Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Real-World Safety And Effectiveness Of OnabotulinumtoxinA Treatment Of Crow’s Feet Lines And Glabellar Lines: Results Of A Korean Postmarketing Surveillance Study
Authors Yi DJ , Hwang S, Son J, Yushmanova I , Anson Spenta K , St.Rose S
Received 16 August 2019
Accepted for publication 26 October 2019
Published 19 November 2019 Volume 2019:12 Pages 851—856
DOI https://doi.org/10.2147/CCID.S227493
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Dong-Jin Yi,1 Seongjin Hwang,2 JunHyuk Son,3 Irina Yushmanova,4 Krystal Anson Spenta,5 Suzanne St.Rose6
1BLS Clinic, Seoul, Republic of Korea; 2Fulore Clinic, Seoul, Republic of Korea; 3Department of Ophthalmology, Yeungnam University College of Medicine, Daegu, Republic of Korea; 4Global Patient Safety and Epidemiology, Allergan plc, Irvine, CA, USA; 5Global Medical Trial Management, Allergan plc, Irvine, CA, USA; 6Global Patient Safety and Epidemiology, Allergan Holdings Ltd, Marlow, Buckinghamshire, UK
Correspondence: Suzanne St.Rose
Allergan Holdings, Ltd., Marlow International, The Parkway, Marlow, Buckinghamshire SL7 1YL, UK
Tel +44 16 2849 4299
Email [email protected]
Purpose: OnabotulinumtoxinA is approved in the Republic of Korea for the treatment of moderate-to-severe crow’s feet lines (CFL) and glabellar lines (GL), separately or in combination. We assessed safety and effectiveness of onabotulinumtoxinA in real-world clinical practice.
Patient and methods: This 4-year postmarketing surveillance study was conducted in the Republic of Korea in subjects with moderate-to-severe CFL. Subjects aged 18 to 75 years received onabotulinumtoxinA injections for CFL alone or in combination with GL. Safety assessments included adverse events (AEs), serious AEs (SAEs), and unexpected AEs (not noted in Korean prescribing information). Investigators assessed effectiveness via change from baseline in CFL.
Results: The full analysis set comprised 695 subjects; 667 were in the safety set and 376 in the effectiveness set. In the safety set, mean ± SD age was 40.9±13.0 years; most subjects (87.3%) were female. More subjects were treated for CFL (69.9%) than CFL and GL simultaneously (30.1%). Eleven subjects experienced 14 AEs; 12 were mild in severity and 11 resolved without sequelae. Two cases of injection site pain in 2 subjects each were deemed possibly related to onabotulinumtoxinA. One unexpected SAE (acute renal failure) occurred in 1 subject (0.15%). All unexpected AEs (n=4) were mild and considered unrelated to treatment. Overall change from baseline showed CFL was improved in 375 subjects (99.7%) and unchanged in 1 subject (0.3%).
Conclusion: OnabotulinumtoxinA was well tolerated and effective for treatment of CFL with or without GL in a real-world Korean population. No new safety concerns were identified.
Keywords: aesthetics, Botox, botulinum toxins, type A, skin aging
Introduction
Botulinum toxin A treatment for facial lines is one of the most widely used aesthetic treatments worldwide. According to the International Society of Aesthetic Plastic Surgery (ISAPS), more than 5 million aesthetic procedures involving botulinum toxin A were performed globally in 2017 alone.1 The efficacy and safety of onabotulinumtoxinA (Botox, Allergan plc; supplied in 50 U vials) are well established, with data from clinical trials described in approximately 500 peer-reviewed journal articles spanning almost 3 decades. Pivotal trials conducted in North America and Europe2–5 and in Asia6–9 demonstrated that onabotulinumtoxinA is well tolerated and efficacious for treatment of upper facial lines. Currently, onabotulinumtoxinA is approved in the United States for the treatment of moderate-to-severe glabellar lines (GL), lateral canthal lines (crow’s feet lines [CFL]), and forehead lines.10
Based on results from the pivotal trials, onabotulinumtoxinA was approved in the Republic of Korea in 2008 for temporary improvement in the appearance of moderate-to-severe GL associated with corrugator and/or procerus muscle activity in adults aged 18 to 75 years, and in 2014 for moderate-to-severe CFL associated with orbicularis oculi activity and for moderate-to-severe CFL and GL when treated simultaneously. Per requirement of the Korean Ministry of Food and Drug Safety (MFDS),11 a prospective postmarketing surveillance (PMS) study was conducted to evaluate the safety and effectiveness of onabotulinumtoxinA for treatment of individuals with moderate-to-severe CFL, with or without simultaneous treatment of GL, through active surveillance under routine clinical practice over a 4-year period. This active surveillance study provided the first opportunity, in a large population of more than 600 Korean subjects, to identify any unexpected adverse reactions that were not observed during the clinical development programs for onabotulinumtoxinA in CFL. Additionally, any potentially rare adverse events (AEs) for which no causal relationship to onabotulinumtoxinA had been established could be assessed in a real-world setting representative of the general Korean population.
Materials And Methods
Study Design
This prospective PMS study (NCT02248844) was conducted at 26 sites in the Republic of Korea from February 4, 2014, to February 3, 2018 (Table S1). Investigators collected safety and effectiveness data at all follow-up office visits within 3 months after the index onabotulinumtoxinA treatment or before the subject received additional onabotulinumtoxinA treatment, if it occurred within the 3-month period. All safety data for this study were collected by interviewing subjects and/or reviewing medical charts during office visits, or by the investigator or a designee contacting the subject by telephone to collect follow-up data if no office visits occurred within 3 months. The effectiveness data were collected by the investigator only during office visits and were not collected via telephone interview with the subject.
Study Population
Korean subjects aged 18 to 75 years with moderate-to-severe CFL were recruited to join this PMS study until the planned number of subjects (N=600, per the Korean MFDS) were enrolled. All subjects had to be receiving treatment with onabotulinumtoxinA injections according to the approved label from a 50-U vial either for CFL alone or for CFL and GL simultaneously. All enrolled subjects from whom a case report form was retrieved comprised the full analysis set. Subjects from the full analysis set were included in the safety analysis set if they received an index onabotulinumtoxinA treatment and had safety data collected during the follow-up contact. Subjects were excluded from the effectiveness analysis set if they were missing an overall assessment of change in CFL by the investigator, had received a subsequent onabotulinumtoxinA injection before the overall assessment of change in CFL by the investigator, or had effectiveness evaluations collected beyond 3 months after the index onabotulinumtoxinA injection. Each study site obtained approval from an independent ethics committee, and all patients provided written informed consent prior to enrollment. This postmarketing surveillance study was conducted in compliance with Article 32 of the Republic of Korea’s Pharmaceutical Affairs Act and Article 23 of the Regulations on Safety of Pharmaceuticals.
Outcome Measures
Safety assessments included AEs, serious AEs (SAEs), and unexpected AEs. All AEs that occurred during the entire surveillance period were reported, regardless of their causal relationship with the study drug. All SAEs were also reported to the Korean Institute of Drug Safety and Risk Management. For this study, unexpected AEs were defined as any AE or suspected adverse reaction that was not included in the approved Korean onabotulinumtoxinA prescribing information. Investigators assessed the causal relationship between each AE and the drug itself (onabotulinumtoxinA), as well as the injection procedure, as certainly, probably/likely, possibly, or unlikely related to treatment, based on the Korean MFDS Guidelines for PMS studies.
Per MFDS PMS regulations, treatment effectiveness was assessed via the investigator’s evaluation of overall improvement in CFL and was recorded by the investigator as improved, unchanged, or worsened.11 Subjects who were assessed as having improved CFL were categorized as having effective treatment, whereas those with unchanged or worsened CFL were categorized as having ineffective treatment. Subjects who were not assessed for overall improvement were excluded from the effectiveness cohort, and the reasons for not performing the assessment were recorded by the investigator (eg, subject lost to follow-up, subject contacted via telephone).
Statistical Analysis
Data for demographic characteristics, effectiveness, and safety outcomes were summarized using descriptive statistics. All AEs were categorized according to World Health Organization Adverse Reaction Terminology. The incidence of AEs was calculated with a 95% CI estimated and analyzed using the χ2-test or Fisher’s exact test. No imputations for missing data were conducted.
Results
Study Subjects
A total of 695 subjects were included in the full analysis set, with 667 in the safety analysis set and 376 in the effectiveness analysis set (Figure 1). Demographics across the 3 analysis sets were comparable, although the mean age was slightly higher in the effectiveness analysis set (49.1 years). Subjects in the safety analysis set had a mean age of 40.9 years, the majority of subjects were female, and none of the female subjects were pregnant or became pregnant after receiving the study drug (Table 1). Treatment indications for the subjects in the safety analysis set included moderate-to-severe CFL associated with orbicularis oculi activity accounting for 69.9% (466/667) of the subjects, and moderate-to-severe CFL and GL treated simultaneously, accounting for 30.1% (201/667) of the subjects. The 466 subjects treated for CFL only received onabotulinumtoxinA in 6 prespecified injection sites (total, 24 U), whereas the 201 subjects treated for CFL and GL simultaneously received onabotulinumtoxinA in 6 + 5 sites with 24 U plus 20 U. Two subjects (0.3%) received a subsequent dose of onabotulinumtoxinA after the initial study treatment. Among the 667 subjects in the safety analysis set, 56 (8.4%) had received concomitant medications and/or other botulinum toxins besides onabotulinumtoxinA.
 |
Table 1 Demographics and Treatment History |
 |
Figure 1 Subject disposition. Abbreviation: CRF, case report form. |
Safety
A total of 14 AEs occurred in 11 of 667 (1.7%) subjects in the safety analysis set (Table 2). This is similar to the incidence in the full analysis set, in which AEs were reported in 1.6% of the subjects. The majority of the 14 AEs were assessed as mild (85.7%; 12/14); 1 event was moderate and 1 was severe (both eyelid ptosis). Most AEs (11/14) resolved without sequelae. The only AEs reported in more than 1 subject were eyelid ptosis, injection site pain, and bruising, which occurred in 2 subjects (0.3%), with 2 cases each. Only the 2 cases of injection site pain reported in 2 subjects were deemed by the investigators as possibly related to onabotulinumtoxinA. One SAE (acute renal failure) considered unlikely to be related to onabotulinumtoxinA treatment by the investigator occurred in 1 subject (0.2%); this event was considered to be attributable to symptoms worsening due to treatment for recurrent tuberculosis. Four unexpected AEs were reported in 3 (0.5%) subjects (1 case each of tuberculosis, burning sensation, nail disorder, and acute renal failure). All unexpected AEs were assessed as mild by the investigator and were considered unlikely to be related to onabotulinumtoxinA treatment.
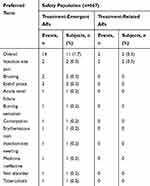 |
Table 2 Incidence of Adverse Events |
Effectiveness
Effectiveness was assessed by the investigators in 376 subjects. The overall change from baseline showed CFL was improved (effective treatment) in 99.7% (375/376) of the subjects and unchanged (ineffective treatment) in 1 subject (0.3%).
Discussion
This is the first prospective PMS study of onabotulinumtoxinA for treatment of CFL, with or without simultaneous treatment of GL, in a Korean population. Postmarketing studies are progressively becoming mandatory in many countries where regulatory agencies require additional postmarketing data to confirm the continued safe use of a medication in the treated population.12 This real-world study of 667 Korean subjects provided new information for this specific population, showing that onabotulinumtoxinA treatment of CFL with or without GL is well tolerated, with no new safety concerns. The majority of AEs were mild in severity, and the only AE that was considered to be related to treatment was mild injection site pain. A total of 4 unexpected AEs in 3 subjects were reported for onabotulinumtoxinA; none was considered to be related to onabotulinumtoxinA or to the injection procedure.
Our results are in accordance with postmarketing AE data for onabotulinumtoxinA as reported to the US Food and Drug Administration covering the first year after its initial approval for aesthetic use in treating GL.13 OnabotulinumtoxinA was not associated with any new safety concerns, and unexpected AEs each constituted less than 1% of the 995 nonserious AEs reported for aesthetic procedures.13 However, the overall incidence of AEs reported in this surveillance study was lower than that previously reported in clinical trials. In a prospective, multicenter, randomized, double-blind, active-controlled study conducted in a Korean population to treat GL of at least moderate severity at maximum frown, treatment-emergent AEs of 18.1% (24/133 subjects; 36 AEs) and treatment-related AEs of 4.5% (6/133 subjects; 8 AEs) were reported for onabotulinumtoxinA.9 Additionally, a meta-analysis of safety and tolerability data of onabotulinumtoxinA in more than 1600 participants treated for CFL and GL from high-quality, placebo-controlled studies conducted prior to 2009 reported an overall incidence of onabotulinumtoxinA-related AEs of 26.6%.14 The most common treatment-related AEs reported in the meta-analysis were headache (7.0%), eyelid sensory disorder (4.1%), eyelid ptosis (3.6%), and injection site pain (2.1%). The higher incidence of AEs in clinical trials of onabotulinumtoxinA compared with PMS studies may be attributable to differences in the monitoring of AEs within the setting of a clinical trial in which study conditions are rigorously controlled compared with postmarketing surveillance.12
The effectiveness data showed that treatment with onabotulinumtoxinA improved the appearance of CFL in more than 99% of the subjects with available data. These results are consistent with data from a 2-center, open-label, single-group, 14-day study that reported a response rate of 100% after 4 days for treatment of moderate-to-severe GL with onabotulinumtoxinA in treatment-naive subjects.15 Our findings are also comparable to the response rates of 88.6%, 94.1%, and 88.6% reported at maximum frown from clinical studies of onabotulinumtoxinA in different Asian populations.7–9 However, it should be noted that these data7–9,15 were generated from clinical trials that were conducted in standardized conditions, which differs from the current PMS study design. Consequently, discrepancies in patient selection or treatment conditions may alter the efficacy/effectiveness outcomes.
There are several limitations to this study. First, AEs were only monitored at follow-up visits for up to 3 months after the index treatment. This may have led to recall bias and to potential under-reporting of AEs. In addition, only 376 of 695 subjects were assessed for effectiveness, potentially leading to withdrawal bias. Finally, the PMS study design makes it difficult to control for all confounding factors, which may have impacted study outcomes (eg, telephone contact to collect safety data for subjects who did not have office visits within 3 months of the index treatment). Despite these caveats, our study provides valuable real-world data on onabotulinumtoxinA treatment of CFL and/or GL in a large population in Korea and has helped to provide a more accurate understanding of the safety and effectiveness of onabotulinumtoxinA in this country.
Conclusions
OnabotulinumtoxinA treatment of CFL, with or without simultaneous treatment of GL, was well tolerated and effective in the postmarketing setting in Korea. No new safety concerns were identified for onabotulinumtoxinA in this real-world setting compared with the safety profile identified in the clinical development program for CFL treatment. OnabotulinumtoxinA may be safely used in Korean subjects for the treatment of moderate-to-severe CFL with or without simultaneous treatment of GL.
Abbreviations
AE, adverse event; CFL, crow’s feet lines; CI, confidence interval; GL, glabellar lines; ISAPS, International Society of Aesthetic Plastic Surgery; MFDA, Korean Ministry of Food and Drug Safety; PMS, postmarketing surveillance; SAE, serious adverse event.
Data Sharing Statement
Data reported in this manuscript are available within the article and/or its supplementary materials. Allergan will share de-identified patient-level data and/or study-level data, including protocols and clinical study reports, for phase 2–4 trials completed after 2008 that are registered on ClinicalTrials.gov or EudraCT. The primary manuscript from the trial must be published prior to data sharing. To request access to the data, the researcher must sign a data user agreement. All shared data are to be used for noncommercial purposes only. More information can be found at www.allerganclinicaltrials.com/.
Acknowledgments
The authors thank Daniel Chung for providing useful insight that facilitated the research, Zhanying Bai for biostatistical support, and Suzanne Magante for study management. Writing and editorial assistance was provided to the authors by Peloton Advantage, LLC, an OPEN Health company, and funded by Allergan plc, Dublin, Ireland. This research was funded by Allergan plc, Dublin, Ireland. Medical writing and editorial support were provided by Peloton Advantage, LLC, an OPEN Health company, and were sponsored by Allergan plc. No honoraria or other forms of payment were made for authorship.
Author Contributions
All authors contributed to conceptualization and methodology. D-JY, SH, and JHS were study investigators. IY, KAS, and SSt.R participated in data curation and formal analysis. All authors had full access to the data, contributed to the interpretation of the data, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
IY, KAS, and SSt.R are employees of Allergan plc and may own stock/options in the company. JHS has served as a lecturer for Alcon and Novartis and as a consultant for Allergan plc. He also reports personal fees from Allergan, during the conduct of the study and outside the submitted work. D-JY reports personal fees from Allergan plc, during the conduct of the study; personal fees from Medytox, Hugel, lnc., LG Chem, and Aesthetics, outside the submitted work. SH reports personal fees from Allergan, during the conduct of the study; personal fees from Wonik, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. ISAPS international survey on aesthetic/cosmetic procedures performed in 2017. 2018. Available from: https://www.isaps.org/wp-content/uploads/2018/10/ISAPS_2017_International_Study_Cosmetic_Procedures.pdf.
2. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840–849. doi:10.1067/mjd.2002.121356
3. Moers-Carpi M, Carruthers J, Fagien S, et al. Efficacy and safety of onabotulinumtoxinA for treating crow’s feet lines alone or in combination with glabellar lines: a multicenter, randomized, controlled trial. Dermatol Surg. 2015;41(1):102–112. doi:10.1097/DSS.0000000000000220
4. Carruthers J, Rivkin A, Donofrio L, et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of repeated onabotulinumtoxinA treatments in subjects with crow’s feet lines and glabellar lines. Dermatol Surg. 2015;41(6):702–711. doi:10.1097/DSS.0000000000000357
5. Baumann L, Dayan S, Connolly S, et al. Duration of clinical efficacy of onabotulinumtoxinA in crow’s feet lines: results from two multicenter, randomized, controlled trials. Dermatol Surg. 2016;42(5):598–607. doi:10.1097/DSS.0000000000000757
6. Harii K, Kawashima M, Furuyama N, Lei X, Hopfinger R, Lee E. OnabotulinumtoxinA (Botox) in the treatment of crow’s feet lines in Japanese subjects. Aesthetic Plast Surg. 2017;41(5):1189–1197. doi:10.1007/s00266-017-0844-9
7. Harii K, Kawashima M. A double-blind, randomized, placebo-controlled, two-dose comparative study of botulinum toxin type A for treating glabellar lines in Japanese subjects. Aesthetic Plast Surg. 2008;32(5):724–730. doi:10.1007/s00266-008-9199-6
8. Wu Y, Zhao G, Li H, et al. Botulinum toxin type A for the treatment of glabellar lines in Chinese: a double-blind, randomized, placebo-controlled study. Dermatol Surg. 2010;36(1):102–108. doi:10.1111/j.1524-4725.2009.01390.x
9. Won CH, Kim HK, Kim BJ, et al. Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxinA in the treatment of glabellar lines: a multicenter, randomized, double-blind, active-controlled study. Int J Dermatol. 2015;54(2):227–234. doi:10.1111/ijd.2015.54.issue-2
10. Botox Cosmetic [package insert]. Dublin, Ireland: Allergan plc; 2017.
11. Post-marketing safety management of drugs etc—guideline on re-evaluation tasks of new drugs etc [Handbook-0019-03]. 2018. Available from: https://www.mfds.go.kr/eng/index.do.
12. Haque A, Daniel S, Maxwell T, Boerstoel M. Postmarketing surveillance studies-an industry perspective on changing global requirements and implications. Clin Ther. 2017;39(4):675–685. doi:10.1016/j.clinthera.2017.03.011
13. Cote TR, Mohan AK, Polder JA, Walton MK, Braun MM. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53(3):407–415. doi:10.1016/j.jaad.2005.06.011
14. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61(6):961–970. doi:10.1016/j.jaad.2009.06.040
15. Beer KR, Boyd C, Patel RK, Bowen B, James SP, Brin MF. Rapid onset of response and patient-reported outcomes after onabotulinumtoxinA treatment of moderate-to-severe glabellar lines. J Drugs Dermatol. 2011;10(1):39–44.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
