similarly, 6-month clinical cure was respectively found in 100% of acute septic arthritis, 60% of osteomyelitis, 50% of spondylodiscitis, and 38% of prosthetic joint infections in 50 patients affected by bone and joint infections retrospectively collected.53 Notably, Almangour et al.56 found a trend for higher 90-day clinical cure in 11 patients receiving dalbavancin for the treatment of Staphylococcus aureus osteomyelitis compared with 11 subjects treated with SOC, including vancomycin, daptomycin, or cefazolin according to Staphylococcus aureus susceptibility (100.0% vs 72.7%; p = 0.06). Different retrospective studies and case reports documented the efficacy of dalbavancin for the treatment of bone and joint infections caused by Enterococcus faecium or Corynebacterium striatum.33,38,40,54,57,59,63
 |
 |
 |
 |
 |
 |
 |
Table 4 Summary of the Evidence Investigating the off-Label Use of Dalbavancin for the Treatment of Bone and Joint Infections |
Other off-Label Indications
Four retrospective cohort studies and two case reports34,37,41,68–70 assessed the efficacy and safety of dalbavancin for other off-label indications (Table 5). Overall, 16 cases of deep sternal wound infection associated with mediastinitis, three cases of intra-abdominal infection, two cases of mediastinitis, two cases of pneumonia, and one case each of sinusitis and pyelonephritis were reported. Clinical cure was documented in 92.0% of cases. Relapse was reported in a patient affected by deep sternal wound infection,68 and in a case of mediastinitis.34 No occurrence of resistance development to dalbavancin was found, and no safety issues emerged.
 |
Table 5 Summary of the Evidence Investigating the off-Label Use of Dalbavancin for the Treatment of Other Infection Typologies |
Quality of Life
The favourable PK/PD properties of dalbavancin resulting in a single weekly administration and abbreviated dosing schedule may allow for the treatment of endocarditis, osteomyelitis, and vascular infections in an outpatient setting, thus leading to shorter length of hospital stay, reduction in cost and healthcare resource use, and improvement in patient satisfaction. Although different studies37,71–76 assessed the advantages of dalbavancin from a pharmacoeconomic point of view, the consequent impact on quality of life has been less investigated. Early discharge and shorter hospital stays are associated with improved patient quality of life, mobility, and the prevention of non-infectious and infectious catheter-related complications.8 In the ENHANCE pre-post trial,77 McCarthy et al. found a significant improvement in work productivity and activity impairment (impairment while working [47.9% vs 8.9%; p = 0.01], overall work impairment [59.3% vs 18.0%; p = 0.01], and non-work related impairment of activity [60.2% vs 18.5%; p<0.001]) in 43 patients treated with dalbavancin for acute bacterial skin and skin structure infections (ABSSSIs) in the post-period compared with 48 subjects receiving usual care in the pre-period. Furthermore, a trend to significant improvement in quality-of-life outcomes was reported with dalbavancin (p = 0.07). However, no difference in absenteeism between pre- and post-period was found. In a post-hoc analysis78 of a phase 3 RCT involving 698 adult patients with ABSSSIs and treated with dalbavancin (386 and 312 respectively managed in outpatient and inpatient settings), outpatients reported significantly greater convenience and satisfaction with antibiotic treatment and care setting than inpatients. Specifically, a greater number of outpatients versus inpatients reported that antibiotic treatment did not interfere at all with daily activities (74% vs 42%; p<0.001) and that they were easily able to modify their schedule to receive antibiotic therapy (97% vs 76%; p<0.001).
Safety
Dalbavancin exhibited an excellent safety profile both in RCTs and observational studies investigating in- and off-label indications.79 Dunne et al.80 performed a pooled analysis of 3002 patients enrolled in seven phase II/III RCTs receiving dalbavancin (N = 1778) or comparators (N = 1224), including vancomycin, linezolid, nafcillin, oxacillin, and cephalosporins. Patients treated with dalbavancin experienced a significantly lower number of overall treatment-emergent AEs compared with those receiving other antibiotics (44.9% vs 46.8%; p = 0.012). AEs reported in at least 2% of subjects receiving dalbavancin in RCTs were: nausea, headache, diarrhoea, vomiting, and rash.80 Furthermore, no late-onset AEs were reported.80 A recent meta-analysis including seven RCTs investigating dalbavancin in different settings (ABSSSIs, CR-BSIs, and osteomyelitis) found no significant difference in terms of any AEs (OR 1.58; 95% CI 0.82–3.02), adverse drug reactions (OR 0.85; 95% CI 0.61–1.19), and specific AEs (including nausea, headache, constipation, hypertension, vomiting, rash, pyrexia, anaemia, fungal infection, alanine aminotransferase elevation and gamma-glutamyl transferase elevation) between dalbavancin and comparators.81 When compared with vancomycin or linezolid, the incidence of diarrhoea was significantly lower in patients receiving dalbavancin (OR 0.38; 95% CI 0.21–0.68).81 Notably, there was no significant difference in serious AEs (OR 0.80; 95% CI 0.55–1.17).81
The excellent safety profile was also confirmed in real-life studies evaluating dalbavancin use in off-label settings (e.g., endocarditis, BSIs, osteomyelitis, joint infections), in which the overall proportion of AEs ranged from 0% to 13%.28–34,36–38,40,41,54 Furthermore, most AEs were not serious, with serious AEs ranging between 0.0% and 2.9%.28–38,40,41,54 Notably, Veve et al.30 found a significantly lower proportion of AEs in patients treated with dalbavancin for off-label indications (namely osteoarticular infections, IE, and BSIs) compared with vancomycin or daptomycin (3% vs 14%; p = 0.013). Dalbavancin was well-tolerated by children ranging in age from 3 months to 17 years.82,83 Additionally, Dunne et al. found no QTc interval prolongation with dalbavancin administration at a dosage up to 1500 mg in healthy volunteers.84 Finally, Mahoney et al.85 reported no short-term or long-term AEs in a case of unintentional receipt of 3000 mg of dalbavancin within 20 hours, with the possible exception of mild diarrhoea.
Expert Opinion
In the last five years, several reports assessing the use of dalbavancin in challenging off-label clinical scenarios have emerged, highlighting the innovative role of this agent for the management of Gram-positive infections usually requiring long-term therapy. Overall, in the 800 identified patients receiving dalbavancin for off-label indications, a positive clinical outcome was reported in a remarkable proportion of subjects, namely in 84.3% of bone and joint infections, 81.3% of BSIs (mainly CR-BSIs or endovascular infections), 81.1% of IE, and 92.0% of other indications (including deep sternal wound infections associated with mediastinitis, intra-abdominal infections, pneumonia, pyelonephritis, and sinusitis; Table 6). Notably, in these settings dalbavancin showed a significantly higher clinical cure rate and lower infection-related readmission with respect to comparators (namely vancomycin and daptomycin). Furthermore, the occurrence of relapse was limited (below 10%), while resistance development to dalbavancin was reported in only four patients (three cases of infection caused by MRSA and one due to MSSA). Similarly, the emergence of serious safety issues with dalbavancin was negligible.
 |
Table 6 Cumulative Efficacy Reported with the Use of Dalbavancin for off-Label Therapeutic Indications |
Notably, the use of dalbavancin in these challenging scenarios exhibits some relevant advantages, specifically allowing for avoiding daily in-hospital intravenous antibiotic treatment or complications associated with outpatient antimicrobial therapy. Indeed, the unique PK/PD properties of dalbavancin resulting in a single weekly administration and abbreviated dosing schedule may allow for the treatment of Gram-positive infections in an outpatient setting. This represents a crucial aspect in view of the current COVID-19 era, in which patients requiring prolonged (e.g., IE or bone and joint infections) or long-term suppressive (endovascular infections) antibiotic therapy after hospital discharge due to severe Gram-positive infections are at increased risk for contracting and/or transmitting COVID-19 due to extensive contact with the healthcare system. Furthermore, dalbavancin allows a limited healthcare resource use coupled with a lower length of hospital stay, resulting in non-negligible cost savings. In this scenario, dalbavancin could play a key role in enhancing outpatient treatment of several infections requiring long-term antibiotic therapy.
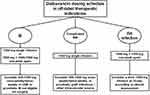
Real-world evidence showed a wide heterogeneity in dalbavancin dosing schedule and treatment duration in the different clinical scenarios. Consequently, an attempt to standardize the therapeutic approach in patients requiring dalbavancin for the management of IE, BSI, or bone and joint infections could be made (Figure 1). A single infusion of 1500 mg, or 1500 mg followed by a second dose of 1000–1500 mg one-week apart could be proposed as the dalbavancin dosing schedule in patients affected by IE. In subjects with prosthetic IE not eligible for surgical treatment or affected by cardiac device-related endocarditis requiring long-term chronic suppression therapy, once-weekly or twice-weekly infusion of dalbavancin 500–1500 mg could be suggested. A single infusion of dalbavancin 1500 mg could be adequate for the treatment of complicated BSI, as well as in the case of CR-BSI. In patients affected by BSI due to prosthetic graft infection or other intravascular source (e.g., left ventricular assistance device infection), a long-term suppression therapy consisting in once-weekly or twice-weekly infusion of dalbavancin 500–1500 mg could be suggested. Dalbavancin dosing schedule proposed by Dunne et al.2 (1500 mg followed by a second infusion of 1500 mg one-week apart) could be proposed in patients affected by bone and joint infections, considering that this regimen ensures great efficacy against Staphylococcus aureus for up to 5 weeks. In the fifth week, a clinical assessment may allow for considering the administration of an additional dose for prolonging effective treatment.27 In this case, a therapeutic drug monitoring-guided approach could also be implemented to assess the achievement of optimal dalbavancin PK/PD target.
The role of dalbavancin combination therapy in off-label indications remains an unmet clinical need. Several in vitro studies showed the synergistic effect of dalbavancin in combination with ceftaroline, cefazolin, daptomycin, rifampicin, and linezolid in reducing the MIC for MRSA, daptomycin non-susceptible, and heterogeneous VISA strains.86–89 However, real-world experiences are limited to a single case report of dalbavancin and linezolid combination therapy for the management of a prosthetic joint infection.60
Conclusion
Dalbavancin shows remarkable efficacy and good tolerability in different challenging scenarios, emerging as a promising alternative in the management of IE, complicated BSI, and osteoarticular infections. The role of dalbavancin is further enhanced in the current COVID-19 era, in which ineluctable hospitalization and empowerment of territorial medicine are strongly required. Further studies assessing the best dosing schedule and the role of combination therapy in each specific scenario are warranted.
Acknowledgment
This work was supported by an unrestricted grant from Angelini S.P.A.
Disclosure
Dr M Gatti reports grants from Angelini S.p.A., during the conduct of the study. Prof. M Andreoni has participated in advisory boards and/or received speaker honoraria from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, ViiV Healthcare and has received study grants from Merck Sharp & Dohme, outside the submitted work. Prof. F Pea reports personal fees from Angelini, during the conduct of the study; personal fees from Angelini, Basilea Pharmaceutica, Gilead, Hikma, MSD, Pfizer, Sanofi-Aventis, Shionogi, Thermo Fisher, and Accelerate Diagnostics, outside the submitted work; has participated in speaker’s bureau for Accelerate Diagnostics, Angelini, Basilea Pharmaceutica, Gilead, Hikma, MSD, Pfizer, Sanofi-Aventis, Shionogi, Thermo Fisher, and as consultant for Angelini, Basilea Pharmaceutica, Gilead, MSD, Pfizer, Shionogi, outside the submitted work. Prof P Viale has served as a consultant for bioMérieux, Gilead, Merck Sharp & Dohme, Nabriva, Nordic Pharma, Pfizer, Thermo-Fisher, and Venatorx, and received payment for serving on the speaker’s bureaus for Correvio, Gilead, Merck Sharp & Dohme, Nordic Pharma, and Pfizer, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Andreoni M, Bassetti M, Corrao S, De Rosa FG, Esposito V, Falcone M, Grossi P, Pea F, Petrosillo N, Tascini C, Venditti M, Viale P. The role of dalbavancin for Gram positive infections in the COVID-19 era: state of the art and future perspectives. Expert Rev Anti Infect Ther. 2021 Mar 16:1–10. doi:10.1080/14787210.2021.1894130. Epub ahead of print.
2. Dunne MW, Puttagunta S, Sprenger CR, Rubino C, Van Wart S, Baldassarre J. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother. 2015;59(4):1849–1855. doi:10.1128/AAC.04550-14
3. Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169–2179. doi:10.1056/NEJMoa1310480
4. Dunne MW, Puttagunta S, Giordano P, Krievins D, Zelasky M, Baldassarre J. A randomized clinical trial of single-dose versus weekly dalbavancin for treatment of acute bacterial skin and skin structure infection. Clin Infect Dis. 2016;62(5):545–551. doi:10.1093/cid/civ982
5. Doi Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis. 2019;69(Suppl 7):S565–S575. doi:10.1093/cid/ciz830
6. Thomas G, Henao-Martínez AF, Franco-Paredes C, Chastain DB. Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: a systematic review. Int J Antimicrob Agents. 2020;56(3):106069. doi:10.1016/j.ijantimicag.2020.106069
7. Herrera-Hidalgo L, de Alarcón A, López-Cortes LE, et al. Enterococcus faecalis endocarditis and outpatient treatment: a systematic review of current alternatives. Antibiotics (Basel). 2020;9(10). doi:10.3390/antibiotics9100657
8. Durante-Mangoni E, Gambardella M, Iula VD, et al. Current trends in the real-life use of dalbavancin: report of a study panel. Int J Antimicrob Agents. 2020;56(4):106107. doi:10.1016/j.ijantimicag.2020.106107
9. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393–407. doi:10.1093/cid/cir842
10. Luque Paz D, Lakbar I, Tattevin P. A review of current treatment strategies for infective endocarditis. Expert Rev Anti Infect Ther. 2021;19(3):297–307. doi:10.1080/14787210.2020.1822165
11. Mansour O, Keller S, Katz M, Townsend JL. Outpatient parenteral antimicrobial therapy in the time of COVID-19: the urgent need for better insurance coverage. Open Forum Infect Dis. 2020;7(8):ofaa287. doi:10.1093/ofid/ofaa287
12. Dash RP, Babu RJ, Srinivas NR. Review of the pharmacokinetics of dalbavancin, a recently approved lipoglycopeptide antibiotic. Infect Dis (Lond). 2017;49(7):483–492. doi:10.1080/23744235.2017.1296968
13. Carrothers TJ, Chittenden JT, Critchley I. Dalbavancin population pharmacokinetic modeling and target attainment analysis. Clin Pharmacol Drug Dev. 2020;9(1):21–31. doi:10.1002/cpdd.695
14. Jones RN, Stilwell MG, Sader HS, Fritsche TR, Goldstein BP. Spectrum and potency of dalbavancin tested against 3322 Gram-positive cocci isolated in the United States surveillance program (2004). Diagn Microbiol Infect Dis. 2006;54(2):149–153. doi:10.1016/j.diagmicrobio.2005.08.015
15. Pfaller MA, Flamm RK, Castanheira M, Sader HS, Mendes RE. Dalbavancin in-vitro activity obtained against Gram-positive clinical isolates causing bone and joint infections in US and European hospitals (2011–2016). Int J Antimicrob Agents. 2018;51(4):608–611. doi:10.1016/j.ijantimicag.2017.12.011
16. Buckwalter M, Dowell JA. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J Clin Pharmacol. 2005;45(11):1279–1287. doi:10.1177/0091270005280378
17. Andes D, Craig WA. In vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob Agents Chemother. 2007;51(5):1633–1642. doi:10.1128/AAC.01264-06
18. Lepak A, Marchillo K, VanHecker J, Andes D. Impact of glycopeptide resistance in staphylococcus aureus on the dalbavancin in vivo pharmacodynamic target. Antimicrob Agents Chemother. 2015;59(12):7833–7836. doi:10.1128/AAC.01717-15
19. Marbury T, Dowell JA, Seltzer E, Buckwalter M. Pharmacokinetics of dalbavancin in patients with renal or hepatic impairment. J Clin Pharmacol. 2009;49(4):465–476. doi:10.1177/0091270008330162
20. Van Matre ET, Teitelbaum I, Kiser TH. Intravenous and intraperitoneal pharmacokinetics of dalbavancin in peritoneal dialysis patients. Antimicrob Agents Chemother. 2020;64(5). doi:10.1128/AAC.02089-19
21. Corona A, Agarossi A, Veronese A, Cattaneo D, D’Avolio A. Therapeutic drug monitoring of dalbavancin treatment in severe necrotizing fasciitis in 3 critically ill patients: a grand round. Ther Drug Monit. 2020;42(2):165–168. doi:10.1097/FTD.0000000000000729
22. Di Pilato V, Ceccherini F, Sennati S, et al. In vitro time-kill kinetics of dalbavancin against Staphylococcus spp. biofilms over prolonged exposure times. Diagn Microbiol Infect Dis. 2020;96(2):114901. doi:10.1016/j.diagmicrobio.2019.114901
23. Fernández J, Greenwood-Quaintance KE, Patel R. In vitro activity of dalbavancin against biofilms of staphylococci isolated from prosthetic joint infections. Diagn Microbiol Infect Dis. 2016;85(4):449–451. doi:10.1016/j.diagmicrobio.2016.05.009
24. Neudorfer K, Schmidt-Malan SM, Patel R. Dalbavancin is active in vitro against biofilms formed by dalbavancin-susceptible enterococci. Diagn Microbiol Infect Dis. 2018;90(1):58–63. doi:10.1016/j.diagmicrobio.2017.09.015
25. Nicolau DP, Sun HK, Seltzer E, Buckwalter M, Dowell JA. Pharmacokinetics of dalbavancin in plasma and skin blister fluid. J Antimicrob Chemother. 2007;60(3):681–684. doi:10.1093/jac/dkm263
26. Rappo U, Dunne MW, Puttagunta S, et al. Epithelial lining fluid and plasma concentrations of dalbavancin in healthy adults after a single 1500-milligram infusion. Antimicrob Agents Chemother. 2019;63(11). doi:10.1128/AAC.01024-19
27. Cojutti PG, Rinaldi M, Zamparini E, et al. Population pharmacokinetics of dalbavancin and dosing consideration for optimal treatment of adult patients with staphylococcal osteoarticular infections. Antimicrob Agents Chemother. 2021. doi:10.1128/AAC.02260-20
28. Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2019;6(1):ofy331. doi:10.1093/ofid/ofy331
29. Raad I, Darouiche R, Vazquez J, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis. 2005;40(3):374–380. doi:10.1086/427283
30. Veve MP, Patel N, Smith ZA, Yeager SD, Wright LR, Shorman MA. Comparison of dalbavancin to standard-of-care for outpatient treatment of invasive Gram-positive infections. Int J Antimicrob Agents. 2020;56(6):106210. doi:10.1016/j.ijantimicag.2020.106210
31. Hidalgo-Tenorio C, Vinuesa D, Plata A, et al. DALBACEN cohort: dalbavancin as consolidation therapy in patients with endocarditis and/or bloodstream infection produced by gram-positive cocci. Ann Clin Microbiol Antimicrob. 2019;18(1):30. doi:10.1186/s12941-019-0329-6
32. Tobudic S, Forstner C, Burgmann H, et al. Dalbavancin as primary and sequential treatment for gram-positive infective endocarditis: 2-year experience at the general hospital of vienna. Clin Infect Dis. 2018;67(5):795–798. doi:10.1093/cid/ciy279
33. Wunsch S, Krause R, Valentin T, et al. Multicenter clinical experience of real life Dalbavancin use in gram-positive infections. Int J Infect Dis. 2019;81:210–214. doi:10.1016/j.ijid.2019.02.013
34. Dinh A, Duran C, Pavese P, et al. French national cohort of first use of dalbavancin: a high proportion of off-label use. Int J Antimicrob Agents. 2019;54(5):668–672. doi:10.1016/j.ijantimicag.2019.08.006
35. Bryson-Cahn C, Beieler AM, Chan JD, Harrington RD, Dhanireddy S. Dalbavancin as secondary therapy for serious staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect Dis. 2019;6(2):ofz028. doi:10.1093/ofid/ofz028
36. Vazquez Deida AA, Shihadeh KC, Preslaski CR, Young HL, Wyles DL, Jenkins TC. Use of a standardized dalbavancin approach to facilitate earlier hospital discharge for vulnerable patients receiving prolonged inpatient antibiotic therapy. Open Forum Infect Dis. 2020;7(8):ofaa293. doi:10.1093/ofid/ofaa293
37. Bouza E, Valerio M, Soriano A, et al. Dalbavancin in the treatment of different gram-positive infections: a real-life experience. Int J Antimicrob Agents. 2018;51(4):571–577. doi:10.1016/j.ijantimicag.2017.11.008
38. Bai F, Aldieri C, Cattelan A, et al. Efficacy and safety of dalbavancin in the treatment of acute bacterial skin and skin structure infections (ABSSSIs) and other infections in a real-life setting: data from an Italian observational multicentric study (DALBITA study). Expert Rev Anti Infect Ther. 2020;18(12):1271–1279. doi:10.1080/14787210.2020.1798227
39. Ajaka L, Heil E, Schmalzle S. Dalbavancin in the treatment of bacteremia and endocarditis in people with barriers to standard care. Antibiotics (Basel). 2020;9(10). doi:10.3390/antibiotics9100700
40. Núñez-Núñez MP, Casas-Hidalgo I, García-Fumero R, et al. Dalbavancin is a novel antimicrobial against Gram-positive pathogens: clinical experience beyond labelled indications. Eur J Hosp Pharm. 2020;27(5):310–312. doi:10.1136/ejhpharm-2018-001711
41. Bork JT, Heil EL, Berry S, et al. Dalbavancin use in vulnerable patients receiving outpatient parenteral antibiotic therapy for invasive gram-positive infections. Infect Dis Ther. 2019;8(2):171–184. doi:10.1007/s40121-019-0247-0
42. Spaziante M, Franchi C, Taliani G, et al. Serum bactericidal activity levels monitor to guide intravenous dalbavancin chronic suppressive therapy of inoperable staphylococcal prosthetic valve endocarditis: a case report. Open Forum Infect Dis. 2019;6(11):ofz427. doi:10.1093/ofid/ofz427
43. Hakim A, Braun H, Thornton D, Strymish J. Successful treatment of methicillin-sensitive Staphylococcus aureus tricuspid-valve endocarditis with dalbavancin as an outpatient in a person who injects drugs: a case report. Int J Infect Dis. 2020;91:202–205. doi:10.1016/j.ijid.2019.12.008
44. Steele JM, Seabury RW, Hale CM, Mogle BT. Unsuccessful treatment of methicillin-resistant Staphylococcus aureus endocarditis with dalbavancin. J Clin Pharm Ther. 2018;43(1):101–103. doi:10.1111/jcpt.12580
45. Kussmann M, Karer M, Obermueller M, et al. Emergence of a dalbavancin induced glycopeptide/lipoglycopeptide non-susceptible Staphylococcus aureus during treatment of a cardiac device-related endocarditis. Emerg Microbes Infect. 2018;7(1):202. doi:10.1038/s41426-018-0205-z
46. Hitzenbichler F, Mohr A, Camboni D, Simon M, Salzberger B, Hanses F. Dalbavancin as long-term suppressive therapy for patients with Gram-positive bacteremia due to an intravascular source-a series of four cases. Infection. 2021;49(1):181–186. doi:10.1007/s15010-020-01526-0
47. Jones BM, Keedy C, Wynn M. Successful treatment of Enterococcus faecalis bacteremia with dalbavancin as an outpatient in an intravenous drug user. Int J Infect Dis. 2018;76:4–5. doi:10.1016/j.ijid.2018.07.016
48. Cho JC, Estrada SJ, Beltran AJ, Revuelta MP. Treatment of methicillin-sensitive Staphylococcus aureus bacteremia secondary to septic phlebitis using dalbavancin. J Clin Pharm Ther. 2015;40(5):604–606. doi:10.1111/jcpt.12306
49. Werth BJ, Jain R, Hahn A, et al. Emergence of dalbavancin non-susceptible, vancomycin-intermediate Staphylococcus aureus (VISA) after treatment of MRSA central line-associated bloodstream infection with a dalbavancin- and vancomycin-containing regimen. Clin Microbiol Infect. 2018;24(4):
50. Ciccullo A, Giuliano G, Segala FV, Taddei E, Farinacci D, Pallavicini F. Dalbavancin as a second-line treatment in methicillin-resistant Staphylococcus aureus prosthetic vascular graft infection. Infection. 2020;48(2):309–310. doi:10.1007/s15010-019-01379-2
51. Howard-Anderson J, Pouch SM, Sexton ME, et al. Left ventricular assist device infections and the potential role for dalbavancin: a case report. Open Forum Infect Dis. 2019;6(9):ofz235. doi:10.1093/ofid/ofz235
52. Martínez-Sanz J, Gijón de la Santa L, Torralba M. Treatment with dalbavancin in a patient with septic thrombophlebitis of the internal jugular vein due to Staphylococcus aureus after insertion of an implantable cardioverter defibrillator. Enferm Infecc Microbiol Clin. 2018;36(6):389–390. doi:10.1016/j.eimc.2017.10.002
53. Tobudic S, Forstner C, Burgmann H, et al. Real-world experience with dalbavancin therapy in gram-positive skin and soft tissue infection, bone and joint infection. Infection. 2019;47(6):1013–1020. doi:10.1007/s15010-019-01354-x
54. Morata L, Cobo J, Fernández-Sampedro M, et al. Safety and efficacy of prolonged use of dalbavancin in bone and joint infections. Antimicrob Agents Chemother. 2019;63(5). doi:10.1128/AAC.02280-18
55. Almangour TA, Perry GK, Terriff CM, Alhifany AA, Kaye KS. Dalbavancin for the management of gram-positive osteomyelitis: effectiveness and potential utility. Diagn Microbiol Infect Dis. 2019;93(3):213–218. doi:10.1016/j.diagmicrobio.2018.10.007
56. Almangour TA, Perry GK, Alhifany AA. Dalbavancin versus standard of care for the treatment of osteomyelitis in adults: a retrospective matched cohort study. Saudi Pharm J. 2020;28(4):460–464. doi:10.1016/j.jsps.2020.02.007
57. Buzón Martín L, Mora Fernández M, Perales Ruiz JM, et al. Dalbavancin for treating prosthetic joint infections caused by Gram-positive bacteria: a proposal for a low dose strategy. A retrospective cohort study. Rev Esp Quimioter. 2019;32(6):532–538.
58. Azamgarhi T, Donaldson J, Shah A, Warren S. Dalbavancin to treat infected massive endoprostheses: a case report and cost comparison analysis. J Bone Jt Infect. 2019;4(5):234–237. doi:10.7150/jbji.37980
59. Loupa CV, Lykoudi E, Meimeti E, et al. Successful treatment of diabetic foot osteomyelitis with dalbavancin. Med Arch. 2020;74(3):243–245. doi:10.5455/medarh.2020.74.243-245
60. Carrión Madroñal IM, Sánchez Del Moral R, Abad Zamora JM, Martínez Marcos FJ. Dalbavancin combined with linezolid in prosthetic-hip infection. Rev Esp Quimioter. 2020;33(2):147–148. doi:10.37201/req/087.2019
61. Vates R, Rodríguez SJ, Martínez ME, Martínez JA. Experiencia clínica sobre un caso de osteomielitis tratado con dalbavancina [Clinical experience on a case of osteomyelitis treated with dalbavancin]. Rev Esp Quimioter. 2018. Spanish. Epub ahead of print.
62. Almangour TA, Fletcher V, Alessa M, Alhifany AA, Tabb D. Multiple weekly dalbavancin dosing for the treatment of native vertebral osteomyelitis caused by methicillin-resistant staphylococcus aureus: a case report. Am J Case Rep. 2017;18:1315–1319. doi:10.12659/ajcr.905930
63. Molina Collada J, Rico Nieto A, Díaz de Bustamante Ussia M, Balsa Criado A. Septic arthritis in a native knee due to Corynebacterium striatum. Reumatol Clin. 2018;14(5):301–302. doi:10.1016/j.reuma.2017.01.013
64. Trujillano Ruiz A, Mesquida Riera J, Serrano Fabiá MA, Riera Pérez E, Mejía Benard A, Taberner Ferrer MD. Tratamiento prolongado con dalbavancina en infección protésica de cadera por Staphylocuccus epidermidis resistente a meticilina [Prolonged treatment with dalbavancin in prosthetic hip infection by methicillin-resistant Staphylococcus epidermidis]. Rev Esp Quimioter. 2019;32(2):203–204. Spanish. Epub 2019 Mar 13.
65. Barbero Allende JM, García Sánchez M, Culebras López AM, Agudo Alonso R. Suppressive antibiotic treatment with dalbavancin. A case report. Rev Esp Quimioter. 2021;34(2):151–153. doi:10.37201/req/105.2020
66. Ramírez Hidalgo M, Jover-Sáenz A, García-González M, Barcenilla-Gaite F. Dalbavancin treatment of prosthetic knee infection due to oxacillin-resistant Staphylococcus epidermidis. Enferm Infecc Microbiol Clin. 2018;36(2):142–143. doi:10.1016/j.eimc.2017.04.009
67. Álvarez Otero J, Sanjurjo Rivo A, Lamas Ferreiro JL, de la Fuente Aguado J. Dalbavancin treatment of methicillin-susceptible Staphylococcus aureus pyomyositis with torpid evolution: a case report. Rev Esp Quimioter. 2019;32(3):276–277.
68. Bartoletti M, Mikus E, Pascale R, et al. Clinical experience with dalbavancin for the treatment of deep sternal wound infection. J Glob Antimicrob Resist. 2019;18:195–198. doi:10.1016/j.jgar.2019.03.015
69. Barber KE, Tirmizi A, Finley R, Stover KR. Dalbavancin use for the treatment of methicillin-resistant Staphylococcus aureus pneumonia. J Pharmacol Pharmacother. 2017;8(2):77–79. doi:10.4103/jpp.JPP_2_17
70. Guzek A, Suwalski G, Tomaszewski D, Rybicki Z. Dalbavancin treatment in a deep sternal wound MRSA infection after coronary artery bypass surgery: a case report. J Cardiothorac Surg. 2018;13(1):3. doi:10.1186/s13019-017-0690-5
71. Streifel AC, Sikka MK, Bowen CD, Lewis JS. Dalbavancin use in an academic medical centre and associated cost savings. Int J Antimicrob Agents. 2019;54(5):652–654. doi:10.1016/j.ijantimicag.2019.08.007
72. Bookstaver PB, Milgrom A. Stewarding the costly antibiotic: considerations for dalbavancin. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1730
73. Poliseno M, Bavaro DF, Brindicci G, et al. Dalbavancin efficacy and impact on hospital length-of-stay and treatment costs in different gram-positive bacterial infections. Clin Drug Investig. 2021;41:437–448. doi:10.1007/s40261-021-01028-3
74. Wilke M, Worf K, Preisendörfer B, Heinlein W, Kast T, Bodmann K-F. Potential savings through single-dose intravenous Dalbavancin in long-term MRSA infection treatment - a health economic analysis using German DRG data. GMS Infect Dis. 2019;7:Doc03. doi:10.3205/id000043
75. Morrisette T, Miller MA, Montague BT, Barber GR, McQueen RB, Krsak M. Long-acting lipoglycopeptides: “lineless antibiotics” for serious infections in persons who use drugs. Open Forum Infect Dis. 2019;6(7):ofz274. doi:10.1093/ofid/ofz274
76. Agarwal R, Bartsch SM, Kelly BJ, et al. Newer glycopeptide antibiotics for treatment of complicated skin and soft tissue infections: systematic review, network meta-analysis and cost analysis. Clin Microbiol Infect. 2018;24(4):361–368. doi:10.1016/j.cmi.2017.08.028
77. McCarthy MW, Keyloun KR, Gillard P, et al. Dalbavancin reduces hospital stay and improves productivity for patients with acute bacterial skin and skin structure infections: the ENHANCE trial. Infect Dis Ther. 2020;9(1):53–67. doi:10.1007/s40121-019-00275-4
78. Rappo U, Gonzalez PL, Puttagunta S, et al. Single-dose dalbavancin and patient satisfaction in an outpatient setting in the treatment of acute bacterial skin and skin structure infections. J Glob Antimicrob Resist. 2019;17:60–65. doi:10.1016/j.jgar.2019.02.007
79. Simonetti O, Rizzetto G, Molinelli E, Cirioni O, Offidani A. Review: a safety profile of dalbavancin for on- and off-label utilization. Ther Clin Risk Manag. 2021;17:223–232. doi:10.2147/TCRM.S271445
80. Dunne MW, Talbot GH, Boucher HW, Wilcox M, Puttagunta S. Safety of dalbavancin in the treatment of skin and skin structure infections: a pooled analysis of randomized, comparative studies. Drug Saf. 2016;39(2):147–157. doi:10.1007/s40264-015-0374-9
81. Wang Y, Wang J, Wang R, Li Y, Cai Y. Efficacy and safety of dalbavancin in the treatment of Gram-positive bacterial infections. J Glob Antimicrob Resist. 2021;24:72–80. doi:10.1016/j.jgar.2020.11.018
82. Bradley JS, Puttagunta S, Rubino CM, Blumer JL, Dunne M, Sullivan JE. Pharmacokinetics, safety and tolerability of single dose dalbavancin in children 12–17 years of age. Pediatr Infect Dis J. 2015;34(7):748–752. doi:10.1097/INF.0000000000000646
83. Gonzalez D, Bradley JS, Blumer J, et al. Dalbavancin pharmacokinetics and safety in children 3 months to 11 years of age. Pediatr Infect Dis J. 2017;36(7):645–653. doi:10.1097/INF.0000000000001538
84. Dunne MW, Zhou M, Darpo B. A thorough QT study with dalbavancin: a novel lipoglycopeptide antibiotic for the treatment of acute bacterial skin and skin-structure infections. Int J Antimicrob Agents. 2015;45(4):393–398. doi:10.1016/j.ijantimicag.2014.12.021
85. Mahoney MV, Padival S. Receipt of supratherapeutic dalbavancin. Am J Health Syst Pharm. 2020;77(5):326–328. doi:10.1093/ajhp/zxz337
86. Abdul-Mutakabbir JC, Kebriaei R, Stamper KC, et al. Dalbavancin, vancomycin and daptomycin alone and in combination with cefazolin against resistant phenotypes of Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antibiotics (Basel). 2020;9(10). doi:10.3390/antibiotics9100696
87. Kebriaei R, Rice SA, Stamper KC, Rybak MJ. Dalbavancin alone and in combination with ceftaroline against four different phenotypes of Staphylococcus aureus in a simulated pharmacodynamic/pharmacokinetic model. Antimicrob Agents Chemother. 2019;63(4). doi:10.1128/AAC.01743-18
88. Aktas G, Derbentli S. In vitro activity of daptomycin combined with dalbavancin and linezolid, and dalbavancin with linezolid against MRSA strains. J Antimicrob Chemother. 2017;72(2):441–443. doi:10.1093/jac/dkw416
89. Baldoni D, Furustrand Tafin U, Aeppli S, et al. Activity of dalbavancin, alone and in combination with rifampicin, against meticillin-resistant Staphylococcus aureus in a foreign-body infection model. Int J Antimicrob Agents. 2013;42(3):220–225. doi:10.1016/j.ijantimicag.2013.05.019
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.