Back to Journals » Cancer Management and Research » Volume 13
Real-World Data on Osimertinib in Chinese Patients with Pretreated, EGFR T790M Mutation Positive, Advanced Non-Small Cell Lung Cancer: A Retrospective Study
Authors Peng D, Shan D, Dai C, Li J, Wang Z, Huang Z, Peng R, Zhao P, Ma X
Received 18 October 2020
Accepted for publication 3 December 2020
Published 26 February 2021 Volume 2021:13 Pages 2033—2039
DOI https://doi.org/10.2147/CMAR.S287466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Da Peng,1 Dongfeng Shan,2 Chengcheng Dai,1 Jie Li,3 Zifan Wang,1 Ziyi Huang,1 Rui Peng,1 Peng Zhao,4 Xuezhen Ma1
1Department of Oncology, Affiliated Qingdao Central Hospital, Qingdao University, Qingdao, Shandong, People’s Republic of China; 2The Affiliated Hospital of Qingdao University, Qingdao, Shandong, People’s Republic of China; 3Department of Oncology, Jiaozhou Central Hospital, Jiaozhou, People’s Republic of China; 4Biotherapy Center, Affiliated Qingdao Central Hospital, Qingdao University, Qingdao, Shandong, People’s Republic of China
Correspondence: Xuezhen Ma
Department of Oncology, Affiliated Qingdao Central Hospital, Qingdao University, No. 127 Siliunan Road, Qingdao, Shandong, 266042, People’s Republic of China
Tel +86-18660229289
Fax +86-532-85953085
Email [email protected]
Peng Zhao
Biotherapy Center, Affiliated Qingdao Central Hospital, Qingdao University, Qingdao, Shandong, People’s Republic of China
Email [email protected]
Purpose: As a third-generation EGFR TKI has been taken orally, Osimertinib effectively inhibits mutant EGFR, including T790M EGFR resistance mutations. Here, we examined real-world efficacy and tolerability of Osimertinib among Chinese patients with advanced EGFR T790M-mutant NSCLC.
Patients and Methods: A total of 106 advanced NSCLC patients who were taking Osimertinib following disease progression after EGFR-TKIs or other treatments were retrospectively recruited in this study. The PFS and OS after Osimertinib treatment were analyzed as the primary endpoints.
Results: Osimertinib was used as a second line and ≥ 3rd line treatment in 22.6% and 77.4% of the patients, respectively. DCR and ORR were 93.4% and 57.5%, respectively. Median PFS was 12.4 12 (95% CI, 10.5– 13.5) months. The PFS was 11 (8.0, 14.0) and 12 (10.3,13.7) months (p = 0.373), in patients with and without CNS metastasis, respectively. PFS in 2nd and ≥ 3rd line treatment was 11 (9.0, 13.0) and 12.4 12 (8.9, 15.1) months (p = 0.799), respectively. In patients with EGFR exon 19 deletion and exon 21 L858 mutation, the median PFS was 11 (9.2, 12.8) and 12 (9.2, 14.8) months, respectively (p = 0.833). Median PFS in the monotherapy group and combined anti-angiogenesis group was 11 (9.9,12.1) and 14 (11.2,16.8) months, respectively. Median OS after Osimertinib initiation was 27 (19.6, 34.4) months: 15 (6.9, 23.1) and 27 (22, 32) months in patients with and without CNS metastasis (p=0.027), 27 (20.3,33.7) months and (undefined) as second line or ≥ 3rd line of treatment (p = 0.421), respectively. In patients with exon 19 deletion, the median OS was not reached, and in patients with exon 21 L858 mutations, the median OS was 23 (19.1,29.9) months (p=0.027). Median OS in the monotherapy group was 27 (21.7,32.3) months, and in combined anti-angiogenesis group was not reached (p=0.68).
Conclusion: Osimertinib can effectively treat advanced NSCLC with T790M mutations independently of previous treatment lines.
Keywords: osimertinib, EGFR-activating mutations, T790M EGFR mutation, tyrosine-kinase inhibitors, non-small cell lung cancer
Introduction
Lung cancer which comprises non-small-cell lung cancer (NSCLC) is the leading cause of cancer-related mortality globally.1 About 50% of the Asian NSCLC patients have mutations which activating EGFR (epidermal growth factor receptor) activating.2 EGFR tyrosine kinase inhibitors (TKIs) like erlotinib gefitinib and icotinib exhibit clinical efficacy against EGFR-mutant NSCLC.3–6 Although the disease initially responds to EGFR-TKIs, drug resistance often develops within 1 year after treatment.7,8 Resistance to first or second generation EGFR-TKIs is mainly driven by a T790M mutation in exon 20. This mutation converts threonine 790 of the EGFR kinase domain to methionine and accounts for 50–60% of the resistant patients.9,10 The AURA trial program investigated the efficacy of Osimertinib (AZD9291), a 3rd generation EGFR TKI that overcomes T790M-mediated TKI resistance.11 AURA2, AURA3 and AURA extension trial uncovered high clinical activity as evidenced by a disease control rate (DCR) of >90% and an overall response rate (ORR) of nearly 70%.12–14
Here, we explored the tolerability and efficacy of Osimertinib in Chinese patients with advanced EGFR T790M-mutant NSCLC. We also analyzed the effects of combination therapy, such as chemotherapy, radiotherapy, and angiogenesis.
Patients and Methods
Data Source and Study Population
Clinical data from 106 advanced EGFR T790M-mutant NSCLC patients were retrospectively collected from Qingdao Central Hospital and the affiliated Hospital of Qingdao University from Mar 1, 2017 to Aug 1, 2020. All patients, who acquired disease progression after treatment with EGFR-TKIs with or without other treatments, received Osimertinib once per day until disease progression, intolerable toxicity or death. All patients with metastatic NSCLC (stage IIIB and IV) or locally advanced were required to undergo histological or cytological confirmation of T790M mutation at least once during the disease period. Patients who received Osimertinib for <3 weeks were not recorded. Patients with an Eastern cooperative oncology group performance status (ECOG PS) of >2 were excluded. There was no limit to the type of treatment (chemotherapy, radiotherapy, or TKI) received prior to Osimertinib. The parameters analyzed included age, smoking status, EGFR mutation status, gender, histological type, first-generation PFS, clinical stage, metastasis site, past therapy and toxicity.
Assessments
Primary endpoints (PFS and OS) were assessed from Osimertinib initiation by a senior oncologist. Secondary endpoints were safety, ORR and DCR. PFS refers to the time between initiation of Osimertinib treatment and progressive disease (PD) or death due to any cause (whichever occurred first). OS refers to the time between initiation of Osimertinib treatment to death from any cause. Patients who were living or without tumor progression at the time of data collection were PFS-counted at the date of the last contact. Patients living at data cut-off were OS-censored at the date of the last contact. The DCR and ORR were assessed using Response evaluation criteria in solid tumor (RECIST), version 1.1. DCR refers to the percentage of patients with stable disease (SD), complete remission (CR) or partial remission (PR) for ≥6 weeks before disease progression, while ORR referred to CR or PR. Tumor response was assessed every 8–12 weeks via radiographic scanning (such as chest/abdomen CT or MRI for brain lesions) based on medical records. Adverse events (AEs) were determined and graded in line with the National cancer institute common terminology criteria for adverse events (CTCAE, version 4.0).
Statistical Analysis
The last date for data collection was Aug 1, 2020. Data analysis was performed using the SPSS version 23.0. Mean values between groups were compared with a two-sided t-test. Alpha = 0.05 served as the significance threshold. The log-rank test was employed to compared Survival curves in various subgroups. Fisher’s exact tests and the Chi-square were employed to analyze the DCR and ORR. The Kaplan-Meier method was utilized to analyze the PFS and OS.
Results
Patient Characteristics
One hundred and six patients who had acquired the T790M mutation prior to Osimertinib initiation met inclusion criteria. Their median age was 60.7 (range: 37–81), 65.1% (69/106) of the patients were women, 84% (88/106) were non-smokers, 76.4% (81/106) had an ECOG PS≤1, and 96.2% (102/106) had adenocarcinoma (Table 1).
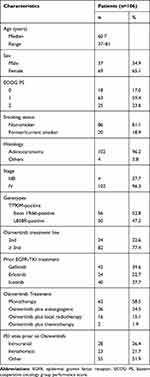 |
Table 1 Baseline Clinical and Demographic Features of Patients |
The key molecular baseline parameters at diagnosis were EGFR exon 19 deletion (52.8%) and EGFR L858R exon 21 L858 mutation (47.2%). All patients had metastatic disease at Osimertinib initiation and had received first generation EGFR-targeting TKI prior to Osimertinib. The patients had received Gefitinib (42 patients; 39.6%), erlotinib (24 patients; 22.7%), and Icotinib (40 patients; 37.7%). One hundred and six patients were eligible for response analysis, 24 as 2nd-line therapy and 82 as ≥3rd line. Twenty-eight patients (26.4%) had brain metastases prior to Osimertinib initiation, while 23 (21.7%) had intrathoracic metastases. Sixty-two patients (58.5%) received Osimertinib monotherapy, 26 (24.5%) received combined anti-angiogenesis therapy, 16 (15.1%) had treatment combined with local radiotherapy, and 2 patients (1.9%) received combined chemotherapy. At Osimertinib initiation, four patients were diagnosed with locally advanced stage IIIB disease and 102 with metastatic, stage IV disease.
Clinical Outcomes
At data cutoff, 22 patients (20,6%) were still on Osimertinib and the median duration of follow-up was 18 months. The Summary of clinical activity for Osimertinib is shown in Table 2.
 |
Table 2 Summary of Clinical Activity for Osimertinib |
Tumor Response
Of the 106 patients, 60 (56.6%) had PR, 38 (35.9%) had SD, and 7 (6.6%) had PD. DCR and ORR were 93.4% and 57.5%, including 1 patient with complete remission (CR). DCR was similar between 2nd line and ≥3rd line treatment groups, and between patients harboring the sensitizing EGFR exon19del and L858R mutations. DCR in patients without CNS metastases was higher than in those with CNS metastases but without significant differences (p = 0.143). ORR was markedly higher in patients with exon19del mutations and in ≥3rd line treatment groups but without significant differences (p = 0.308, p = 0.061). DCR in patients receiving Osimertinib monotherapy plus anti-angiogenics or local radiotherapy was 88.7%, 100%, and 100%, respectively. ORR in patients receiving Osimertinib monotherapy, plus anti-angiogenics or local radiotherapy was 53.2%, 61.5%, and 75.0%, respectively. DCR differences were seen in the monotherapy vs combined treatment group (88.7 vs 100%, p = <0.05). However, no statistical differences were observed in the ORR.
PFS and OS
At data cutoff, 90 of 106 patients (84.9%) had progressed or died. Median PFS was 12 months (95% CI: 10.5–13.5) and median OS was 27 months (95% CI: 19.6–34.4). PFS analysis by treatment line, genotype, CNS metastases, smoking status, age, sex, treatment line, and Osimertinib treatment revealed differences without statistical significance. OS analysis by genotype revealed significantly longer OS in patients who had both the T790M mutation and exon19del (not reached) relative to those with the T790M mutation and L858R mutation (median = 23 months, 95% CI: 19.1–29.9, p = <0.05). In the exon19del group, 40 (75%) patients were alive at data cut-off. Thus, the median OS was not reached. OS analysis by CNS metastases revealed significantly higher survival in patients without CNS metastases relative to those with CNS metastases (median = 27 months, 95% CI: 22–32) vs (median = 15 months (95% CI: 6.9–23.1), p = <0.05). Female patients who received Osimertinib exhibited better median OS relative to male patients (p = <0.05). Comparison between monotherapy and combined therapy groups did not reveal statistically significant differences. The Kaplan-Meier curves of PFS and OS are shown in Figures 1 and 2, respectively.
Safety
Rash (6.6%), low white blood cell count (6.6%), and dry skin (5.7%) were the most frequent AEs. Most AEs were of grade 1 or 2 in severity. One grade 4 event was recorded but no grade 3 cases or AE-associated mortalities were observed. While a dose reduction or drug discontinuation due to AEs was not observed, 3 (2.8%) of the patients suffered from dose interruption (1 due to grade 4 white blood cell decrease, 1 due to grade 3 platelet count decrease, and 1 due to grade 3 headache caused by encephaledema). One patient suffered from pulmonary fibrosis, an unusual side effect that we could not definitively linked to Osimertinib. A summary of AEs is shown in Table 3.
 |
Table 3 Adverse Events (n=106) |
Discussion
This was a real-world analysis of Osimertinib’s efficacy and safety in Chinese patients with EGFR T790M-mutant NSCLC, who fail to respond to prior EGFR‐TKI treatment. All patients had low-performance status and multiple past treatment lines. Our primary endpoint DCR was 93.4% in the overall population and ranged from 88.7% to 100.0% in subgroups. These results were consistent with AURA,11 AURA 2,12 and AURA313 prospective clinical trials. However, the ORR (57.5%) was lower than reported in the randomized trials, possibly because 82/106 (77.4%) patients received Osimertinib as a ≥3rd line therapy while it was used as 2nd line therapy in AURA 3. Our study’s median PFS (12 months) was similar to the median PFS (9.9 months) described in the AURA extension and AURA 2 studies, which included T790M-positive advanced NSCLC cases that underwent Osimertinib pretreatment following failed first or second generation EGFR TKIs. The other primary endpoint, median PFS, was also comparable to the median PFS of Osimertinib (10.1 months) described in AURA3 trial, a Phase 3 trial comparing Osimertinib to patients with advanced NSCLC progressing harboring T790M mutation after first-generation TKI.13 Here, we observed a median OS of 27 months after Osimertinib initiation, which is consistent with pooled AURA extension and AURA3 finding of a median OS of 26.8 months.14,15
In a subset analysis, we noted that patients carrying both T790M and exon19del mutations prior to Osimertinib initiation had longer median OS relative to those carrying both T790M and L858R mutations, which is consistent with findings that patients with exon19del benefit more from early-generation EGFR-TKIs relative to those with L858R mutations.16 It may be that the EGFR-TKIs inhibited exon 19del mutation more effectively. Additionally, Osimertinib exhibited clinical efficacy independently of the number of treatment lines used or previous treatment strategies.
Past studies showed EGFR mutations increase the risk of CNS metastases in NSCLC patients.17 Preclinical studies found that Osimertinib penetrates the blood-brain barrier more efficiently than afatinib or gefitinib,18 and exhibited better intracranial efficacy in patients with advanced NSCLC.13,19 Here, patients with or without CNS metastases exhibited similar DCR, ORR and PFS, but the latter had longer median OS (12 months). Even for NSCLC patients with CNS metastases, we observed an encouraging median PFS of 11 months. These results confirmed the promising intracranial efficacy of Osimertinib in T790M-mutant NSCLC. Better intracranial efficacy was observed in T790M-mutant NSCLC. Notably, we observed that in patients without CNS metastases, no new CNS metastases occurred following Osimertinib resistance, indicating Osimertinib is active in the CNS, which is in line with preclinical and clinical studies.20–22
In our analysis, we put greater emphasis on patient subgroups that were previously underreported and found that monotherapy did not exhibit any difference in efficacy relative to therapy combined with bevacizumab. This observation differs from past findings,23–25 which may be attributable to the following: a) 83% (88/106) of patients in our study had an ECOG PS≤1, b) most patients in our study were on third-line treatment, and c) the study’s small sample size.
Among the limitations include the retrospective nature of data collection, possibility of selection biases, inconsistent definition of disease progression across studies.
In summary, Osimertinib is an effective and safe therapy for advanced T790M-mutant NSCLC, independently of prior treatment lines.
Ethical Statement
The study was approved by the ethics committee of Qingdao Central Hospital and affiliated Hospital of Qingdao University. This study complied with Declaration of Helsinki guidelines. Given the retrospective design of the study, some patients had passed away before the study was conducted and therefore could not give informed consent. Patient-specific information was not used in the study. Thus, the ethics committee exempted our study from requiring informed consent.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892–2911.
3. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi:10.1056/NEJMoa0909530
4. Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14(10):953–961. doi:10.1016/S1470-2045(13)70355-3
5. Zhou C, Wu Y, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi:10.1016/S1470-2045(11)70184-X
6. Zhou C, Wu Y, Chen G, et al. Final overall survival results from a randomised, Phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol. 2015;26(9):1877–1883. doi:10.1093/annonc/mdv276
7. Ercan D, Choi HG, Yun CH, et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res. 2015;21(17):3913–3923. doi:10.1158/1078-0432.CCR-14-2789
8. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi:10.1158/1078-0432.CCR-12-2246
9. Cortot A, Jänne P. Molecular mechanisms of resistance in epidermal growth factor receptor-mutant lung adenocarcinomas. Eur Respir Rev. 2014;23(133):356–366. doi:10.1183/09059180.00004614
10. Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. 2015;5(5):390–401. doi:10.1016/j.apsb.2015.07.001
11. Jänne P, Yang J, Kim D, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–1699. doi:10.1056/NEJMoa1411817
12. Goss G, Tsai C, Shepherd F, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, Phase 2 study. Lancet Oncol. 2016;17(12):1643–1652. doi:10.1016/S1470-2045(16)30508-3
13. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi:10.1056/NEJMoa1612674
14. Yang J, Ahn M, Kim D, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study Phase II extension component. J Clin Oncol. 2017;35(12):1288–1296. doi:10.1200/JCO.2016.70.3223
15. Wu Y-L, Mok TSK, Han J-Y, et al. Overall survival (OS) from the AURA3 phase III study: osimertinib vs platinum-pemetrexed (plt-pem) in patients (pts) with EGFR T790M advanced non-small cell lung cancer (NSCLC) and progression on a prior EGFR-tyrosine kinase inhibitor (TKI). Ann Oncol. 2019;30:ix158. doi:10.1093/annonc/mdz437.001
16. Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12(13):3908–3914. doi:10.1158/1078-0432.CCR-06-0462
17. Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–111. doi:10.1016/j.lungcan.2015.01.020
18. Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–5140. doi:10.1158/1078-0432.CCR-16-0399
19. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018:Jco2018783118.
20. Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–1061. doi:10.1158/2159-8290.CD-14-0337
21. Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol. 2018;29(3):687–693. doi:10.1093/annonc/mdx820
22. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized Phase III trial (AURA3). J Clin Oncol. 2018;36(26):2702–2709. doi:10.1200/JCO.2018.77.9363
23. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non-small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229–237. doi:10.1007/s40264-017-0596-0
24. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi:10.1016/S1470-2045(19)30035-X
25. Yu HA, Schoenfeld AJ, Makhnin A, et al. Effect of osimertinib and bevacizumab on progression-free survival for patients with metastatic EGFR-mutant lung cancers: a phase 1/2 single-group open-label trial. JAMA Oncol. 2020;6(7):1–8. doi:10.1001/jamaoncol.2020.1260
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


