Back to Journals » Journal of Asthma and Allergy » Volume 15
Real-World Effectiveness of Dupilumab for Patients with Severe Asthma: A Retrospective Study
Authors Numata T , Araya J, Miyagawa H , Okuda K, Takekoshi D, Hashimoto M, Minagawa S, Ishikawa T, Hara H, Kuwano K
Received 8 January 2022
Accepted for publication 23 March 2022
Published 1 April 2022 Volume 2022:15 Pages 395—405
DOI https://doi.org/10.2147/JAA.S357548
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Amrita Dosanjh
Takanori Numata, Jun Araya, Hanae Miyagawa, Keitaro Okuda, Daisuke Takekoshi, Mitsuo Hashimoto, Shunsuke Minagawa, Takeo Ishikawa, Hiromichi Hara, Kazuyoshi Kuwano
Department of Respiratory Diseases, The Jikei University School of Medicine, Tokyo, Japan
Correspondence: Takanori Numata, Department of Respiratory Diseases, The Jikei University School of Medicine, 3-25-8 Nishi-Shimbashi, Minato-ku Tokyo, 105-8461, Japan, Tel +81-3-3433-1111 (ext. 3271), Fax +81-3-3433-1020, Email [email protected]
Background: Treatment with dupilumab, an anti-interleukin (IL)-4 receptor α monoclonal antibody that blocks both the IL-4 and IL-13 pathways, has demonstrated efficacy for the treatment of severe asthma (SA) with type 2 inflammation. However, few studies have focused on the efficacy of this biologic for the treatment of SA in a real-world setting.
Methods: From April 2019 to December 2021, 26 Japanese patients with SA received dupilumab at Jikei University Hospital. We retrospectively evaluated the number of moderate-to-severe exacerbations, pulmonary function, maintenance dose of corticosteroids, biomarkers, and adverse events.
Results: During a mean follow-up period of 12.6 months, 10 patients received dupilumab as the first biologic, and 16 switched to dupilumab from other biologics. Dupilumab treatment significantly reduced the number of annual exacerbations from 3.4 ± 4.1 to 1.6 ± 2.7 (/person-year, p < 0.01) at the last follow-up regardless of previous biologic use. The Asthma Control Test score significantly improved in all patients by six months after administration but tended to worsen by 24 months in patients with previous biologic use. On the other hand, blood eosinophil counts (BECs) transiently increased and peaked three to six months after administration. The peak timing can be affected by previous biologic use. Adverse events included wheezing immediately after injection, hypereosinophilia, mild conjunctivitis, and relapse of chronic eosinophilic pneumonia in the patient switched from benralizumab.
Conclusion: Dupilumab treatment was useful for patients with SA in a real-world setting. However, the BEC should be monitored carefully, especially in patients who previously received anti-IL-5/IL-5 receptor antibody.
Keywords: dupilumab, severe asthma, exacerbation, transient eosinophilia, real-world
Introduction
Bronchial asthma affects approximately 300 million people worldwide,1 and 3–10% of patients have severe and uncontrolled asthma.2–4 Patients with asthmatic exacerbation requiring oral corticosteroids (OCSs) or hospitalization have diminished health-related quality of life and high healthcare costs, which influences socioeconomic activity.4,5 The pathophysiology of asthma is often explained by type 2 and non-type 2 inflammation. Severe asthma is recognized as a heterogeneous syndrome and non-type 2 inflammation, mainly composed of neutrophilic inflammation, is responsible for the refractory phenotype. However, there are few applicable treatments for non-type 2 inflammation, including macrolide, bronchial thermoplasty, and weight loss.6,7 On the other hand, various biologics targeting IgE and type 2 cytokines have been developed for the type 2 endotype and are now clinically available, showing high efficacy.8 The relevant cytokines in type 2 inflammation are interleukin (IL)-5, IL-4 and IL-13; IL-5 is involved in eosinophil differentiation and proliferation,9 and IL-4 and 13 are associated with tissue migration, smooth muscle contraction, thickening of the basement membrane, remodeling, mucus production and B cell class switching.10 Recently, many biologics have become available for patients with severe asthma (SA): omalizumab (anti-IgE antibody), mepolizumab and reslizumab (anti-IL-5 antibody), benralizumab (anti-IL-5 receptor α chain antibody) and dupilumab (anti-IL-4 receptor α chain antibody). In Japan, all of these biologics except for reslizumab were available in 2021. These biologics demonstrated efficacy in preventing exacerbation, reducing the OCS maintenance dose, and improving pulmonary function.11–19 Unlike other biologics, multiple biomarkers have been shown to predict the efficacy of dupilumab in SA treatment, and the efficacy of dupilumab has also been demonstrated in the treatment of atopic dermatitis (AD)20 and chronic rhinosinusitis with nasal polyps (CRSwNPs)/eosinophilic chronic rhinosinusitis (ECRS).21
Only 3 to 10% of patients with severe and uncontrolled asthma received biologics from 2014 to 2016 in Japan.4,22 Although the frequency of biologic use is increasing based on the existence of predictive biomarkers, clinical data predicting response and long term efficacy including biologic switching for SA treatment with dupilumab remain to be established in a real world setting.23 Accordingly, we conducted this single-center, retrospective study to examine the clinical efficacy of dupilumab in terms of changes in asthmatic exacerbations and the OCS maintenance dose. We also attempted to elucidate the clinical characteristics of super responders to dupilumab treatment among Japanese patients with SA in a real-world setting.
Methods
Subjects
From April 2019 to December 2021, 26 adult patients with SA received dupilumab injection (600 mg at first, followed by 300 mg every two weeks using a prefilled syringe or autoinjector) at Jikei University Hospital, Tokyo, Japan. All patients with asthma were diagnosed by respiratory physicians based on the Japanese guidelines24 or the Global Initiative of Asthma (GINA) guidelines.6 SA was defined according to the GINA guidelines6,7 and required high doses of ICSs plus at least one of the following additional controllers: long-acting beta-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), leukotriene receptor antagonists (LTRAs), xanthine derivative, and daily OCSs. However, a moderate dose of ICS was acceptable, as in the randomized controlled trial (RCT).18 Severity at baseline and during exacerbation was defined according to the GINA guidelines.6,7 The present study was approved by the Ethical Committee of Jikei University [33-075(1687)]. Based on the ethics guidelines of Jikei University, the need to obtain informed consent was waived because of the retrospective study design, and we posted an opt-out consent statement on the website of our hospital. This study was conducted in accordance with the Declaration of Helsinki. The rules for the prescription of dupilumab were based on the guidelines of the Pharmaceuticals and Medical Devices Agency in Japan. The criteria for initiating treatment with this biologic were as follows: patients had uncontrolled asthma despite receiving standard therapy based on the guidelines, at least one exacerbation requiring OCS, or patients had received OCS maintenance therapy or another biologic therapy. This retrospective study included patients treated with mepolizumab and benralizumab who were analyzed in our previous studies.25–28
Regarding the laboratory examination, serum non-specific IgE and allergen-specific IgE were examined by fluorescence enzyme immunoassay. Pulmonary function was measured using a spirometer, SpiroshiftTM SP-790COPD (FUKUDA DENSHI, Tokyo, Japan), and the FeNO level was measured using a NIOX VEROTM device (Aerocrine AB, Stockholm, Sweden). These examinations were performed by experienced technicians.
Data Collection and Evaluation
We retrospectively examined the following characteristics: sex, age, comorbidities of eosinophilic Diseases, smoking status, body mass index (BMI), baseline treatments including biologics, and duration of asthma. We examined and evaluated the following parameters at baseline, after one and three (±1) months, and if available after 6 (± 2), 12 (± 3), 18 (± 3) and 24 (± 3) months: peripheral blood eosinophil counts (BECs) and basophil counts, serum IgE, fractional exhaled nitric oxide (FeNO), Asthma Control Test (ACT) scores, pulmonary function test results [forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FEV1/FVC, %FEV1 and % peak expiratory flow (%PEF)], and daily OCS maintenance doses as prednisone equivalents (mg). The timing of each evaluation was every one to three months based on the hospital visit schedule of the clinical practice. Clinical data were collected only during the dupilumab treatment. We extrapolated several missing data values using the last-observation-carried-forward approach. The number of annual exacerbations of asthma symptoms requiring systemic CS was defined as the total number of exacerbations x 12/the total duration of the observation period (months). To evaluate asthmatic symptoms, we utilized the ACT score; the ACT score is clinically useful as a simple scoring system, and scores of 20–25 are classified as well-controlled asthma.6 The minimal clinically important difference (MCID) was an ACT score of three points.29
The primary endpoints were the change in the number of asthma exacerbations per year and the reduction in the daily OCS dose at the last follow-up. Systemic CS doses are presented as prednisone equivalents (mg). The secondary endpoints included factors predictive of super responders and the time course of each of the following parameters or biomarkers starting from baseline: annual exacerbations, ACT score and BEC. To accurately assess the predictive biomarkers for patients with previous biologic use, we also included data obtained prior to the use of any biologics. A responder was defined by one of the following: 50% or greater decrease in exacerbations, 50% or greater decrease in the OCS maintenance dose, or an improvement in the ACT score of three or greater. A super responder satisfied two or more of the following criteria: exacerbation-free after administration (for at least six months), discontinuation of maintenance OCS, and a major improvement of asthma control with an MCID or ACT > 19.30,31 To examine sinusitis symptoms and findings, we utilized nasal discharge, nasal congestion and olfactory loss as reported in the medical records.
Statistical Analyses
All statistical analyses were performed using StatView version 5 (SAS Institute, Inc., Cary, NC, USA). All values are expressed as the means ± standard deviation (SD) or standard error (SE). A p value < 0.05 was considered statistically significant. The factors associated with patient characteristics were analyzed using the Mann–Whitney U-test, Fisher’s exact test, analysis of variance (ANOVA), or the Wilcoxon signed rank test (univariate model). Furthermore, subgroup analysis of the clinical data was performed based on several biomarker cutoffs as follows: BEC ≥ 150 or not, BEC ≥ 300 or not, FeNO ≥ 25 or not, and IgE ≥ 167 or not at baseline before dupilumab treatment. Logistic regression analysis was performed to evaluate factors predictive of super responders (in a multivariate model), including variables that achieved a value of p < 0.20 in the univariate models. To ensure the accuracy of the assessments, multivariate logistic analysis was performed separately for each biomarker prior to the use of any biologics and prior to dupilumab administration.
Results
Patient Characteristics and the Changes in Parameters
The baseline characteristics of 26 asthmatic patients who received dupilumab treatment are shown in Table 1. There were 16 patients who previously received other biologics: 10 patients received mepolizumab, seven patients received omalizumab, and five patients received benralizumab. The reasons for biologic switching were as follows: no improvement in asthmatic symptoms (n = 9), no improvement in CRSwNP/ECRS or eosinophilic otitis media (EOM) (n = 3), no improvement in asthma and CRS/EOM (n = 2), and others (n = 2). Comorbidities included CRSwNP/ECRS (n = 17), aspirin-exacerbated respiratory disease (AERD) (n = 10), AD (n = 6) and EOM (n = 5). No patients suffered from coronavirus infectious disease-2019 (COVID-19) during the observational period in the present study. Among nine patients (35%) who received maintenance OCS doses (mean 11.6 mg/day, equivalent prednisone), five received the doses solely for SA, and four received them for both SA and comorbid eosinophilic diseases. The clinical parameters of all patients at baseline and after six months, except for those of the early discontinuation case, are shown in Table 2. Regarding three biomarkers of type 2 inflammation (BEC, serum IgE and FeNO), the number of patients with BEC ≥ 150 was 16 (62%), that with IgE ≥ 167 was 13 (50%) and that with FeNO ≥ 25 was 16 (62%). Four patients were not positive for any of the biomarkers and 6 were positive for one of the biomarkers. Sixteen (62%) patients were positive for multiple biomarkers, including 10 cases of double positive and 6 cases of triple positive. The BEC, basophil count, serum IgE level and FeNO significantly changed after treatment. Although the ACT score significantly increased in all patient groups and each subgroup, no clear improvement in pulmonary function tests was demonstrated, but significance was detected in the FEV1/FVC in the previous biologics (-) group.
 |
Table 1 Patient Characteristics at Baseline (n = 26) |
 |
Table 2 Change from Baseline to Last Follow-Up in Asthma Patients Who Received Dupilumab Treatment |
Efficacy of Dupilumab Treatment (Primary Outcomes)
We compared each parameter before and after administration of dupilumab treatment in all patients in the previous biologics (-) and (+) groups, as shown in Table 2. After dupilumab administration, the number of annual exacerbations significantly decreased from 3.4 ± 4.1 to 1.6 ± 2.7 (/person-year) (−53%, p < 0.01) by the last follow-up period (Table 2). The subgroup analysis of the annual exacerbation rate based on the levels of type 2 biomarkers is shown in Figure 1. Regardless of prior biologic use, the annual exacerbation rate significantly decreased in the subgroups with BEC ≥ 150 or 300, FeNO ≥ 25 and IgE ≥ 167. Furthermore, in nine patients who received OCS maintenance, the mean dose of OCS significantly decreased from 11.6 to 5.3 (mg/day) (−54%, p < 0.05) (Table 2). To evaluate the effect of the COVID-19 pandemic on exacerbation, we compared the number of annual exacerbations prior to dupilumab administration among the 2019, 2020, and 2021 groups. The number of annual exacerbations in the 2019, 2020, and 2021 groups prior to administration was 7.3, 2.8, and 1.3/person-year, respectively (p = 0.015 by ANOVA and p = 0.0059 between 2019 and 2021 by Bonferroni correction). On the other hand, no significant difference in the reduction rate of annual exacerbation was observed among the 2019, 2020, and 2021 groups (−58.4%, −42.2%, and −50.0%, respectively, p = 0.92, ANOVA).
Among the 20 patients who experienced exacerbations in the previous year, 13 patients (65%) experienced a reduction in annual exacerbation rate < −50% (Table 3). The efficacy based on a change in ACT score ≥ 3 before and after treatment was 62% (n = 16), and the other four patients obtained the maximum score (ACT = 25) (Table 3). Furthermore, five patients (56%) had a maintenance OCS dose reduction ≤ −50% (data not shown). The efficacy of rhinosinusitis symptoms was approximately 93% in patients who had ECRS.
 |
Table 3 Clinical Efficacy Based on Each Evaluation |
Factors Predictive of Super Responders
Among the 26 assessed patients, 9 (35%) were identified as super responders. The factors predictive of super responders were analyzed by using a multivariate logistic regression method (Table 4). Based on the patient characteristics and each biomarker prior to the use of any biologics, we identified BEC ≥ 300 (/µL) prior to the use of any biologics as significantly associated with super responders [odds ratio (OR) = 10.4, 95% confidence interval (CI) (1.02–106), p = 0.048]. On the other hand, when evaluated by biomarkers prior to dupilumab administration, we found that BEC ≥ 150 (/µL) was significantly associated with super responders, regardless of previous biologic treatment [OR = 9.8, 95% CI (1.2–78.7), p = 0.031].
 |
Table 4 Factors Predicting Super Responders According to the Logistic Regression Analysis |
Time Course of the Clinical Parameters
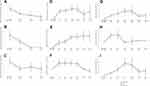
The time course changes in the number of annual exacerbations and the ACT score from baseline are shown in Figure 2A–F. These two parameters significantly improved, and the efficacy was maintained for two years, especially in patients without use of previous biologics (Figure 2A–F). Although the number of annual exacerbations decreased, the ACT score tended to worsen at 18 and 24 months in patients with previous biologic use, which can be attributed to the small number of patients (n = 5 at 18 months and n = 2 at 24 months). The time course changes in BEC after dupilumab administration are shown in Figure 2G–I. In the patients without previous biologic treatment, the BEC peaked at three or four months. On the other hand, in the group with previous biologic treatment mainly composed of anti-IL-5/IL-5R antibody (anti-IL-5/IL-5R antibody: n = 11, anti-IgE antibody: n = 5), the BEC peaked at six to 12 months, and the counts were higher than those in the previous biologics (-) group (Figure 2I and Supplementary Figure 1). In addition, the time course of FeNO was also examined as shown in Supplementary Figure 2, indicating that FeNO was not strongly linked with BEC during dupilumab treatment.
Adverse Events and Discontinuation Cases
There were four patients with mild conjunctivitis, one with hypereosinophilia (patient who switched from mepolizumab), one with moderate wheezing immediately after injection (requiring additional bronchodilator), and one with relapse of chronic eosinophilic pneumonia (CEP) requiring hospitalization (patient who switched from benralizumab). For the following reasons, eight patients discontinued dupilumab use during the observation period: asthma exacerbations (n = 5), CEP (n = 1), hypereosinophilia (n = 1) and wheezing due to an immediate allergic reaction (n = 1).
Discussion
This retrospective, real-world study of dupilumab treatment for SA showed that the clinical efficacy of dupilumab was comparable to that observed in major RCTs18,19 and a subgroup analysis of Japanese patients.32 The rates of annual exacerbation reductions in the present study, a major RCT and a Japanese subgroup analysis were −53%, −46%18 and −75%,32 respectively, and the rates of maintenance OCS dose reduction were −54%, −70%19 and not available, respectively. In line with major RCTs, in our study, the reduction in annual exacerbations significantly decreased in the subgroups with BEC ≥ 150 or 300, FeNO ≥ 25 and serum IgE ≥ 167 (Figure 1). Because of the real-world nature of this study, compared with major RCTs, there are several important differences. First, in terms of the inclusion criteria, a history of biologics use was not permitted in the previous RCTs. Our study included patients with or without previous biologic treatment and demonstrated a significant reduction in exacerbations and maintenance OCS doses in both all patients and biologic-switched patients, and a tendency toward a reduction was detected in biologic-naïve patients, suggesting the potential efficacy of switching biologics especially in patients who have persistent asthmatic symptoms in the clinical setting. Second, with respect to patient characteristics, the pulmonary function at baseline of our patients was better than those in a previous RCT18 and several real-world studies:23,33 91.1 (%) vs 58.4–73.5 (%) in mean %FEV1, and 2303 (mL) vs 1780–2130 (mL) in mean FEV1, respectively. We observed no remarkable improvement of pulmonary function after dupilumab administration, which can be attributed to the better baseline pulmonary function in this study. Additionally, it is plausible that the significant improvement in FEV1/FVC in the previous biologics (-) group was affected by the slight decrease in FVC. Third, we firstly demonstrated the predictive factors for super responders, including BEC ≥ 300 prior to the use of any biologics and BEC ≥ 150 in patients who switched to dupilumab from other biologics. Fourth, compared to biologic-naïve patients, the trajectory of eosinophilia after dupilumab administration was different in the biologic-switched patients. It is plausible that potential adverse events associated with eosinophilia can occur at a later phase in biologic-switched patients.
Among BEC, serum IgE, and FeNO, only BEC is reported as a predictive factor for exacerbation.34,35 Therefore, it is not surprising that BEC is also a predictor of responders. To increase the reliability of the multivariate logistic analysis of the predictive factors for response to dupilumab, we selected variables from previously reported RCTs for the univariate model and variables that achieved a value of p < 0.20 in the univariate model were further evaluated. Transient eosinophilia is a characteristic finding after dupilumab administration. IL-4/13 plays an important role in the tissue migration of eosinophils by regulating vascular cell adhesion molecule 1 (VCAM1) expression in endothelial cells.36 Thus, dupilumab-mediated inhibition of IL-4/13 may elevate eosinophil levels, resulting in reduced tissue migration but not egress from bone marrow, which is regulated by IL-5. Most eosinophilia cases are asymptomatic, but several cases that develop eosinophilic pneumonia or eosinophilic granulomatous polyangiitis have been reported, especially in patients who switch from anti-IL-5/IL-5R antibody treatment.18,37 We experienced one case of relapse of chronic eosinophilic pneumonia (CEP) after switching from benralizumab. In previous studies, the peak BEC elevation was reported at approximately 3 months after dupilumab initiation in biologic-naïve patients and decreased to baseline at 12 months.18,38 Intriguingly, the eosinophil peak was observed at six to 12 months in biologic-switched patients, who mainly switched from anti-IL-5/IL-5R antibody treatment in the present study. One possible explanation is the gradual disappearance of the anti-IL-5/IL-5R antibody effect. According to previous reports, mepolizumab is generally considered to be effective for approximately three to four months,39,40 while benralizumab is effective for approximately six months.41 Another explanation is that since dupilumab reduces tissue migration of eosinophils by inhibiting the IL-4/13 pathway and does not directly regulate IL-5 signaling, it may take for longer to obtain an BEC reduction in IL-5-dependent asthma patients with higher basal eosinophil counts. Accordingly, compared to biologic-naïve patients, we propose that long-term careful observation for one year be necessary when patients shift biologics from anti-IL-5/IL-5R antibody to dupilumab.
Clinical examinations such as pulmonary function tests including the FeNO test have been limited by the COVID-19 pandemic to prevent aerosol transmission. Moreover, this pandemic may also have influenced the frequency of asthma exacerbations, a primary outcome of our study. In line with previous reports, we observed significantly decreased numbers of annual exacerbations prior to dupilumab administration in 2021, which can be attributed to reduced viral infections through infection prevention measures such as universal masking.42,43 However, no significant difference in the reduction rate of annual exacerbations was demonstrated during the observational period, suggesting the constant efficacy of dupilumab during COVID-19 pandemic.
There are several limitations in the present study. First, this is a single-center, retrospective study of a small number of patients. Approximately 600–800 asthma cases are seen at our hospital as outpatients and 80–90 cases are treated with any biologic, suggesting that the majority of SA cases are treated with biologics. Because four biologics are available, we speculate that number of patients treated with dupilumab in the present study reasonably reflects the real-word setting in Japan. Furthermore, since dupilumab was administrated based on the use of biomarkers and comorbidities as predictive factors, selection bias cannot be excluded in this study. However, there is little evidence of the efficacy of dupilumab for SA in the real world, and evidence should be accumulated with more large-scale studies in the future. Second, although we observed a delayed peak in eosinophils in biologic-switched patients compared to biologic-naïve patients, the exact mechanisms for this difference remain uncertain and should be elucidated by examining changes in type 2 cytokine levels in future studies. However, we believe that the efficacy of switching biologics and the presence of long-term eosinophilia after switching from anti-IL-5/IL-5R antibody are useful to know when prescribing and monitoring dupilumab administration in the real world.
In conclusion, dupilumab treatment for patients with SA showed effectiveness in terms of reducing exacerbations and OCS maintenance doses and improving symptoms of asthma and comorbidities. Baseline BEC (≥150 before dupilumab administration, or ≥300 prior to the use of any biologics) is predictive of super responders to dupilumab treatment. Although switching from other biologics can be effective, it is necessary to check for eosinophilia for a long time, especially in the setting of switching biologics.
Abbreviations
ACT, Asthma Control Test; BEC, blood eosinophil count; CRSwNPs, chronic rhinosinusitis with nasal polyps; ECRS, eosinophilic chronic rhinosinusitis; FeNO, fractional exhaled nitric oxide; ICS, inhaled corticosteroid; IL, interleukin; LABA, long-acting β-2 agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; MCID, minimal clinically important difference; OCS, oral corticosteroids; SA, severe asthma.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
Due to the retrospective nature of this study, we provided an opt-out consent on the website of our hospital. This study was approved by the Ethical Committee of the Jikei University School of Medicine [33-075(10687)] on Oct 1, 2021. The director/administer of Jikei University Hospital granted us permission to access the medical records. The data used in this study were anonymized before use.
Author Contributions
All authors made significant contributions to the work reported, whether to the study conception, design, and execution; data acquisition, analysis, and interpretation; or all these areas. All authors contributed to drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. World Health Organization. Global surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach; World Health Organization. 2007:1–146.
2. Nagase H. Severe asthma in Japan. Allergol Int. 2019;68(2):167–171. doi:10.1016/j.alit.2019.02.004
3. Hekking PPW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042
4. Nagase H, Adachi M, Matsunaga K, et al. Prevalence, disease burden, and treatment reality of patients with severe, uncontrolled asthma in Japan. Allergol Int. 2020;69(1):53–60. doi:10.1016/j.alit.2019.06.003
5. Gon Y, Ohyanagi N, Kobayashi A. The association between control level and self-reported treatment adherence across different treatment types in Japanese asthma patients. Respir Investig. 2021;59(4):454–463. doi:10.1016/j.resinv.2021.02.003
6. Global Initiative for Asthma. Global Strategy Asthma Management and Prevention, 2019. Available from: www.ginasthma.org. Accessed Oct 21, 2019.
7. Global Initiative for Asthma. GINA Difficult-To-Treat & Severe Asthma in adolescent and adult patients Diagnosis and Management, 2019. Available from: www.ginasthma.org. Accessed April 18, 2019.
8. Brusselle GG, Koppelman GH. Biologic therapies for severe asthma. N Engl J Med. 2022;386(2):157–171. doi:10.1056/nejmra2032506
9. Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119(6):1303–1310. doi:10.1016/j.jaci.2007.03.048
10. Israel E, Reddel H, Drazen JM. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377(10):965–976. doi:10.1056/NEJMra1608969
11. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316. doi:10.1111/j.1398-9995.2004.00772.x
12. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi:10.1056/NEJMoa1403290
13. Bel EH, Ortega HG, Pavord ID. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291
14. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, Phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. doi:10.1016/S2213-2600(15)00042-9
15. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi:10.1016/S0140-6736(16)31324-1
16. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi:10.1016/S0140-6736(16)31322-8
17. Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi:10.1056/NEJMoa1703501
18. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi:10.1056/NEJMoa1804092
19. Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi:10.1056/NEJMoa1804093
20. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi:10.1056/nejmoa1610020
21. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi:10.1016/S0140-6736(19)31881-1
22. Adachi M, Hozawa S, Nishikawa M, Yoshida A, Jinnai T, Tamura G. Asthma control and quality of life in a real-life setting: a cross-sectional study of adult asthma patients in Japan (ACQUIRE-2). J Asthma. 2019;56(9):1016–1025. doi:10.1080/02770903.2018.1514628
23. Campisi R, Crimi C, Nolasco S, et al. Real-world experience with dupilumab in severe asthma: one-year data from an Italian named patient program. J Asthma Allergy. 2021;14:575–583. doi:10.2147/JAA.S312123
24. Ichinose M, Sugiura H, Nagase H, et al. Japanese guidelines for adult asthma 2017. Allergol Int. 2017;66(2):163–189. doi:10.1016/j.alit.2016.12.005
25. Numata T, Nakayama K, Utsumi H, et al. Efficacy of mepolizumab for patients with severe asthma and eosinophilic chronic rhinosinusitis. BMC Pulm Med. 2019;19(1):176. doi:10.1186/s12890-019-0952-1
26. Numata T, Miyagawa H, Kawamoto H, et al. Predictors of the enhanced response to mepolizumab treatment for severe eosinophilic asthma: a retrospective, long-term study. Cogent Med. 2020;7(1). doi:10.1080/2331205X.2020.1776468
27. Numata T, Miyagawa H, Nishioka S, et al. Efficacy of benralizumab for patients with severe eosinophilic asthma: a retrospective, real-life study. BMC Pulm Med. 2020;20(1):207. doi:10.1186/s12890-020-01248-x
28. Numata T, Araya J, Miyagawa H, et al. Effectiveness of switching biologics for severe asthma patients in Japan: a single-center retrospective study. J Asthma Allergy. 2021;14:609–618. doi:10.2147/JAA.S311975
29. Schatz M, Kosinski M, Yarlas AS, et al. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4):719–723. doi:10.1016/j.jaci.2009.06.053
30. Kavanagh JE, AP Hearn, Elstad M, et al. Real-world effectiveness and the characteristics of a “Super-Responder” to mepolizumab in severe eosinophilic asthma. Chest. 2020;158(2):491–500. doi:10.1016/j.chest.2020.03.042
31. Upham JW, Le Lievre C, Jackson DJ, et al. Defining a severe asthma super-responder: findings from a delphi process. J Allergy Clin Immunol Pract. 2021;9(11):3997–4004. doi:10.1016/j.jaip.2021.06.041
32. Tohda Y, Nakamura Y, Fujisawa T, et al. Dupilumab efficacy and safety in Japanese patients with uncontrolled, moderate-to-severe asthma in the phase 3 LIBERTY ASTHMA QUEST study. Allergol Int. 2020;69(4):578–587. doi:10.1016/j.alit.2020.04.002
33. Dupin C, Belhadi D, Guilleminault L, et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin Exp Allergy. 2020;50(7):789–798. doi:10.1111/cea.13614
34. Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–313. doi:10.1164/rccm.201602-0419OC
35. Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3(11):849–858. doi:10.1016/S2213-2600(15)00367-7
36. Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov. 2013;12(2):117–129. doi:10.1038/nrd3838
37. Eger K, Pet L, Weersink EJM, Bel EH. Complications of switching from anti-IL-5 or anti-IL-5R to dupilumab in corticosteroid-dependent severe asthma. J Allergy Clin Immunol Pract. 2021;9(7):2913–2915. doi:10.1016/j.jaip.2021.02.042
38. Wechsler ME, Ford LB, Maspero JF, et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med. 2021;2600(21):1–15. doi:10.1016/s2213-2600(21)00322-2
39. Ortega H, Lemiere C, Llanos JP, et al. Outcomes following mepolizumab treatment discontinuation: real-world experience from an open-label trial. Allergy Asthma Clin Immunol. 2019;15(1):4–7. doi:10.1186/s13223-019-0348-z
40. Moore WC, Kornmann O, Humbert M, et al. Stopping versus continuing long-term mepolizumab treatment in severe eosinophilic asthma (COMET study). Eur Respir J. 2021:2100396. doi:10.1183/13993003.00396-2021
41. Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132(5):1086–1096.e5. doi:10.1016/j.jaci.2013.05.020
42. Klompas M, Morris CA, Sinclair J, Pearson M, Shenoy ES. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020;382(21):e63. doi:10.1056/NEJMp2006372
43. Abe K, Miyawaki A, Nakamura M, Ninomiya H, Kobayashi Y. Trends in hospitalizations for asthma during the COVID-19 outbreak in Japan. J Allergy Clin Immunol Pract. 2021;9(1):494–496.e1. doi:10.1016/j.jaip.2020.09.060
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.