Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Real-World Effectiveness and Safety of Antipsychotics in Individuals at Clinical High-Risk for Psychosis: Study Protocol for a Prospective Observational Study (ShangHai at Risk for Psychosis-Phase 2)
Authors Wu G, Gan R, Li Z, Xu L, Tang X, Wei Y, Hu Y, Cui H, Li H, Tang Y, Hui L, Liu X, Li C, Wang J, Zhang T
Received 13 September 2019
Accepted for publication 16 December 2019
Published 24 December 2019 Volume 2019:15 Pages 3541—3548
DOI https://doi.org/10.2147/NDT.S230904
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
GuiSen Wu,1 RanPiao Gan,1 ZhiXing Li,1 LiHua Xu,1 XiaoChen Tang,1 YanYan Wei,1 YeGang Hu,1 HuiRu Cui,1 HuiJun Li,2 YingYing Tang,1 Li Hui,3 XiaoHua Liu,1 ChunBo Li,1 JiJun Wang,1,4,5 TianHong Zhang1
1Shanghai Mental Health Center, Shanghai Jiaotong University School of Medicine, Shanghai Key Laboratory of Psychotic Disorders, Shanghai 200030, People’s Republic of China; 2Florida a & M University, Department of Psychology, Tallahassee, FL 32307, USA; 3Institute of Mental Health, The Affiliated Guangji Hospital of Soochow University, Soochow University, Suzhou 215137, Jiangsu, People’s Republic of China; 4Bio-X Institutes, Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders (Ministry of Education), Shanghai, People’s Republic of China; 5Brain Science and Technology Research Center, Shanghai Jiao Tong University, Shanghai, People’s Republic of China
Correspondence: TianHong Zhang; JiJun Wang
Shanghai Key Laboratory of Psychotic Disorders (No.13dz2260500), Bio-X Institutes, Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders (Ministry of Education), Shanghai Mental Health Center, Shanghai Jiaotong University School of Medicine, 600 Wanping Nan Road, Shanghai 200030, People’s Republic of China
Tel +86-21-34773065
Fax +86-21-64387986
Email [email protected]
Background: The clinical high-risk (CHR) state is identified as a critical period for early prevention and intervention during the development of psychosis and early treatment may reduce the risk of conversion to psychosis. However, it remains controversial whether antipsychotics are effective in CHR populations. Limited previous randomised controlled trials of antipsychotic treatment of CHR individuals indicated possible short-term efficacy on psychotic symptoms with unclear long-term effects. To answer this question, it is necessary to establish a high-quality real-world cohort study with large sample size to explore the effectiveness and safety of antipsychotics in CHR individuals.
Methods: We plan to consecutively recruit 600 CHR individuals from Shanghai Mental Health Centre in the ongoing SHARP-2 (ShangHai At Risk for Psychosis-Phase 2) project between 2019 and 2022. At baseline, participants will be assessed by the Structured Interview for Prodromal Syndromes, the MATRICS Consensus Cognitive Battery, demographic information, and clinical medication history. They will be followed up in a naturalistic way in which the research team will not prescribe antipsychotics or provide pharmacological consultation. First, CHR participants and their families will be trained to record their medication daily and self-evaluate symptoms through smart-phone application-based assessment and report their information weekly. Second, telephone calls will be arranged monthly so that the researchers are informed about the participants’ symptoms, medications and daily functions. Third, face-to-face interviews will be conducted annually for repeating assessment of baseline. The primary outcomes will include conversion to psychosis and functional outcome (scored with less than 60 in the Global Assessment of Function) at the end of the follow-up period.
Conclusion: The current study will improve our knowledge on the effectiveness and safety of the use of antipsychotics at the prodromal phase, and will eventually facilitate optimisation of individualised interventions for psychosis prevention and treatment.
Keywords: clinical high-risk, early interventions, antipsychotics, subgroup
Introduction
The concept and criteria of clinical high-risk (CHR) for psychosis have long been a global consensus and the clinical features of CHR individuals are clear. Overall, the CHR subjects have attenuated positive psychotic symptoms such as hallucinations or delusions, but to a smaller degree and in a short course. Three critical features make CHR state the best time for early identification. Positive symptoms, compared with negative and non-specific symptoms, are easier and more operable to recognise. Good insight discriminates CHR from the first-episode of psychosis and urges CHR subjects to seek help from clinicians. Additionally, a short course and acute paroxysm are evident.
Not only is the CHR state identifiable, but the probability that the CHR group will develop a psychotic disorder in the next 1–3 years is considerably higher than that of the general population and genetic risk group. Approximately one-third of the CHR individuals will present conversion to psychosis within the following 2–3 years.1,2 Furthermore, the CHR state is the best time for intervention. Individuals in the CHR phase have insight of their psychotic symptoms, which indicates that they are active in seeking treatment, the most effective prerequisite for early intervention. In previous studies on the effectiveness of antipsychotics, poor compliance was a major contributor to the low efficacy.3 Hence, appropriate guidance for the CHR populations may result in sufficient compliance with preventive interventions, and in turn, improve the effectiveness of early interventions. Besides, the earlier the intervention for psychotic disorders is implemented, the better the prognosis, especially functional prognosis, will be. This thesis has been repeatedly verified in previous studies on untreated mental disorders.4 In our previous reports, we demonstrated that the shorter the untreated period of prodromal symptoms, the better the recovery of general functions was.5,6 In addition, the plasticity of cognitive function and brain function in pre-morbid phase CHR individuals is better than in post-morbid phase patients with the first episode of psychosis. This indicates that, although early cognitive impairment7,8 or neurobiological changes9,10 already exist, they are reversible. If effective intervention is implemented at this stage, the functions can be significantly improved.11
Effective interventions in the CHR populations have not been well documented yet, and “monitoring” is often the only recommendation for this population in clinical guidelines globally. In 2000, Amminger et al12 conducted a trial of a small CHR cohort and reported that unsaturated fatty acids had preventive effects, but the result was overthrown by McGorry et al13 in 2016 that used data from a larger CHR cohort. Since the progression from CHR to the first episode of psychosis usually takes months or years, the establishment of a long-term high-quality large cohort study of CHR populations is necessary.
A crucial question remains open because of controversy: are antipsychotics effective in the CHR population? Since CHR is a pre-morbid state, few clinical trials on the efficacy and safety of antipsychotics have been carried out. McGorry et al14 conducted a randomised controlled trial in 2002. They randomly divided 59 CHR cases into two groups to compare the efficacy of needs-based intervention and specific intervention comprising low-dose risperidone and cognitive behaviour therapy. It turned out that 10 out of 28 subjects presented with conversion to psychosis in the demand-based therapy group, while 6 out of 31 presented in the specific therapy group. Yet, there were no significant differences between the two groups. Although the use of antipsychotics seemed to delay the onset of psychosis, it was difficult to demonstrate the effectiveness of drugs in studies of small samples with low conversion rates. Moreover, based on real-world data, only approximately 20% of the CHR population in Western countries take antipsychotics, whereas non-pharmaceutical treatment is highly implemented in these countries, which may not adequately answer the question on drug effectiveness. Thus, the ShangHai At Risk for Psychosis (SHARP) cohort may be the best choice to answer this question at present. The SHARP program launched in 2010, and the first phase (2010–2018) of SHARP included 717 CHR individuals in whom antipsychotics seemed to most frequently applied by clinicians (74.7% of 600 CHR individuals with at least 1 year of follow-up in SHARP-1 took antipsychotics for at least 2 weeks).
In brief, there are uncertainties on the effectiveness, safety, clinical course, management and outcomes of administration of antipsychotics in CHR individuals; thus, this large prospective cohort study is designed to investigate into these areas in a real-world setting in the largest mental health-services centre in China. Our previous experience on recruitment and follow-up in SHARP-1 guarantees the feasibility of the project. SHARP-2 will explore predictors for estimating the efficacy of antipsychotics and identifying subgroups of CHR individuals that may benefit from specific medications. A personal calculator for the effectiveness of medication will be developed and may assist the clinicians in decision-making on whether and how to use antipsychotics.
Objectives
- To demonstrate the effectiveness and safety of antipsychotics in the prevention of psychosis onset by analysing antipsychotics use records and the clinical and functional outcomes during the follow-up.
- To analyse and screen out the CHR subgroup in which antipsychotics are effective, and construct an individualised prediction model of the effectiveness and safety of antipsychotics in CHR populations.
Methods
Study Design
This will be a real-world, single-centre, non-randomised, prospective observational cohort study conducted in the Shanghai Mental Health Centre (SMHC). We will recruit 600 participants at CHR from 2019 to 2022. The participants will be subjected to baseline investigation and will receive meticulous follow-up (see Figure 1 for the flowchart of the study design). The Research Ethics Committees at the SMHC have approved this study. All participants will provide written informed consent at the recruitment stage of the study. Subjects younger than 18-year-old will have their consent forms signed by their parents and the adolescents will give their assent.
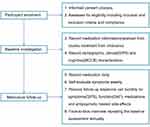 |
Figure 1 Trial flow chart. Abbreviations: GAF, Global Assessment of Function score; MCCB, MATRICS Consensus Cognitive Battery; SIPS, Structured Interview for Prodromal Syndromes. |
Participants
In the SHARP-2 project, help-seeking first-visit participants will be consecutively recruited from the Shanghai Psychotherapy and Psychological Counselling Centre (SPPCC) at the SMHC. They will be screened for eligibility by their clinicians.
The inclusion criteria are listed below. The participants should:
- Be aged 14 to 45-year-old;
- Have had at least 6 years of primary education;
- Be drug-naïve;
- Be understanding the survey, be willing to enrol in the study and sign the informed consent.
- Through the Structured Interview for Prodromal Syndromes/Scale of Prodromal Symptoms (SIPS/SOPS), the participants should meet the Criteria of Prodromal Syndrome. Participants should fulfil at least one of the prodromal syndrome criteria: (1) brief intermittent psychotic syndrome, (2) attenuated positive symptom syndrome, or (3) genetic risk and deterioration syndrome.
The exclusion criteria will be the following:
- Through the Mini-International Neuropsychiatric Interview (MINI), Axis I mental disorders such as schizophrenia, affective disorders, and anxiety spectrum disorders will be excluded.
- Acute or chronic renal failure; liver cirrhosis or active liver diseases.
- Abnormal laboratory tests results judged by the researchers to be clinically significant and considered to affect the efficacy of the test drugs or the safety of the subjects.
- Severe or unstable physical diseases, including: neurological disorders (delirium, dementia, stroke, epilepsy, etc.), congestive heart failure, angina pectoris, myocardial infarction, arrhythmia, hypertension (including untreated or uncontrolled hypertension), immune compromise, and blood glucose above 12 mmol/L.
- Drug (such as methamphetamine) abuse or dependence.
- Pregnant or lactating women, or women in childbearing age who are positive in urine human chorionic gonadotropin test, or men and women who do not take effective contraceptive measures or plan for pregnancy within 3 months after the initiation of the trial.
- Stroke within the last month.
- Participating in any clinical trial within 30 days before the baseline.
- Other situations judged by the investigators not to be suitable for the clinical trial.
Sample Size Estimation
Based on our experience from the sampling process in the SHARP-1 project, we will recruit 600 participants at CHR. Considering a dropout rate of 20%, 510 cases of CHR will be followed up, of which 75% will be treated with antipsychotics, resulting in 383 cases in the antipsychotics group and 127 cases in the control group without antipsychotics. We can conduct stratified analysis on the antipsychotics group. Considering a significance level of 0.05, an 85% statistical power, according to the sample size calculation formula in superiority clinical trials of new drugs:
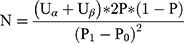
(Uα = 1.65, Uβ = 1.28, P1 = 0.5, P0 = 0.25, P = 0.375)
the sample size of 600 cases is adequate for the demonstration of the effectiveness and safety of antipsychotics in CHR subjects.
Procedure
We plan to enrol 600 CHR cases in total, 150 per year. Initial recruitment and screening will be performed according to the methods applied in the SHARP-1 project, as detailed in our previous reports.15–18 Two senior nurses that will conduct the initial screenings were employed to collect all diagnostic and medication information from medical records on every follow-up visit. The four psychiatrists are qualified and well-trained and will conduct the SIPS/SOPS interview at baseline and follow-up. The internal rate of return for the SIPS/SOPS positive symptoms ranged from 0.86 (P5) to 0.98 (P4) among the four raters in SHARP-1. Participants who meet the inclusion criteria will be consecutively recruited to participate in the study. They will be informed that this is not a treatment study and it involves naturalistic follow-up without any extra intervention or financial remuneration. They will otherwise follow the routine clinical treatment procedure at the SPPCC. After consent and initial assessment, all the participants will be informed that the plan involves at least 2 years of follow-up. The participants and their families will be trained to record their medication daily and self-evaluate symptoms weekly in a smart-phone application. Follow-up telephone calls will be arranged monthly so that the researchers are informed about the participants’ symptoms, functions, medications, and antipsychotic-related side effects. Face-to-face interviews will be conducted annually for repeating the assessment of baseline. The assessment will follow the schedule shown in Table 1.
 |
Table 1 Investigation and Assessment |
We will systematically record medication information including the types of antipsychotics, drug response, reduction/withdrawal time, side effects (including heart rate, blood pressure, body weight, body mass index, female menstrual state, etc.), and drug combination. A model will be established to correlate antipsychotics with clinical and functional outcomes from the SHARP-2 CHR cohort and demonstrate whether antipsychotics are useful and safe for preventing CHR individuals from converting to psychosis.
Assessment
Assessment of Clinical Symptoms and Functions
The assessment of changes in mental symptoms will mainly be based on the Chinese version18 of SIPS.19,20 The SHARP project team introduced SIPS, revised it into the Chinese version, and took the lead in the training of its clinical use in China. Further, our team has developed a symptom self-report online questionnaire based on SIPS/SOPS, which has a good consistency with SIPS interview. MINI will be applied for screening out other psychiatric disorders. Depressive symptoms will be assessed using the Hamilton Depression Scale-17 and anxious symptoms with the Hamilton Anxiety Scale.
The functions of the CHR individuals will be assessed with the Social Function Scale, Role Function Scale, and the Global Assessment of Function (GAF).
We will use the Clinical General Impression to evaluate the treatment during the follow-up and NIMH-developed Treatment Emergent Symptom Scale to assess the side effects of antipsychotics. Metabolic syndrome-related indicators such as body weight, and cardiovascular system-related indicators such as heart rate and blood pressure will be recorded in detail at the baseline and follow-up.
Assessment of Cognitive Function
For the assessment of general cognitive function, we will use Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) and part of the Wechsler Adult Intelligence Scale – Revised China (WAIS-RC). We will select the vocabulary and graphic patchwork tests in WAIS to evaluate the IQ required for the enrolment. The Chinese version of the MCCB21 will be administered with the standardised guidelines provided in the test manual to assess cognition. Consistent with the original version of the MCCB,22,23 the Chinese version covers the following seven domains and nine subtests: 1) speed of processing (Part A of Trail Making Test, Symbol Coding Test and Category Fluency Test), 2) attention/vigilance (Continuous Performance Test-Identical Pairs), 3) working memory (the spatial span of the Wechsler Memory Scale-III), 4) verbal learning (Revised Hopkins Verbal Learning Test), 5) visual learning (Revised Brief Visuospatial Memory Test), 6) reasoning and problem solving (Neuropsychological Assessment Battery: Mazes), and 7) social cognition. The Chinese version has also been used to identify cognitive deficits in different populations, especially in patients with psychosis, with a test–retest reliability of subtests ranging from 0.73 to 0.94.21
Outcome Definition
Conversion to psychosis will be one of the major outcomes in this study, which will be determined using the criteria for the Presence of Psychotic Symptoms24 from SIPS. Specifically, the conversion will be defined by the presence of level 6 positive symptoms (the rating “6” refers to severe and psychotic symptoms) identified as either dangerous, disorganised, or occurring at least 1 hour a day on average, over 4 days a week for at least 16 hours.
Poor function is another major outcome in this study, determined by GAF score at the 3-year follow-up point. Specifically, poor function outcome is defined as the GAF score of less than 60 at the follow-up point.25,26
Patient and Public Involvement
Patients and public were not involved in the study design or the development of the research question. The research results will be disseminated to the study participants.
Ethics and Dissemination
This study was approved by the Research Ethics Committees at the Shanghai Mental Health Centre (2019–03). Every participant meeting the inclusion criteria will be fully informed of the study and asked to sign the written informed consent before enrolment. The study will be conducted in full compliance with the Declaration of Helsinki. We will present the study findings in peer-reviewed journals, and national and international conferences.
Discussion
Although the widely adopted psychological treatments, including cognitive behavioural therapy and integrated psychological approaches, appear to decrease the severity of attenuated symptoms in CHR subjects, none of them has yet been proven to reduce the transition to psychosis in the long term.27,28 The promising treatment of nutrition supplements such as omega-3 polyunsaturated fatty acids also shows no improvements in transition rates. Effective interventions in CHR individuals, especially medication, have not been well documented and it remains controversial whether antipsychotics are appropriate since there are only few clinical trials on the long-term efficacy and safety of antipsychotics. This paper describes the design of a prospective, large-scale, observational cohort study that provides a real-world setting to explore the effectiveness of antipsychotics and safety issues, risk factors, clinical course, management, and outcomes in CHR individuals. To our knowledge, this will be the largest observational study that will systematically investigate the effectiveness of antipsychotics in preventing psychosis. By exploring the use of antipsychotics and clinical course during the pre-morbid phase of psychosis, including conversion to psychosis and poor function, this study will inform on whether antipsychotics are appropriate to be administered in CHR subjects. Our findings will suggest the timing and ways for applying antipsychotics in CHR individuals and optimal strategies for treatment. This study will also provide information on whether a subgroup of CHR individuals could benefit from conventional treatments by clinicians, thus optimise individualised treatment. Furthermore, by investigating factors existing prior to the use of antipsychotics, during the prodromal phase, that are associated with the subsequent effectiveness of prevention and safety, we will be able to focus on modifiable factors, such as positive symptoms, as they represent potential targets for preventive interventions. In addition, we will analyse factors that influence the effects of available treatment strategies for psychosis onset and poor function, aiming at identifying individuals who may benefit from certain types of antipsychotics. In summary, the current study will improve our knowledge on the effectiveness of antipsychotics during the prodromal phase of psychosis and factors influencing their effectiveness, which will eventually facilitate optimisation of early individualised interventions for the prevention and treatment of psychosis.
Trial Status
This trial (Clinicaltrials.gov identifier NCT04010864) was registered on July 4, 2019, and is currently recruiting participants. Outcome results are expected at the end of 2022.
Abbreviations
CGI, Clinical General Impression; CHR, clinical high risk; GAF, Global Assessment of Function score; HAMA, Hamilton Anxiety Scale; HAMD-17, Hamilton Depression Scale-17; MCCB, MATRICS Consensus Cognitive Battery; MINI, Mini-International Neuropsychiatric Interview; RFS, Role Function Scale; SFS, Social Function Scale; SHARP, ShangHai At Risk for Psychosis; SHARP-2, ShangHai At Risk for Psychosis-Phase 2; SIPS/SOPS, Structured Interview for Prodromal Syndromes/Scale of Prodromal Symptoms; SMHC, Shanghai Mental Health Center; SPPCC, Shanghai Psychotherapy and Psychological Counselling Centre; TESS, NIMH-developed Treatment Emergent Symptom Scale; WAIS-RC, Wechsler Adult Intelligence Scale - Revised China.
Author Contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by Ministry of Science and Technology of China, National Key R&D Program of China (2016YFC1306803), National Natural Science Foundation of China (81671329, 81671332), Shanghai Key Laboratory of Psychotic Disorders (13dz2260500), Science and Technology Commission of Shanghai Municipality (19441907800), Shanghai Jiaotong University Foundation (ZH2018ZDB03), The Clinical Research Center at Shanghai Mental Health Center (CRC2018ZD01, CRC2018ZD04 and CRC2018YB01), Shanghai Mental Health Center Foundation (2016-FX-01, 2017-TSXK-03).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107–120. doi:10.1001/jamapsychiatry.2013.269
2. Nelson B, Yuen HP, Wood SJ, et al. Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry. 2013;70(8):793–802. doi:10.1001/jamapsychiatry.2013.1270
3. Lindstrom E, Bingefors K. Patient compliance with drug therapy in schizophrenia. Economic and clinical issues. PharmacoEconomics. 2000;18(2):106–124. doi:10.2165/00019053-200018020-00002
4. Crumlish N, Whitty P, Clarke M, et al. Beyond the critical period: longitudinal study of 8-year outcome in first-episode non-affective psychosis. Br j Psychiatry. 2009;194(1):18–24. doi:10.1192/bjp.bp.107.048942
5. Zhang T, Xu L, Tang Y, et al. Duration of untreated prodromal symptoms in a Chinese sample at a high risk for psychosis: demographic, clinical, and outcome. Psychol Med. 2018;48(8):1274–1281. doi:10.1017/S0033291717002707
6. Zhang T, Xu L, Tang Y, et al. Relationship between duration of untreated prodromal symptoms and symptomatic and functional recovery. Eur Arch Psychiatry Clin Neurosci. 2018;269:871–877.
7. Zhang T, Cui H, Wei Y, et al. Progressive decline of cognition during the conversion from prodrome to psychosis with a characteristic pattern of the theory of mind compensated by neurocognition. Schizophr Res. 2018;195:554–559. doi:10.1016/j.schres.2017.08.020
8. Zhang T, Cui H, Tang Y, et al. Correlation of social cognition and neurocognition on psychotic outcome: a naturalistic follow-up study of subjects with attenuated psychosis syndrome. Sci Rep. 2016;6:35017. doi:10.1038/srep35017
9. Collin G, Seidman LJ, Keshavan MS, et al. Functional connectome organization predicts conversion to psychosis in clinical high-risk youth from the SHARP program. Mol Psychiatry. 2018. doi:10.1038/s41380-018-0288-x
10. Shakory S, Watts JJ, Hafizi S, et al. Hippocampal glutamate metabolites and glial activation in clinical high risk and first episode psychosis. Neuropsychopharmacol. 2018;43(11):2249–2255. doi:10.1038/s41386-018-0163-0
11. Rauchensteiner S, Kawohl W, Ozgurdal S, et al. Test-performance after cognitive training in persons at risk mental state of schizophrenia and patients with schizophrenia. Psychiatry Res. 2011;185(3):334–339. doi:10.1016/j.psychres.2009.09.003
12. Amminger GP, Schafer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146–154. doi:10.1001/archgenpsychiatry.2009.192
13. McGorry PD, Nelson B, Markulev C, et al. Effect of omega-3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: the NEURAPRO randomized clinical trial. JAMA Psychiatry. 2017;74(1):19–27. doi:10.1001/jamapsychiatry.2016.2902
14. McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59(10):921–928. doi:10.1001/archpsyc.59.10.921
15. Zhang T, Li H, Woodberry KA, et al. Interaction of social role functioning and coping in people with recent-onset attenuated psychotic symptoms: a case study of three Chinese women at clinical high risk for psychosis. Neuropsychiatr Dis Treat. 2015;11:1647–1654. doi:10.2147/NDT
16. Zhang T, Li H, Woodberry KA, et al. Prodromal psychosis detection in a counseling center population in China: an epidemiological and clinical study. Schizophr Res. 2014;152(2–3):391–399. doi:10.1016/j.schres.2013.11.039
17. Zhang TH, Li HJ, Woodberry KA. et al. Two-year follow-up of a Chinese sample at clinical high risk for psychosis: timeline of symptoms, help-seeking and conversion. Epidemiol Psychiatr Sci;2017. 287–298. doi:10.1017/S2045796016000184
18. Zheng L, Wang J, Zhang T, Li H, Li C, Jiang K. The Chinese version of the SIPS/SOPS: a pilot study of reliability and validity. Chin Mental Health J. 2012;26(8):571–576.
19. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi:10.1093/oxfordjournals.schbul.a007040
20. Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the structured interview for prodromal syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159(5):863–865. doi:10.1176/appi.ajp.159.5.863
21. Shi C, He Y, Cheung EF, Yu X, Chan RC. An ecologically valid performance-based social functioning assessment battery for schizophrenia. Psychiatry Res. 2013;210(3):787–793. doi:10.1016/j.psychres.2013.09.023
22. Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS consensus cognitive battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165(2):214–220. doi:10.1176/appi.ajp.2007.07010043
23. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi:10.1176/appi.ajp.2007.07010042
24. McGlashan TWB, Woods S. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up. New York: Oxford University Press; 2010.
25. Austin SF, Mors O, Secher RG, et al. Predictors of recovery in first episode psychosis: the OPUS cohort at 10 year follow-up. Schizophr Res. 2013;150(1):163–168. doi:10.1016/j.schres.2013.07.031
26. Simonsen C, Faerden A, Romm KL, et al. Early clinical recovery in first-episode psychosis: symptomatic remission and its correlates at 1-year follow-up. Psychiatry Res. 2017;254:118–125. doi:10.1016/j.psychres.2017.04.050
27. Morrison AP, French P, Stewart SL, et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ. 2012;344:e2233.
28. Bechdolf A, Wagner M, Ruhrmann S, et al. Preventing progression to first-episode psychosis in early initial prodromal states. Br j Psychiatry. 2012;200(1):22–29. doi:10.1192/bjp.bp.109.066357
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
